The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Burger Meal
2.2. Burger Antioxidant Composition Analysis
2.3. Subjects
2.4. Study Design
2.5. Anthropometric and Blood Pressure Measurements
2.6. Mediterranean Diet Score
2.7. Biochemical Procedures
2.8. Statistical Analysis
3. Results
3.1. Burger Composition
3.2. Subject Baseline Characteristics
3.3. Anthropometric Measurements and Metabolic Syndrome Component Prevalence over Time
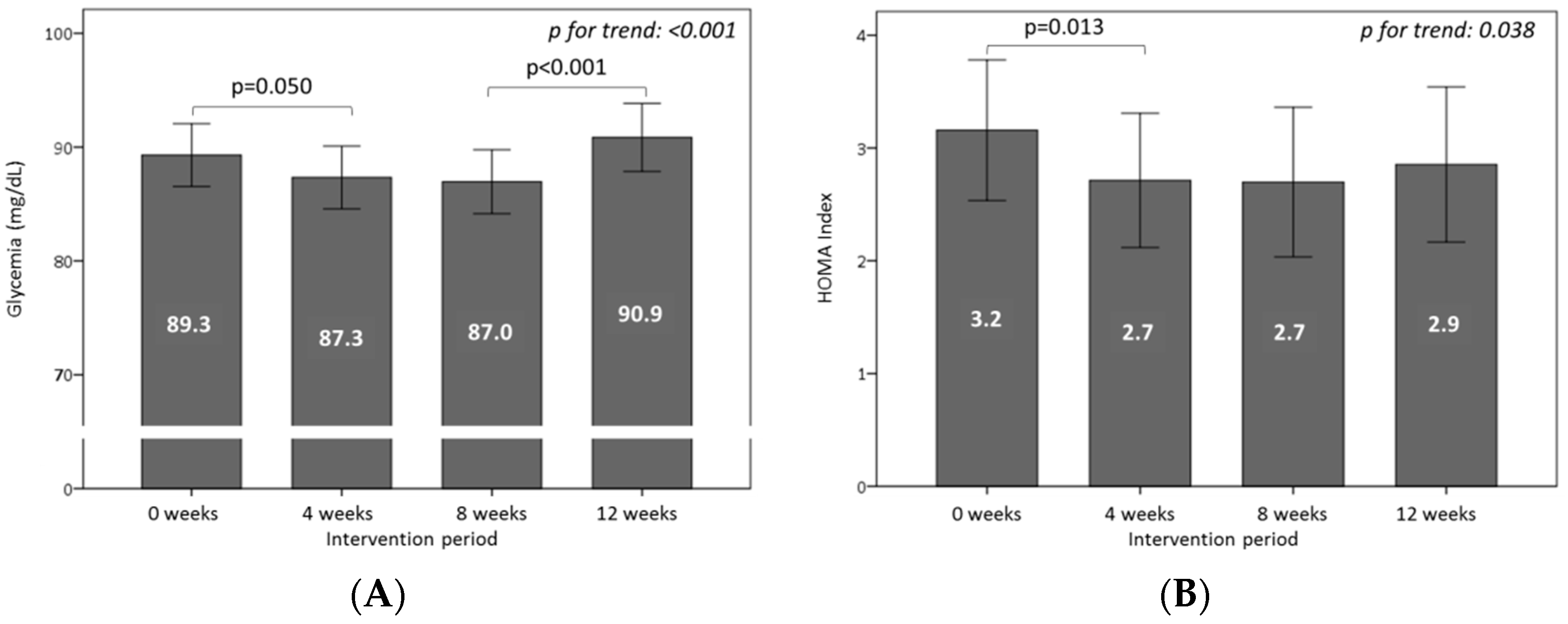
3.4. Effect of Consuming Burgers on Plasma Glucose and Insulin Levels
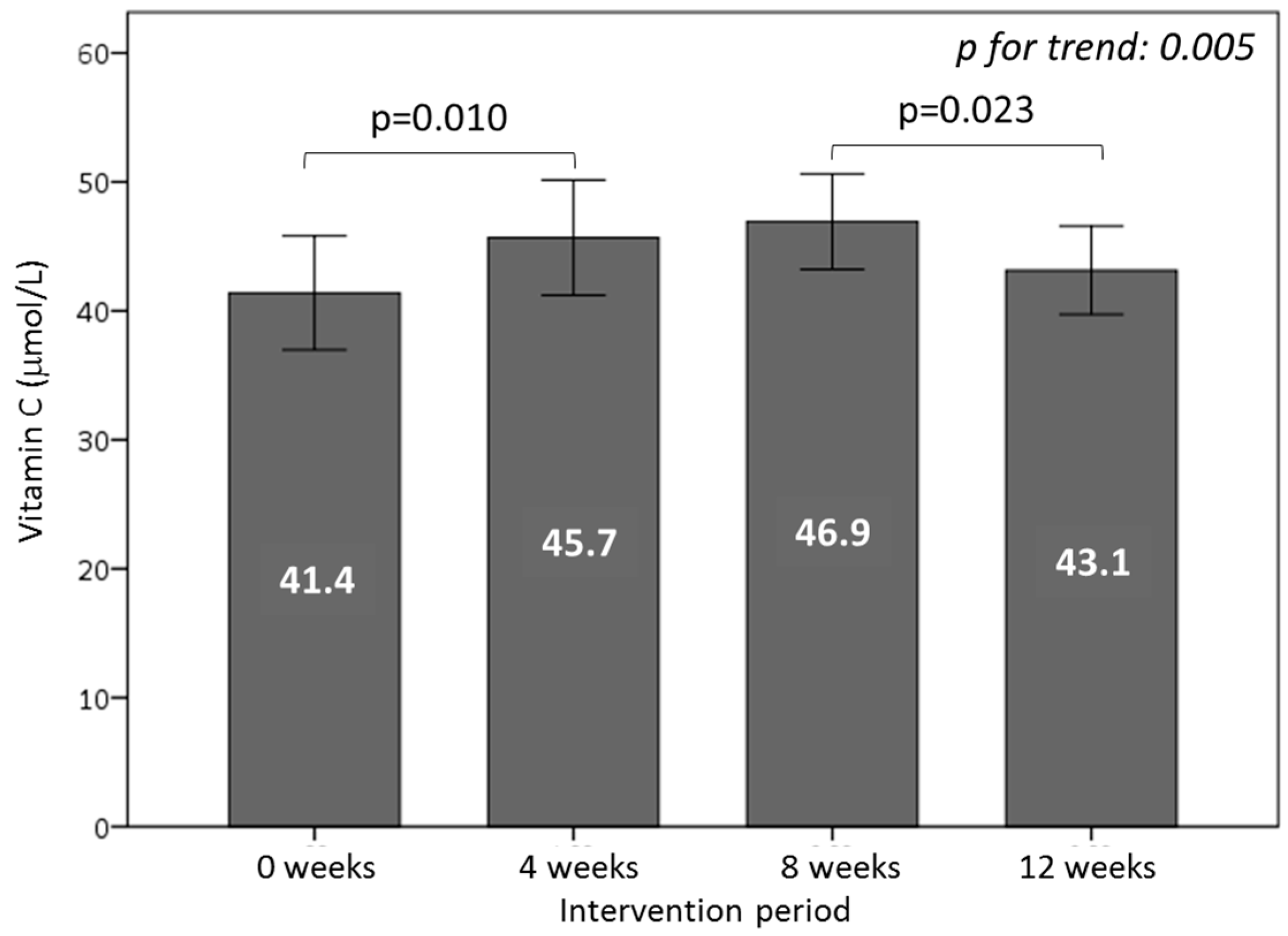
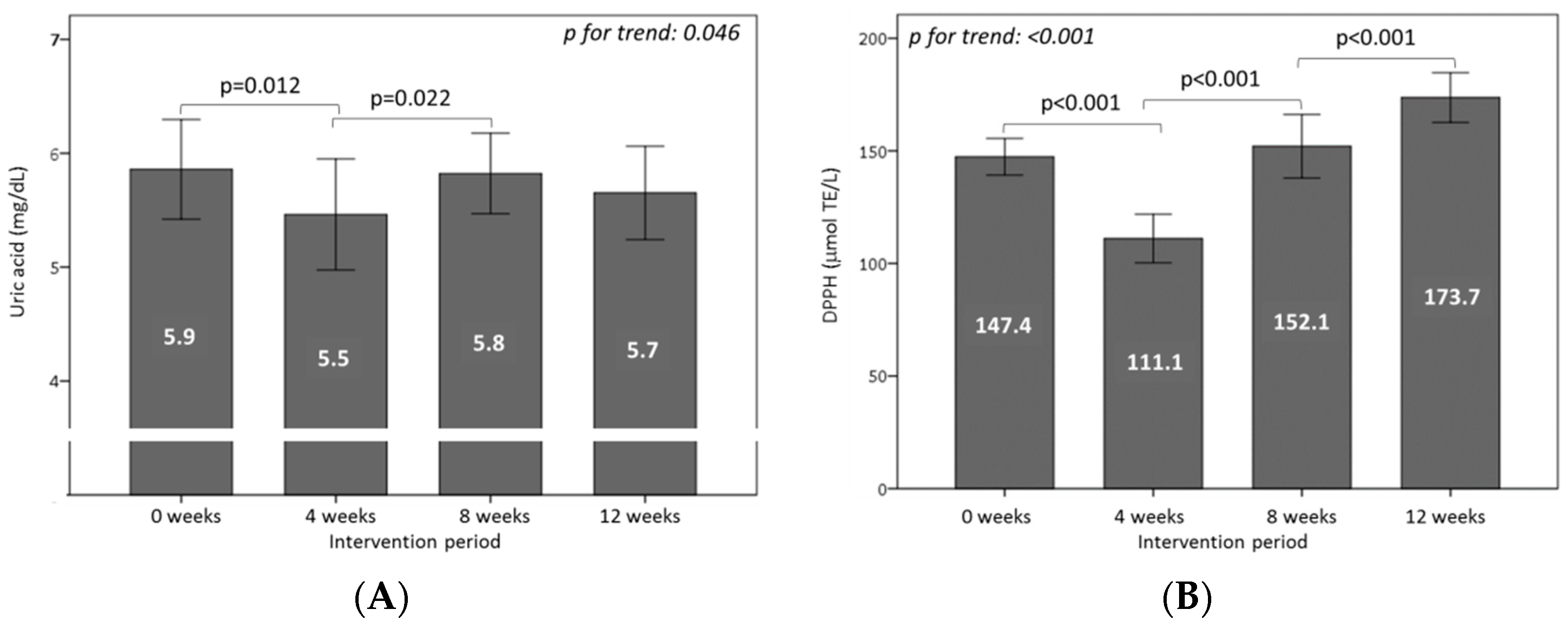
3.5. Effect of Consuming Burgers on Antioxidants and the Total Antioxidant Capacity in Plasma
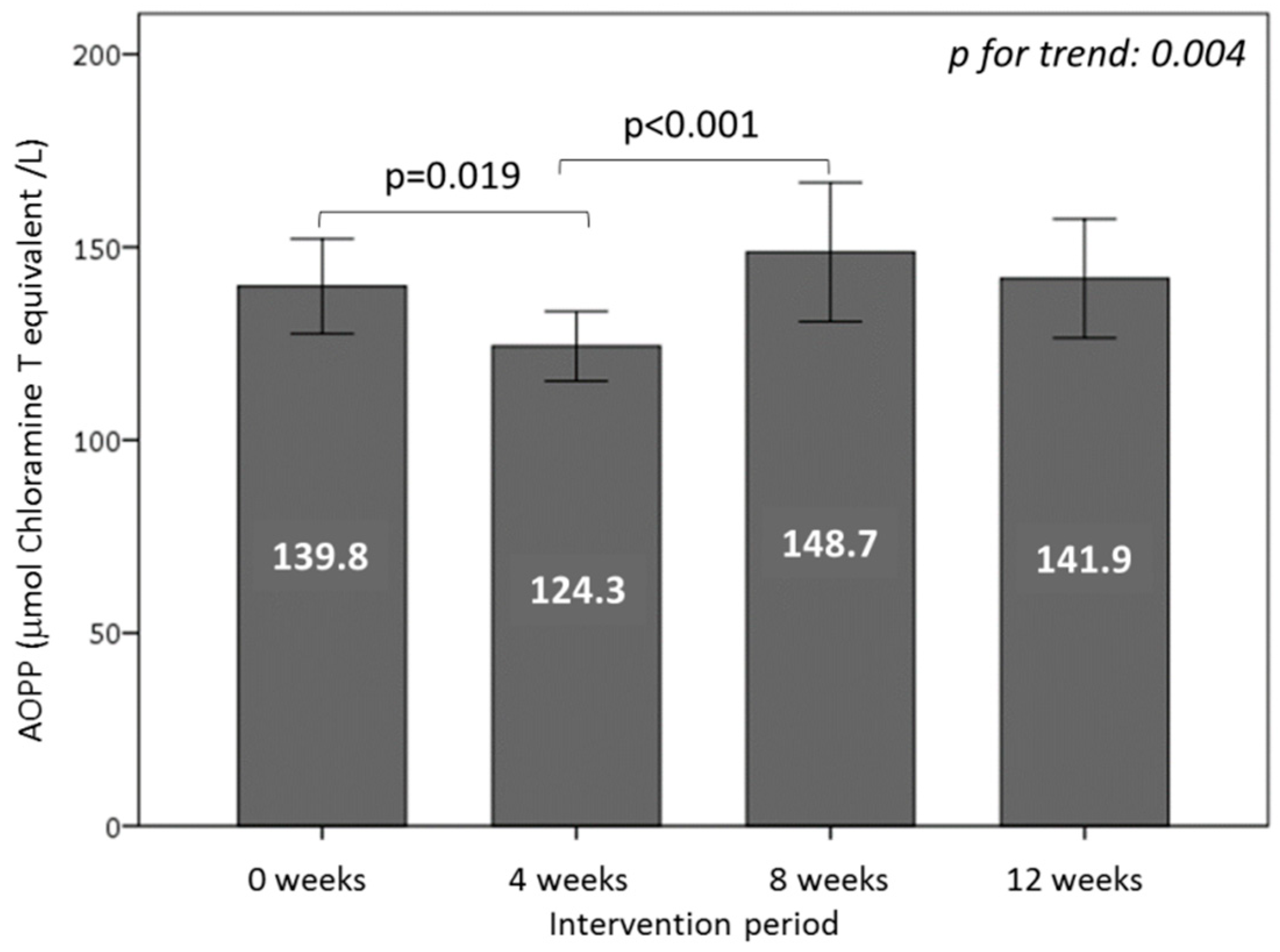
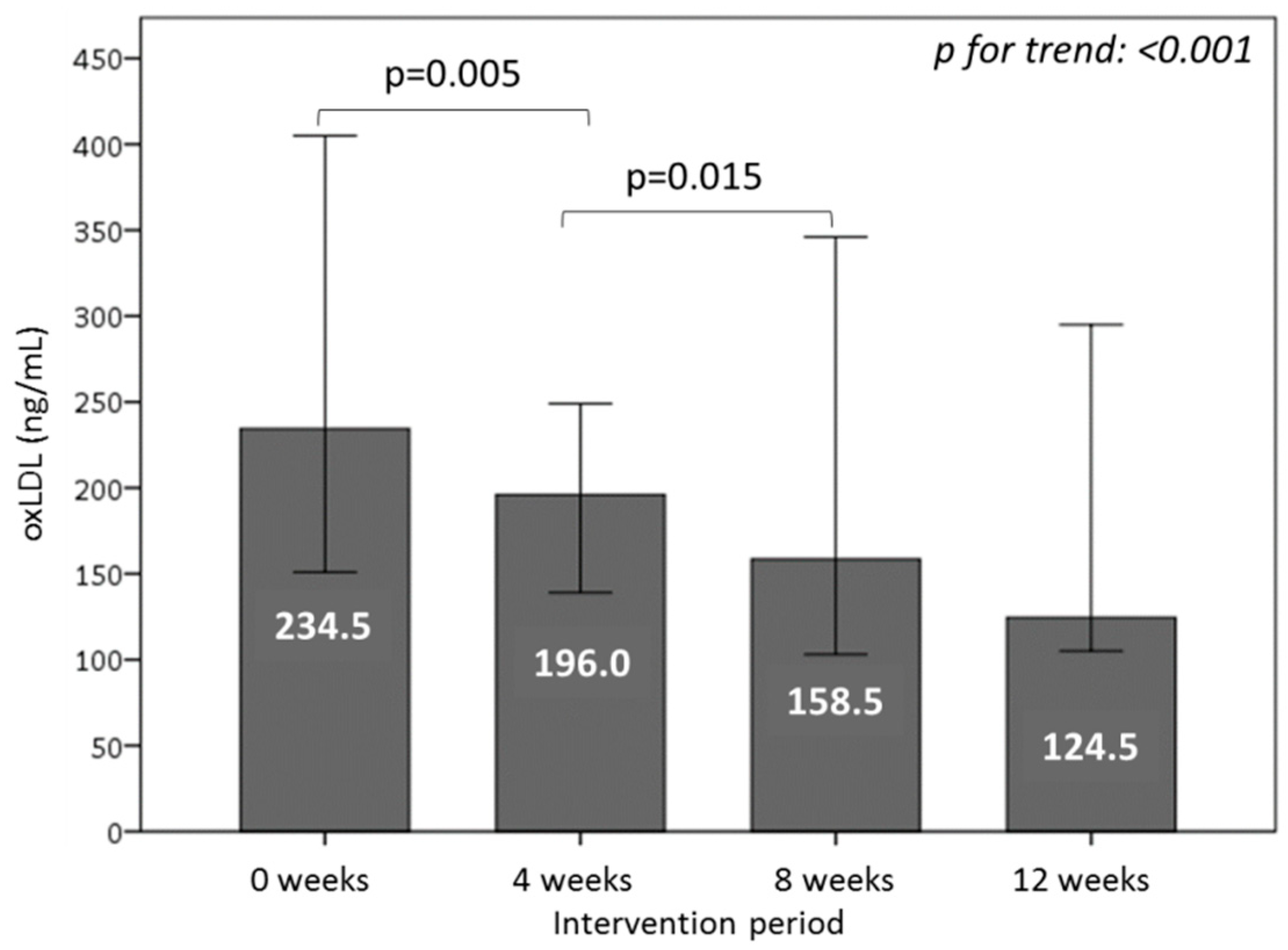
3.6. Effect of Consuming Burgers on Oxidative Damage Markers in Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dussaillant, C.; Echeverria, G.; Urquiaga, I.; Velasco, N.; Rigotti, A. Current evidence on health benefits of the mediterranean diet. Rev. Med. Chil. 2016, 144, 1044–1052. [Google Scholar] [PubMed]
- Urquiaga, I.; Echeverria, G.; Dussaillant, C.; Rigotti, A. origin, components and mechanisms of action of the mediterranean diet. Rev. Med. Chil. 2017, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The mediterranean diets: What is so special about the diet of greece? The scientific evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Kesse, E.; Slimani, N.; Bueno-De-Mesquita, H.B.; Ocke, M.C.; Skeie, G.; Kumle, M.; Dorronsoro Iraeta, M.; Morote Gomez, P.; Janzon, L.; et al. Meat consumption in the european prospective investigation into cancer and nutrition (epic) cohorts: Results from 24-hour dietary recalls. Public Health Nutr. 2002, 5, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Environ. Health Prev. Med. 2002, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Berrino, F. Western diet and alzheimer’s disease. Epidemiol. E Prev. 2002, 26, 107–115. [Google Scholar]
- Mascitelli, L.; Goldstein, M.R.; Zacharski, L.R. Iron, oxidative stress, and the mediterranean diet. Am. J. Med. 2014, 127, e49. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Genoux, A.; Ferrieres, J.; Perret, B.; Carayol, M.; Drouet, L.; Ruidavets, J.B. Iron status is associated with carotid atherosclerotic plaques in middle-aged adults. J. Nutr. 2010, 140, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Gorlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ros: A summary of the findings and prospects for the future from the cost action bm1203. Redox Boil. 2017, 13, 94–162. [Google Scholar] [CrossRef] [PubMed]
- Hosafci, P. Processed Meat. Available online: http://www.globalmeatnews.com/Analysis/Processed-meat-what-is-the-new-Euromonitor-data-telling-us (accessed on 17 August 2014).
- Chile, I.N.E. Carnesy Cecinas. Available online: http://historico.ine.cl/canales/menu/publicaciones/calendario_de_publicaciones/pdf/carnesycecinas.pdf (accessed on 27 August 2014).
- WHO; Shetty, P.S.; Nishida, C. Diet, Nutrition and the Prevention of Chronic Diseases: Scientific Background Papers of the Joint Who/FAO Expert Consultation, Geneva, 28 January—1 February 2002; CABI Publishing: Wallingford, UK, 2004. [Google Scholar]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Whelton, P.K. Dietary fiber intake and reduced risk of coronary heart disease in us men and women: The national health and nutrition examination survey i epidemiologic follow-up study. Arch. Intern. Med. 2003, 163, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Leighton, F. Wine and health: Evidence and mechanisms. World Rev. Nutr. Diet. 2005, 95, 122–139. [Google Scholar] [PubMed]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Garcia-Alonso, A.; Goni, I.; Bravo, L. In vitro determination of the indigestible fraction in foods: An alternative to dietary fiber analysis. J. Agric. Food Chem. 2000, 48, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Tseng, A.; Cavender, G.; Ross, A.; Zhao, Y. Physicochemical, nutritional, and sensory qualities of wine grape pomace fortified baked goods. J. Food Sci. 2014, 79, S1811–S1822. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuna, S.; Perez, D.; Dicenta, S.; Echeverria, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J. Nutr. 1998, 128, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (orac) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Motchnik, P.A.; Frei, B.; Ames, B.N. Measurement of antioxidants in human blood plasma. Method Enzymol. 1994, 234, 269–279. [Google Scholar]
- Kimoto, E.; Terada, S.; Yamaguchi, T. Analysis of ascorbic acid, dehydroascorbic acid, and transformation products by ion-pairing high-performance liquid chromatography with multiwavelength ultraviolet and electrochemical detection. Method Enzymol. 1997, 279, 3–12. [Google Scholar]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.W.; Hill, M.N.; Jones, D.H.; Kurtz, T.; Sheps, S.G.; Roccella, E.J.; et al. Recommendations for blood pressure measurement in humans: An aha scientific statement from the council on high blood pressure research professional and public education subcommittee. J. Clin. Hypertens. 2005, 7, 102–109. [Google Scholar] [CrossRef]
- Echeverria, G.; Urquiaga, I.; Concha, M.J.; Dussaillant, C.; Villarroel, L.; Velasco, N.; Leighton, F.; Rigotti, A. Validation of self-applicable questionnaire for a mediterranean dietary index in chile. Rev. Med. Chil. 2016, 144, 1531–1543. [Google Scholar] [PubMed]
- Wayner, D.D.; Burton, G.W.; Ingold, K.U.; Locke, S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985, 187, 33–37. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total antioxidant capacity of some commercial fruit juices: Electrochemical and spectrophotometrical approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Templar, J.; Kon, S.P.; Milligan, T.P.; Newman, D.J.; Raftery, M.J. Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated hplc method. Nephrol Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 1999, 14, 946–951. [Google Scholar] [CrossRef]
- Anderson, J.W.; Randles, K.M.; Kendall, C.W.; Jenkins, D.J. Carbohydrate and fiber recommendations for individuals with diabetes: A quantitative assessment and meta-analysis of the evidence. J. Am. Coll. Nutr. 2004, 23, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Robles, H.; Rios, A.; Arellano-Mendoza, M.; Escalante, B.A.; Schnoor, M. Antioxidative diet supplementation reverses high-fat diet-induced increases of cardiovascular risk factors in mice. Oxid. Med. Cell. Longev. 2015, 2015, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Jacobs, D.R., Jr.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; Slavin, J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Costabile, G.; Della Pepa, G.; Anniballi, G.; Griffo, E.; Mangione, A.; Cipriano, P.; Viscovo, D.; Clemente, G.; Landberg, R.; et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid peroxidation in a stomach medium is affected by dietary oils (olive/fish) and antioxidants: The mediterranean versus western diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The postprandial oxidative stress index (posi) for balancing nutrition and human health. Redox Boil. 2017, 12, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin c level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The european prospective investigation of cancer--norfolk prospective study. Arc. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; Fenselau, S.; Keppler, I.; Abel, J.; Orth, B.; Laue, C.; Sturmer, W.; Fauth, U.; Halmagyi, M.; Marz, W. Postprandial triglyceride high response and the metabolic syndrome. Ann. N. Y. Acad. Sci. 1997, 827, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Skarbek, A.; Chrzczanowicz, J.; Kostka, J.; Nowak, D.; Drygas, W.; Jegier, A.; Kostka, T. Cardiovascular risk factors and total serum antioxidant capacity in healthy men and in men with coronary heart disease. BioMed Res. Int. 2014, 2014, 216964. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Huang, Z.; Mo, Z.; Xiao, J. The characteristics and roles of advanced oxidation protein products in atherosclerosis. Cardiovasc. Toxicol. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Descamps-Latscha, B. Advanced oxidation protein products: Novel uraemic toxins and pro-inflammatory mediators in chronic renal failure? Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 1997, 12, 1310–1312. [Google Scholar] [CrossRef]
- Iwao, Y.; Anraku, M.; Hiraike, M.; Kawai, K.; Nakajou, K.; Kai, T.; Suenaga, A.; Otagiri, M. The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (aopp). Drug Metab. Pharmacokinet. 2006, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, E.; Biasci, E.; Giampietro, O. Advanced oxidation protein products in plasma: Stability during storage and correlation with other clinical characteristics. Acta. Diabetol. 2001, 38, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A. Advanced oxidation protein products. Part I. Mechanism of the formation, characteristics and property. Polski Merkuriusz Lekarski Organ Polskiego Towarzystwa Lekarskiego 2010, 28, 166–169. [Google Scholar] [PubMed]
- Cao, W.; Hou, F.F.; Nie, J. Aopps and the progression of kidney disease. Kidney Int. Suppl. 2014, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kocak, H.; Gumuslu, S.; Sahin, E.; Ceken, K.; Gocmen, Y.A.; Yakupoglu, G.; Ersoy, F.F.; Tuncer, M. Advanced oxidative protein products are independently associated with endothelial function in peritoneal dialysis patients. Nephrology 2009, 14, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.C.; Xiao, J.; Tang, S.L.; Ouyang, X.P.; He, P.P.; Lv, Y.C.; Long, Z.F.; Yao, F.; Tan, Y.L.; Xie, W.; et al. Advanced oxidation protein products exacerbates lipid accumulation and atherosclerosis through downregulation of atp-binding cassette transporter a1 and g1 expression in apolipoprotein e knockout mice. Circ. J. 2014, 78, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.J.; Arnold, L.; Flowers, D.; Waeg, G.; Malle, E.; Stocker, R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Investig. 1996, 97, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer-Roob, B.M.; Faustmann, G.; Roob, J.M. Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic. Boil. Med. 2017, 111, 38–86. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Margina, D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. J. Prot. 2013, 92, 313–322. [Google Scholar] [CrossRef] [PubMed]
| WGPF-Burger (100 g) | Control-Burger (100 g) | |
|---|---|---|
| Energy (kcal) | 168 | 168 |
| Proteins (g) | 13.3 ± 0.16 | 16.0 ± 0.24 |
| Total fats (g) | 10.9 ± 0.02 | 11.6 ± 0.06 |
| Saturated fatty acids (g) | 5.7 ± 0.04 | 5.9 ± 0.07 |
| Trans fatty acids (g) | 0.0 | 0.0 |
| Monounsaturated fatty acids (g) | 5.0 ± 0.04 | 5.3 ± 0.05 |
| Polyunsaturated fatty acids (g) | 0.2 ± 0.05 | 0.2 ± 0.05 |
| Cholesterol (mg) | 81 ± 4 | 81 ± 4 |
| Available carbohydrates * (g) | 4.1 ± 0.67 | 0.0 |
| Total sugars (g) | 0.1 | 0.0 |
| Fiber (g) | 3.5 ± 0.67 | 0.0 |
| Polyphenols (mg GE/g) | 1.21 ± 0.02 | 0.396 ± 0.019 |
| ORAC (μmol TE/g) | 17.2 ± 1.29 | 1.82 ± 0.23 |
| Parameter | Mean ± SD |
|---|---|
| Age (years) | 43.6 ± 11.2 |
| Anthropometric parameters | |
| Weight (kg) | 87.1 ± 13.5 |
| Body mass index (kg/m2) | 29.5 ± 3.7 |
| Waist circumference (cm) | 101.0 ± 9.6 |
| Blood pressure | |
| Systolic blood pressure (mmHg) | 122.1 ± 15.1 |
| Diastolic blood pressure (mmHg) | 80.2 ± 10.6 |
| Glucose metabolism | |
| Glucose (mg/dL) | 89.3 ± 7.0 |
| Insulin (mg/dL) | 14.2 ± 6.8 |
| Lipid profile | |
| Total cholesterol (mg/dL) | 189.8 ± 30.5 |
| LDL cholesterol (mg/dL) | 109.7 ± 31.1 |
| HDL cholesterol (mg/dL) | 47.3 ± 14.7 |
| Triglycerides (mg/dL) | 163.3 ± 133.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. https://doi.org/10.3390/nu10101388
Urquiaga I, Troncoso D, Mackenna MJ, Urzúa C, Pérez D, Dicenta S, De la Cerda PM, Amigo L, Carreño JC, Echeverría G, et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients. 2018; 10(10):1388. https://doi.org/10.3390/nu10101388
Chicago/Turabian StyleUrquiaga, Inés, Danitza Troncoso, Maria José Mackenna, Catalina Urzúa, Druso Pérez, Sara Dicenta, Paula María De la Cerda, Ludwig Amigo, Juan Carlos Carreño, Guadalupe Echeverría, and et al. 2018. "The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial" Nutrients 10, no. 10: 1388. https://doi.org/10.3390/nu10101388





