Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants Selection
2.3. Randomization
2.4. Baseline Examination
2.5. Dietary Intervention
2.6. Outcomes
2.7. Implementation
2.8. Statistical Analysis
3. Results
3.1. Sample Description
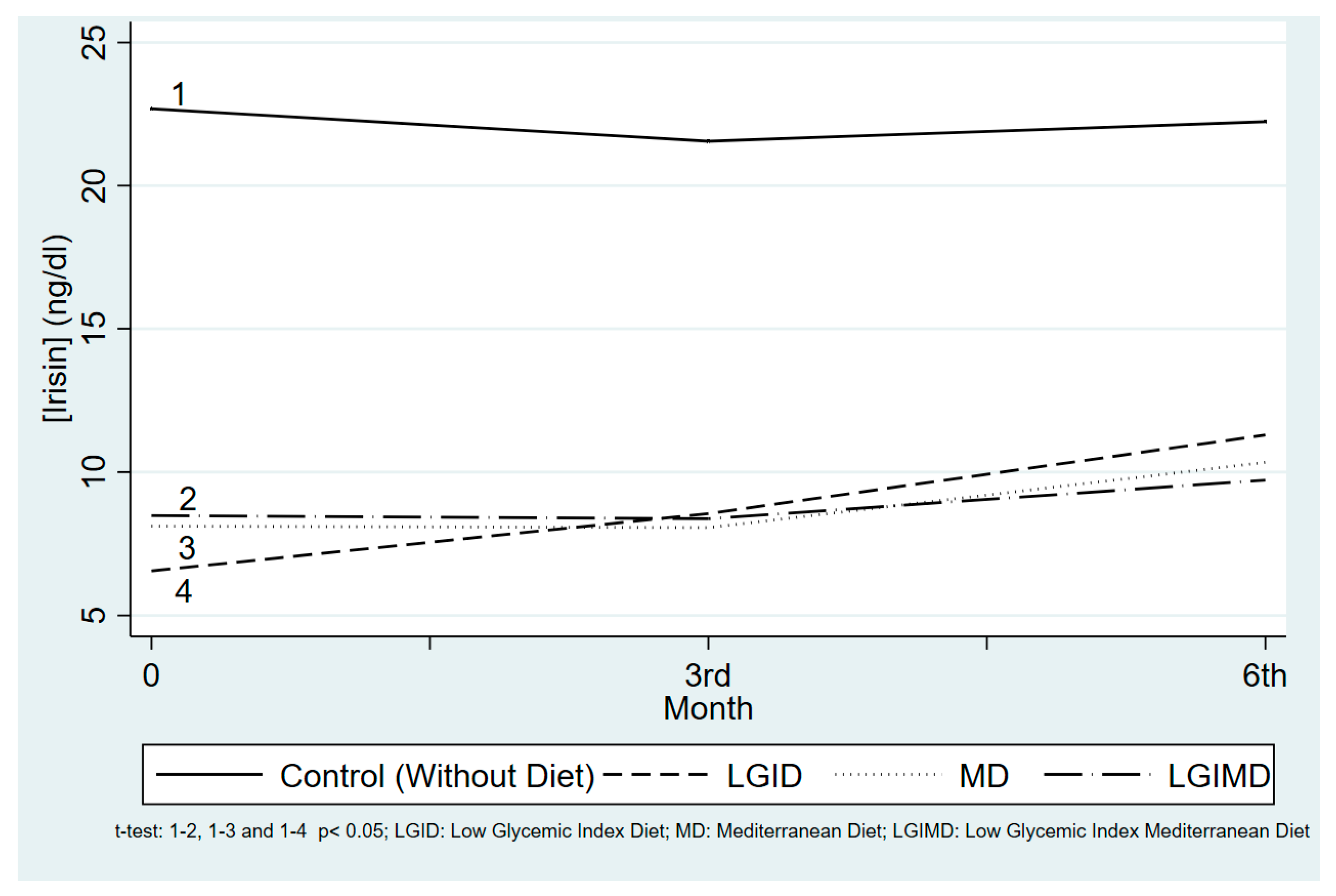
3.2. Time and Compliance with the Diet Influences Serum Concentrations
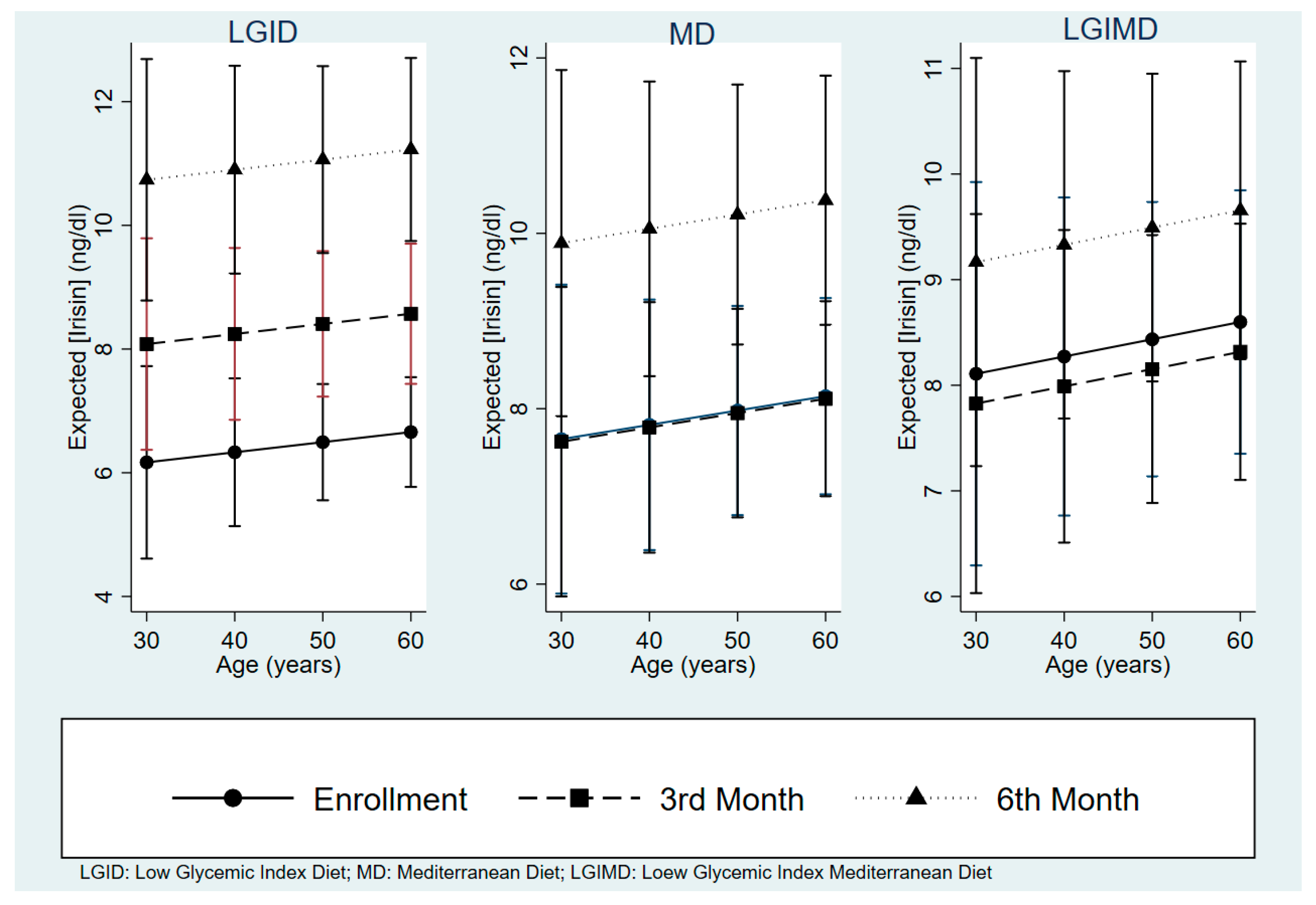
3.3. Effects of Diets and Some Nutrients on Serum Irisin Concentrations
3.4. Each Diet’s Effect on Irisin Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Composition of the Diets
Appendix A.1.1. Food to be Eaten Regularly
Appendix A.1.2. Foods to be Avoided Completely
References
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bergström, G.M. The metabolic syndrome predicts cardiovascular events: Results of a 13-year follow-up in initially healthy 58-year-old men. Metab. Syndr. Relat. Disord. 2012, 10, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Ganotakis, E.S.; Elisaf, M.; Mikhailidis, D.P. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr. Med. Res. Opin. 2005, 21, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Zhao, G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J. Diabetes 2010, 2, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Sánchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Riediger, N.D.; Clara, I. Prevalence of metabolic syndrome in the Canadian adult population. CMAJ 2011, 183, E1127–E1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, K.; Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Singh-Manoux, A.; Tabak, A.G.; Jokela, M.; Virtanen, M.; Ferrie, J.E.; Marmot, M.G.; Shipley, M.J.; Kivimaki, M. Overall diet history and reversibility of the metabolic syndrome over 5 years: The Whitehall II prospective cohort study. Diabetes Care 2010, 33, 2339–2341. [Google Scholar] [CrossRef] [PubMed]
- Case, C.C.; Jones, P.H.; Nelson, K.; O’Brian Smith, E.; Ballantyne, C.M. Impact of weight loss on the metabolic syndrome. Diabetes Obes. Metab. 2002, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.-M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2017, 91, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Jurca, R.; Lamonte, M.J.; Barlow, C.E.; Kampert, J.B.; Church, T.S.; Blair, S.N. Association of muscular strength with incidence of metabolic syndrome in men. Med. Sci. Sports Exerc. 2005, 37, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Akerström, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine Irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.H.; Zhou, Y.B.; Han, Y.; Zhang, Y.M.; et al. FNDC5 overexpression and Irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Corsi Romanelli, M.M. Irisin: A Potential Link between Physical Exercise and Metabolism-An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People. J. Diabetes Res. 2017, 2017, 1039161. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. Available online: https://www.clinicaltrials.gov/ (accessed on 15 November 2017).
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Elia, A. La Rivoluzione Degli Integrali Buoni (Senza L’Aggiunta di Crusca o Fibre) (The Revolution of Good Whole Grains: Without Bran and Fibers Addition); Di Marsico Libri: Bari, Italia, 2010. (In Italian) [Google Scholar]
- Choi, H.Y.; Kim, S.; Park, J.W.; Lee, N.S.; Hwang, S.Y.; Huh, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Youn, B.S.; et al. Implication of circulating Irisin levels with brown adipose tissue and sarcopenia in humans. J. Clin. Endocrinol. Metab. 2014, 99, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic Beta-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum Irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Alis, R.; Pareja-Galeano, H.; Sola, E.; Victor, V.M.; Rocha, M.; Hernandez-Mijares, A.; Romagnoli, M.L. Circulating Irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine 2014, 46, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is Irisin a human exercise gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Folgueira, C.; Sanchez-Rebordelo, E.; Al-Massadi, O. Circulating Irisin Levels Are Not Regulated by Nutritional Status, Obesity, or Leptin Levels in Rodents. Mediat. Inflamm. 2015, 2015, 620919. [Google Scholar] [CrossRef] [PubMed]
- Hee, P.K.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Yan, B.; Shi, X.; Zhang, H.; Pan, L.; Ma, Z.; Liu, S.; Liu, Y.; Li, X.; Yang, S.; Li, Z. Association of serum Irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 2014, 9, e94235. [Google Scholar] [CrossRef] [PubMed]
- Suk Shim, Y.; Jae Kang, M.; Yang, S.; Tae Hwang, I. Irisin is a biomarker for metabolic syndrome in prepubertal children. Endocr. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.B.; Kim, H.J.; Kang, J.H.; Park, S.I.; Park, K.H.; Lee, H.J. Association of circulating Irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism 2017, 73, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Park, K.H.; Shin, S.; Zaichenko, L.; Davis, C.R.; Crowell, J.A.; Joung, H.; Mantzoros, C.S. Diet quality and diet patterns in relation to circulating cardiometabolic biomarkers. Clin. Nutr. 2016, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, M.; Piaggi, P.; Votruba, S.B.; Walter, M.; Krakoff, J.; Thearle, M.S. Increased 24-hour ad libitum food intake is associated with lower plasma Irisin concentrations the following morning in adult humans. Appetite 2015, 90, 154–159. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, R.; Lopez-Legarrea, P.; Crujeiras, A.B.; Pardo, M.; Casanueva, F.F.; Zulet, M.A.; Martinez, J.A. Plasma Irisin depletion under energy restriction is associated with improvements in lipid profile in metabolic syndrome patients. Clin. Endocrinol. 2014, 81, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Zulet, M.A.; Abete, I.; Amil, M.; Carreira, M.C.; Martinez, J.A.; Casanueva, F.F. Interplay of atherogenic factors, protein intake and betatrophin levels in obese-metabolic syndrome patients treated with hypocaloric diets. Int. J. Obes. 2016, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant protein and animal proteins: Do they differentially affect cardiovascular disease risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, T.R.; Ho, B.; Kang, J.; Kaplan, B. Post Hoc Analyses after the Facts. Transplantation 2015, 99, 17–20. [Google Scholar] [CrossRef] [PubMed]
| CONTROL | LGID | MD | LGIMD | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age (years) | ||||||||
| <40 | 16 | 72.7 | 2 | 9.1 | 2 | 9.1 | 2 | 9.1 |
| 40–49 | 20 | 44.4 | 13 | 28.9 | 7 | 15.6 | 5 | 11.1 |
| 50–59 | 13 | 18.3 | 19 | 26.8 | 18 | 25.4 | 21 | 29.6 |
| 60–69 | 20 | 33.3 | 14 | 23.3 | 15 | 25.0 | 11 | 18.3 |
| 70 or more | 11 | 33.3 | 7 | 21.2 | 9 | 27.3 | 6 | 18.2 |
| Gender | ||||||||
| Male | 47 | 34.3 | 29 | 21.2 | 33 | 24.1 | 28 | 20.4 |
| Female | 33 | 35.0 | 26 | 28.0 | 18 | 19.4 | 17 | 18.3 |
| Status | ||||||||
| Single | 5 | 50.0 | 3 | 30.0 | 1 | 10.0 | 1 | 10.0 |
| Married | 72 | 37.9 | 43 | 22.6 | 40 | 21.1 | 35 | 18.4 |
| Widowed | 1 | 9.1 | 4 | 36.4 | 2 | 18.2 | 4 | 36.4 |
| Divorced | 2 | 66.7 | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 |
| Education | ||||||||
| Illiterate | 1 | 16.7 | 2 | 33.3 | 3 | 50.0 | 0 | 0.0 |
| Primary School | 15 | 24.6 | 15 | 24.6 | 15 | 24.6 | 16 | 26.2 |
| Middle School | 26 | 38.2 | 17 | 25.0 | 18 | 26.5 | 7 | 10.3 |
| High School | 29 | 38.2 | 17 | 22.4 | 12 | 15.8 | 18 | 23.7 |
| Graduate | 4 | 26.7 | 4 | 26.7 | 3 | 20.0 | 4 | 26.7 |
| No Information | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 80 | 34.6 | 55 | 23.8 | 51 | 22.1 | 45 | 19.5 |
| Second Month | Fourth Month | ||||
|---|---|---|---|---|---|
| Compliance | Compliance | ||||
| Male | Diet | No | Yes | No | Yes |
| n (%) | n (%) | n (%) | n (%) | ||
| Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| LGID | 44 (53) | 39 (47) | 34 (41) | 49 (59) | |
| [Irisin] # | 8.8 (0.6) * | 7.9 (0.8) | 10.8 (0.7) * | 9.4 (1.1) | |
| MD | 30 (41) | 43 (59) | 30 (41) | 43 (59) | |
| [Irisin] | 8.7 (0.6) * | 8.5 (0.8) ** | 10.2 (0.7) * | 10.5 (1.1) ** | |
| LGIMD | 75 (90) | 8 (10) | 75 (90) | 8 (10) | |
| [Irisin] | 7.5 (0.8) | 6.9 (0.9) ** | 8.3 (0.7) | 9.1 (0.7) ** | |
| Female | Diet | No | Yes | No | Yes |
| n (%) | n (%) | n (%) | n (%) | ||
| Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| LGID | 27 (44) | 35 (56) | 16 (28) | 41 (72) | |
| [Irisin] | 7.6 (0.7) * | 8.2 (0.8) ** | 11.6 (1.1) * | 10.4 (0.8) ** | |
| MD | 20 (39) | 32 (61) | 19 (38.00) | 31 (62.00) | |
| [Irisin] | 7.6 (0.8) * | 8.0 (0.7) ** | 11.2 (1.0) * | 10.7 (0.9) ** | |
| LGIMD | 50 (81) | 12 (19) | 45 (79) | 12 (21) | |
| [Irisin] | 7.7 (1.2) | 8.4 (0.9) | 7.0 (1.0) | 8.5 (0.6) | |
| Variable | Crude Estimates | Adjusted by Age, Gender, BMI, HOMA-IR |
|---|---|---|
| Control (Reference) | 0.00 | 0.00 |
| MD | −14.57 # | −15.77 |
| LGID | −16.13 # | −14.29 |
| LGIMD | −14.20 # | −13.82 |
| 3rd month | 0.00 | 0.00 |
| 6th month | 0.00 | 0.00 |
| MD*Month(3rd) | −0.05 | −0.03 |
| MD* Month (6th) | 2.22 | 2.24 |
| LGID* Month (3rd) | 2.00 | 1.91 |
| LGID* Month (6th) | 4.74 # | 4.57 # |
| LGIMD* Month (3rd) | −0.11 | −0.28 |
| LGIMD* Month (6th) | 1.24 | 1.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osella, A.R.; Colaianni, G.; Correale, M.; Pesole, P.L.; Bruno, I.; Buongiorno, C.; Deflorio, V.; Leone, C.M.; Colucci, S.C.; Grano, M.; et al. Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial. Nutrients 2018, 10, 844. https://doi.org/10.3390/nu10070844
Osella AR, Colaianni G, Correale M, Pesole PL, Bruno I, Buongiorno C, Deflorio V, Leone CM, Colucci SC, Grano M, et al. Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial. Nutrients. 2018; 10(7):844. https://doi.org/10.3390/nu10070844
Chicago/Turabian StyleOsella, Alberto R., Graziana Colaianni, Mario Correale, Pasqua L. Pesole, Irene Bruno, Claudia Buongiorno, Valentina Deflorio, Carla M. Leone, Silvia Concetta Colucci, Maria Grano, and et al. 2018. "Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial" Nutrients 10, no. 7: 844. https://doi.org/10.3390/nu10070844






