Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties
2.2. TPC, TFC, and Antioxidant Activity in Cascara Kombucha Production
2.3. Phenolic Acid and Flavonoid Contents in Cascara Kombucha Production
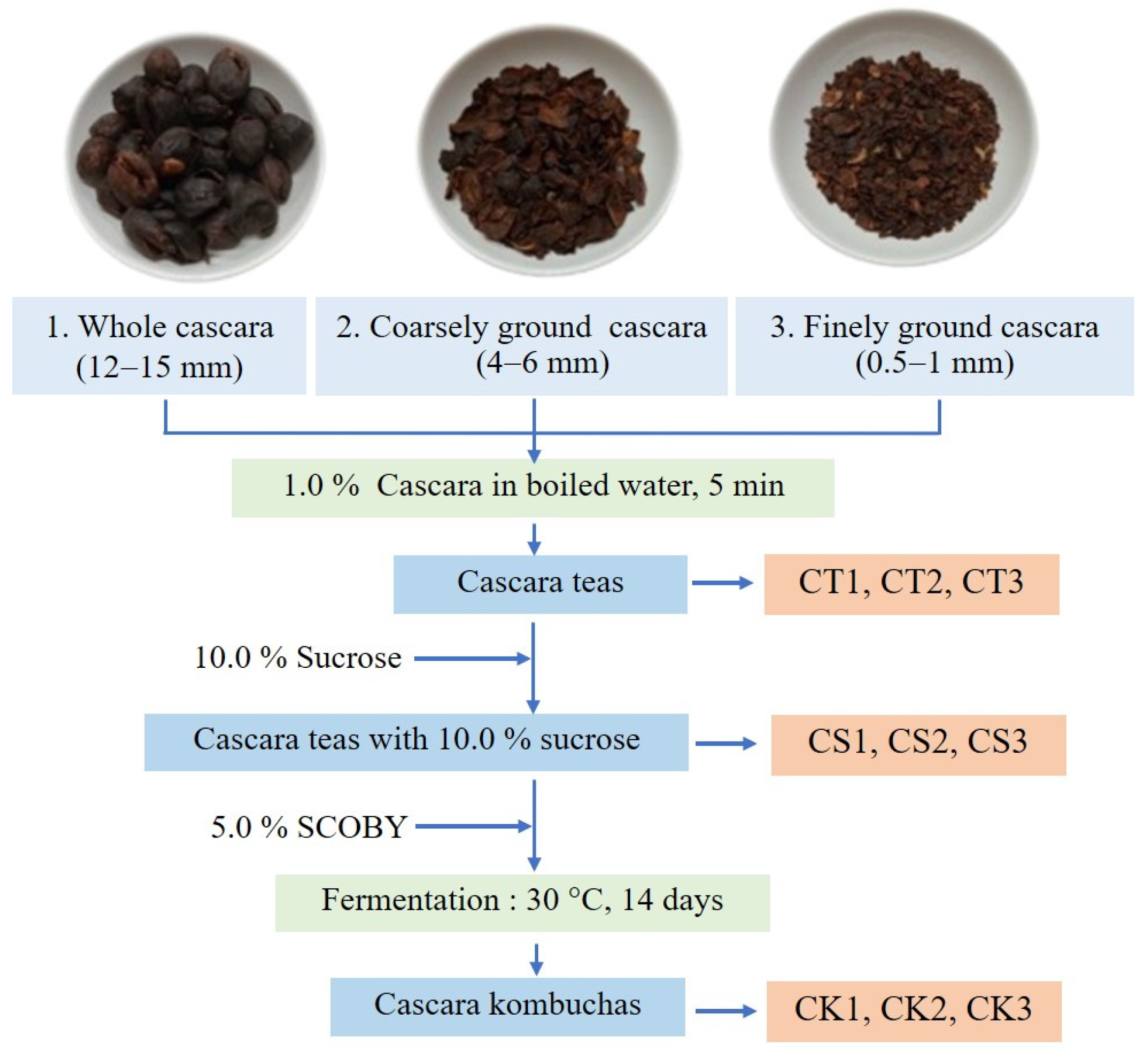
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Kombuchas
3.3. Determination of pH and Total Soluble Solid
3.4. Measurement of Color
3.5. Determination of Total Phenolic Content
3.6. Determination of Total Flavonoid Content
3.7. Radical Scavenging Activity by DPPH Assay
3.8. Ferric–Reducing Antioxidant Power (FRAP) Assay
3.9. HPLC Determination of Phenolic and Flavonoid Compounds
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaashyap, M.; Cohen, M.; Mantri, N. Microbial Diversity and Characteristics of Kombucha as Revealed by Metagenomic and Physicochemical Analysis. Nutrients 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kombucha Market Size, Share & Trends Analysis Report by Product (Hard, Conventional), by Distribution Channel (On-Trade, Off-Trade), by Region (North America, Europe, Asia Pacific), and Segment Forecasts, 2025–2030. Available online: https://www.grandviewresearch.com/industry-analysis/kombucha-market (accessed on 28 March 2025).
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Villarreal–Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT 2022, 168. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Belo, G.M.P.; de Lima, L.S.; Silva, K.A.; Uekane, T.M.; Gonzalez, A.G.M.; Branco, V.N.C.; Pitangui, N.S.; Fernandes, F.F.; Lima, A.R. Arabic coffee infusion based kombucha: Characterization and biological activity during fermentation, and in vivo toxicity. Food Chem. 2023, 412, 135556. [Google Scholar] [CrossRef]
- Charles, A.; Huang, T.; Chang, Y. Structural analysis and characterization of a mucopolysaccharide isolated from roots of cassava (Manihot esculenta Crantz L.). Food Hydrocoll. 2008, 22, 184–191. [Google Scholar] [CrossRef]
- Otsuki, A. Chapter 4; Rheology of Colloidal Particle Suspensions. In Rheology of Polymer Blends and Nanocomposites; Sabu, T., Sarathchandran, C., Chandran, N., Simon, H., Eds.; Elsevier, Inc.: Cambridge, MA, USA, 2020; pp. 49–70. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Wang, H.; Sun, M.; Yu, C.; Liu, Q.; He, Z.; Song, S.; Feng, T.; Yao, L. Flavor and sensory profile of kombucha fermented with raw Pu-erh tea and evaluation of the antioxidant properties. LWT 2024, 200. [Google Scholar] [CrossRef]
- Muzaifa, M.; Abubakar, Y.; Safrida, S.; Nildaa, C.; Irfan, I.; Irfan. Phytochemicals and Sensory Quality of Cascara Kombucha Made from Coffee By-Products. Curr. Res. Nutr. Food Sci. J. 2023, 11, 605–616. [Google Scholar] [CrossRef]
- Le, B.X.N.; Van, T.P.; Phan, Q.K.; Pham, G.B.; Quang, H.P.; Do, A.D. Coffee Husk By-Product as Novel Ingredients for Cascara Kombucha Production. J. Microbiol. Biotechnol. 2023, 34, 673–680. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Booth, I.R.; Stratford, M. Acidulants and low pH. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer US: Boston, MA, USA, 2003; pp. 25–47. ISBN 978-1-4757-1006-9. [Google Scholar]
- Hsieh, Y.; Chiu, M.-C.; Chou, J.-Y. Efficacy of the Kombucha Beverage Derived from Green, Black, and Pu’er Teas on Chemical Profile and Antioxidant Activity. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Sanwal, N.; Gupta, A.; Bareen, M.A.; Sharma, N.; Sahu, J.K. Kombucha fermentation: Recent trends in process dynamics, functional bioactivities, toxicity management, and potential applications. Food Chem. Adv. 2023, 3. [Google Scholar] [CrossRef]
- Vangdal, E. Threshold Values of Soluble Solids in Fruit Determined for the Fresh Fruit Market. Acta Agric. Scand. 1980, 30, 445–448. [Google Scholar] [CrossRef]
- Rohaya, S.; Anwar, S.H.; Amhar, A.B.; Sutriana, A.; Muzaifa, M. Antioxidant Activity and Physicochemical Composition of Coffee Pulp Obtained from Three Coffee Varieties in Aceh, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1182, 012063. [Google Scholar] [CrossRef]
- Yuan, K.; Wu, G.; Li, X.; Zeng, Y.; Wen, X.; Liu, R.; Jiang, X.; Tian, L.; Sun, J.; Bai, W. Anthocyanins degradation mediated by β-glycosidase contributes to the color loss during alcoholic fermentation in a structure-dependent manner. Food Res. Int. 2023, 175, 113732. [Google Scholar] [CrossRef]
- Santos, L.d.M.; Roselino, M.N.; Alves, J.d.C.; Viana, S.N.A.; Requião, E.d.R.; Ferro, J.M.R.B.d.S.; de Souza, C.O.; Ribeiro, C.D.F. Production and characterization of kombucha-like beverage by cocoa (Theobroma cacao) by-product as raw material. Futur. Foods 2024, 11. [Google Scholar] [CrossRef]
- Muralidharan, V.; Jebathomas, C.R.T.; Sundaramoorthy, S.; Madhan, B.; Palanivel, S. Preparation and evaluation of novel biodegradable Kombucha cellulose-based multi-layered composite tableware. Ind. Crop. Prod. 2024, 215. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Van, T.P.; Phan, Q.K.; Quang, H.P.; Pham, G.B.; Thi, N.H.N.; Thi, H.T.T.; Do, A.D. Multi-Strain Probiotics Enhance the Bioactivity of Cascara Kombucha during Microbial Composition-Controlled Fermentation. Prev. Nutr. Food Sci. 2023, 28, 502–513. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of physicochemical properties and antioxidant activities of kombucha “Tea Fungus” during extended periods of fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7. [Google Scholar] [CrossRef]
- König, A.; Sadova, N.; Dornmayr, M.; Schwarzinger, B.; Neuhauser, C.; Stadlbauer, V.; Wallner, M.; Woischitzschläger, J.; Müller, A.; Tona, R.; et al. Combined Acid Hydrolysis and Fermentation Improves Bioactivity of Citrus Flavonoids in Vitro and in Vivo. Commun. Biol. 2023, 6, 1–18. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Anjliany, M.; Syafutri, M.I.; Widowati, T.W. Qualities of arabica and robusta cascara kombucha with different concentrations of starter. Coffee Sci. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.-C. Wheat Bran Particle Size Influence on Phytochemical Extractability and Antioxidant Properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi; Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Makanjuola, S.A. Influence of Particle Size and Extraction Solvent on Antioxidant Properties of Extracts of Tea, Ginger, and Tea-Ginger Blend. Food Sci. Nutr. 2017, 5, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Yen, G.-C. Effects of Phenolic Acids on Human Phenolsulfotransferases in Relation to Their Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 1474–1479. [Google Scholar] [CrossRef]
- Pua, A.; Choo, W.X.D.; Goh, R.M.V.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A Systematic Study of Key Odourants, Non-Volatile Compounds, and Antioxidant Capacity of Cascara (Dried Coffea Arabica Pulp). LWT 2021, 138, 110630. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of Coffee Cherry Pulp and Its Utilisation for Production of Cascara Beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Gunel, N.S.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Abdelkafi, S.; Sayadi, S.; Gam, Z.B.A.; Casalot, L.; Labat, M. Bioconversion of ferulic acid to vanillic acid by Halomonas elongata isolated from table-olive fermentation. FEMS Microbiol. Lett. 2006, 262, 115–120. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Guo, Z.; Lei, J.; Zhou, B. Recent Advancement in Bioeffect, Metabolism, Stability, and Delivery Systems of Apigenin, a Natural Flavonoid Compound: Challenges and Perspectives. Front. Nutr. 2023, 10, 1221227. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-N.; Shi, J.; Fu, Y.; Zhao, X.-H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Boonarsa, P.; Bunyatratchata, A.; Chumroenphat, T.; Thammapat, P.; Chaikwang, T.; Siripan, T.; Li, H.; Siriamornpun, S. Nutritional Quality, Functional Properties, and Biological Characterization of Watermeal (Wolffia Globosa). Horticulturae 2024, 10, 1171. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150. [Google Scholar] [CrossRef]
- Nopparat, J.; Sujipuli, K.; Ratanasut, K.; Weerawatanakorn, M.; Prasarnpun, S.; Thongbai, B.; Laothaworn, W.; Inthima, P. Exploring the excellence of commercial Brahmi products from Thai online markets: Unraveling phytochemical contents, antioxidant properties and DNA damage protection. Heliyon 2024, 10, e24509. [Google Scholar] [CrossRef]
- Li, H.; Chumroenphat, T.; Bunyatratchata, A.; Boonarsa, P.; Wrigley, C.; Siriamornpun, S. Chemical composition and nutritional profile of cicada (Meimuna opalifera Walker) at different developmental stages: Implications for functional food applications. Food Chem. X 2023, 21, 101081. [Google Scholar] [CrossRef]
| Parameters | Cascara Tea and Kombucha Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT1 | CT2 | CT3 | CS1 | CS2 | CS3 | CK1 | CK2 | CK3 | |
| pH | 4.16 ± 0.16 a | 4.25 ± 0.13 a | 4.26 ± 0.16 a | 4.13 ± 0.25 a | 4.185 ± 0.28 a | 4.21 ± 0.28 a | 3.29 ± 0.06 b | 3.34 ± 0.11 b | 3.22 ± 0.03 b |
| TSS (%Brix) | 0.50 ± 0.14 c | 0.60 ± 0.00 c | 0.55 ± 0.07 c | 11.15 ± 0.49 a | 10.70 ± 0.57 ab | 10.55 ± 0.21 ab | 10.90 ± 0.14 ab | 10.15 ± 0.78 b | 10.00 ± 0.14 b |
| Color | |||||||||
| L* | 27.80 ± 0.05 ab | 27.55 ± 0.04 b | 27.73 ± 0.09 ab | 28.11 ± 0.32 a | 28.10 ± 0.10 a | 28.00 ± 0.34 a | 28.10 ± 0.11 a | 28.11 ± 0.12 a | 27.48 ± 0.30 b |
| a* | 0.63 ± 0.01 ab | 0.70 ± 0.04 a | 0.61 ± 0.02 ab | 0.48 ± 0.06 c | 0.48 ± 0.01 c | 0.57 ± 0.05 b | 0.34 ± 0.03 d | 0.33 ± 0.02 d | 0.62 ± 0.08 ab |
| b* | 6.88 ± 0.07 abc | 7.05 ± 0.07 a | 6.79 ± 0.06 bc | 6.71 ± 0.13 cd | 6.54 ± 0.07 d | 6.99 ± 0.28 ab | 6.26 ± 0.06 e | 6.24 ± 0.09 e | 6.94 ± 0.16 abc |
| Antioxidant Activity (µg/mL) | Cascara Tea and Kombucha Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT1 | CT2 | CT3 | CS1 | CS2 | CS3 | CK1 | CK2 | CK3 | |
| TPC | 338.42 ± 3.80 f | 329.30 ± 5.80 e | 372.98 ± 6.66 c | 366.49 ± 6.87 c | 348.60 ± 2.90 d | 392.63 ± 4.11 b | 385.44 ± 4.25 b | 383.51 ± 4.25 b | 424.04 ± 6.05 a |
| TFC | 320.40 ± 0.93 f | 350.89 ± 1.34 d | 432.49 ± 2.17 a | 334.47 ± 1.13 e | 369.53 ± 1.13 c | 426.32 ± 1.50 b | 216.57 ± 2.26 h | 215.33 ± 2.57 h | 240.27 ± 2.52 g |
| DPPH | 233.30 ± 1.73 d | 239.69 ± 1.90 c | 252.01 ± 0.95 b | 250.79 ± 0.46 b | 251.55 ± 0.70 b | 257.33 ± 0.95 a | 228.13 ± 2.06 e | 220.38 ± 2.34 f | 232.39 ± 3.04 d |
| FRAP | 554.88 ± 6.61 g | 561.13 ± 6.61 fg | 630.71 ± 9.38 d | 575.29 ± 6.29 f | 612.79 ± 7.64 e | 678.63 ± 9.92 c | 697.38 ± 13.23 b | 698.63 ± 8.20 b | 725.29 ± 5.05 a |
| Phenolic Acid Content (µg/mL) | Cascara Tea and Kombucha Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT1 | CT2 | CT3 | CS1 | CS2 | CS3 | CK1 | CK2 | CK3 | |
| Hydroxycinnamic acids | |||||||||
| Chlorogenic acid | 39.44 ± 0.23 g | 47.44 ± 0.39 c | 44.16 ± 0.09 d | 44.45 ± 0.35 d | 53.66 ± 0.43 a | 50.12 ± 0.19 b | 40.58 ± 0.28 f | 42.76 ± 0.21 e | 44.28 ± 0.13 d |
| Caffeic acid | 16.40 ± 0.06 f | 19.86 ± 0.17 c | 18.56 ± 0.07 d | 18.34 ± 0.13 d | 21.88 ± 0.75 a | 20.95 ± 0.17 b | 17.48 ± 0.66 e | 18.34 ± 0.45 d | 18.80 ± 0.03 d |
| p-coumaric acid | 9.58 ± 0.82 bc | 8.67 ± 0.08 cd | 8.23 ± 0.02 d | 10.37 ± 0.63 ab | 10.61 ± 0.14 a | 8.95 ± 0.63 cd | 8.97 ± 0.34 cd | 7.18 ± 0.02 e | 8.63 ± 0.01 cd |
| Cinnamic acid | 6.99 ± 0.18 h | 9.51 ± 0.08 d | 10.08 ± 0.11 c | 9.34 ± 0.09 e | 11.11 ± 0.96 b | 11.88 ± 0.01 a | 6.70 ± 0.14 i | 7.41 ± 0.05 g | 8.27 ± 0.04 f |
| Ferulic acid | 4.83 ± 0.18 g | 7.38 ± 0.08 c | 6.49 ± 0.05 d | 5.94 ± 0.13 e | 8.36 ± 0.01 a | 8.09 ± 0.03 b | 4.35 ± 0.16 h | 5.17 ± 0.10 f | 5.69 ± 0.42 e |
| Sinapic acid | 0.17 ± 0.01 a | 0.15 ± 0.00 b | 0.15 ± 0.00 b | 0.15 ± 0.01 b | 0.15 ± 0.00 b | 0.15 ± 0.01 b | 0.14 ± 0.00 b | 0.15 ± 0.01 b | 0.14 ± 0.01 b |
| Hydroxybenzoic acids | |||||||||
| p-hydroxybenzoic acid | 61.68 ± 0.22 c | 62.97 ± 0.45 b | 55.78 ± 0.12 e | 67.22 ± 0.64 a | 67.30 ± 0.36 a | 60.91 ± 0.36 d | 41.72 ± 0.16 f | 41.43 ± 0.10 f | 41.38 ± 0.12 f |
| Gentisic acid | 36.28 ± 3.35 a | 20.98 ± 1.55 h | 22.51 ± 0.55 g | 26.37 ± 0.51 f | 31.74 ± 1.82 c | 35.06 ± 2.43 b | 30.56 ± 0.91 de | 27.87 ± 0.99 ef | 28.09 ± 0.40 ef |
| Gallic acid | 10.62 ± 0.37 c | 10.38 ± 0.21 c | 10.53 ± 0.17 c | 12.89 ± 0.55 b | 12.44 ± 0.09 b | 12.40 ± 1.08 b | 15.08 ± 0.19 a | 8.49 ± 0.14 d | 10.02 ± 0.47 c |
| Protocatechuic acid | 1.02 ± 0.01 f | 1.13 ± 0.02 de | 1.23 ± 0.02 bc | 1.12 ± 0.03 de | 1.41 ± 0.05 a | 1.28 ± 0.04 b | 1.11 ± 0.06 e | 1.19 ± 0.09 cd | 1.20 ± 0.06 cd |
| Syringic acid | 0.13 ± 0.01 f | 0.24 ± 0.01 a | 0.18 ± 0.01 d | 0.19 ± 0.01 c | 0.23 ± 0.01 a | 0.21 ± 0.01 b | 0.11 ± 0.01 f | 0.11 ± 0.01 f | 0.15 ± 0.01 e |
| Vanillic acid | 0.38 ± 0.02 g | 0.45 ± 0.01 e | 0.42 ± 0.00 f | 0.47 ± 0.01 e | 0.52 ± 0.03 d | 0.53 ± 0.01 d | 0.65 ± 0.11 a | 0.61 ± 0.02 b | 0.58 ± 0.01 c |
| Total | 187.52 ± 3.79 d | 189.28 ± 0.59 d | 178.23 ± 0.85 e | 196.85 ± 1.48 c | 219.41 ± 0.81 a | 210.54 ± 2.62 b | 167.47 ± 0.67 f | 160.74 ± 1.05 g | 167.26 ± 1.78 f |
| Flavonoid Content (µg/mL) | Cascara Tea and Kombucha Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT1 | CT2 | CT3 | CS1 | CS2 | CS3 | CK1 | CK2 | CK3 | |
| Apigenin | 4.32 ± 0.28 a | 2.01 ± 0.04 e | 2.06 ± 0.07 e | 2.40 ± 0.02 d | 1.72 ± 0.04 f | 1.68 ± 0.07 f | 3.69 ± 0.12 b | 3.44 ± 0.07 c | 3.43 ± 0.15 c |
| Catechin | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Kaempferol | 1.29 ± 0.02 a | 1.08 ± 0.03 bc | 1.12 ± 0.04 b | 1.09 ± 0.01 bc | 1.07 ± 0.02 c | 1.08 ± 0.03 bc | 1.07 ± 0.01 c | 1.05 ± 0.01 c | 1.05 ± 0.01 c |
| Myricetin | 1.73 ± 0.13 c | 1.80 ± 0.06 c | 1.83 ± 0.01 bc | 1.88 ± 0.08 abc | 1.83 ± 0.05 bc | 1.81 ± 0.01 c | 1.98 ± 0.13 ab | 2.03 ± 0.03 a | 1.90 ± 0.14 abc |
| Quercetin | 4.18 ± 0.11 f | 4.42 ± 0.08 e | 4.73 ± 0.08 c | 4.75 ± 0.13 c | 5.49 ± 0.04 a | 5.53 ± 0.07 a | 4.56 ± 0.06 d | 4.79 ± 0.03 c | 4.95 ± 0.04 b |
| Rutin | 1.00 ± 0.03 e | 1.34 ± 0.05 c | 1.32 ± 0.01 c | 1.20 ± 0.05 d | 1.58 ± 0.03 a | 1.46 ± 0.05 b | 1.35 ± 0.05 c | 1.43 ± 0.01 b | 1.47 ± 0.05 b |
| Total | 12.52 ± 0.29 a | 10.65 ± 0.10 e | 11.08 ± 0.15 d | 11.32 ± 0.23 cd | 11.68 ± 0.09 b | 11.56 ± 0.13 bc | 12.65 ± 0.32 a | 12.74 ± 0.05 a | 12.80 ± 0.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongbai, B.; Sukboonyasatit, D.; Banlue, K.; Inchuen, S.; Chuenta, W.; Siriamornpun, S.; Suwannarong, S. Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties. Molecules 2025, 30, 1934. https://doi.org/10.3390/molecules30091934
Thongbai B, Sukboonyasatit D, Banlue K, Inchuen S, Chuenta W, Siriamornpun S, Suwannarong S. Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties. Molecules. 2025; 30(9):1934. https://doi.org/10.3390/molecules30091934
Chicago/Turabian StyleThongbai, Bussagon, Duljira Sukboonyasatit, Kriangsak Banlue, Sudathip Inchuen, Wanida Chuenta, Sirithon Siriamornpun, and Sarinthorn Suwannarong. 2025. "Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties" Molecules 30, no. 9: 1934. https://doi.org/10.3390/molecules30091934
APA StyleThongbai, B., Sukboonyasatit, D., Banlue, K., Inchuen, S., Chuenta, W., Siriamornpun, S., & Suwannarong, S. (2025). Cascara Kombucha: The Role of Fermentation and Particle Size in Enhancing Antioxidant and Bioactive Properties. Molecules, 30(9), 1934. https://doi.org/10.3390/molecules30091934







