Novel One-Step Total Synthesis of trans-Dehydroosthol and Citrubuntin
Abstract
1. Introduction
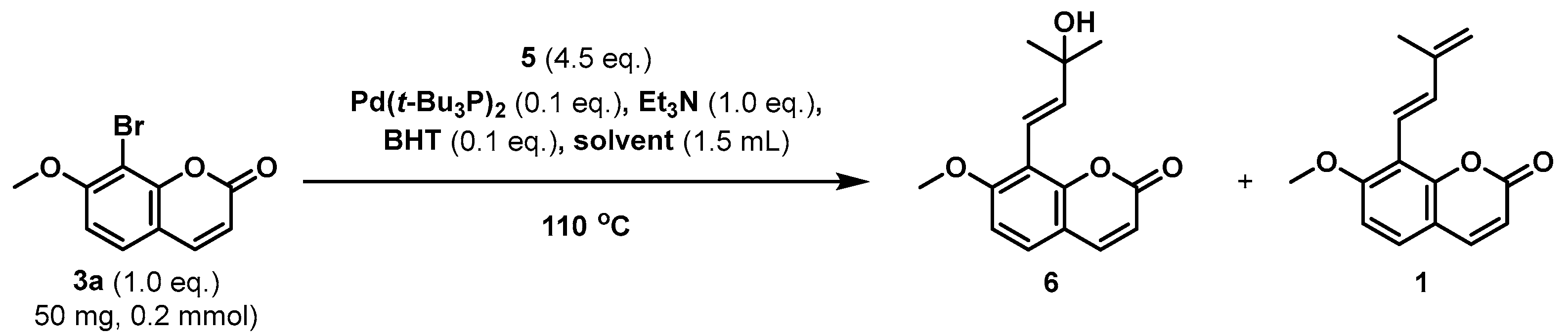
2. Results
2.1. Effect of the Bases
2.2. Effect of the Solvents
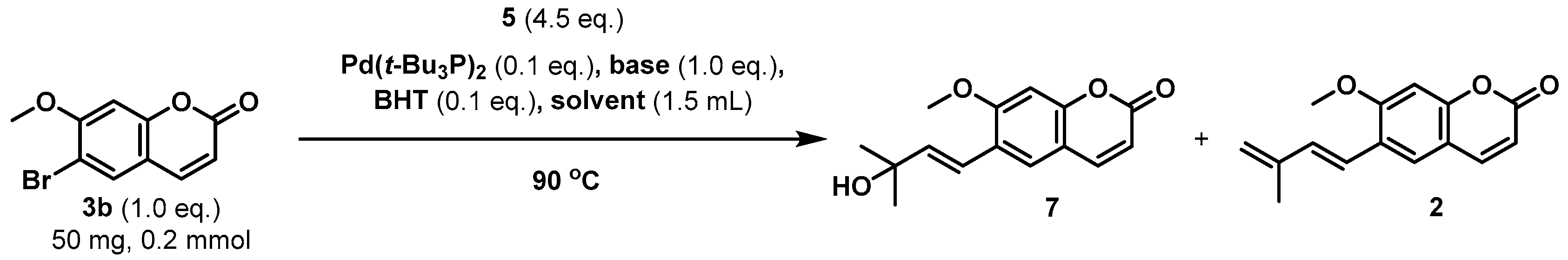
2.3. Further Application
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 3-Bromo-2-hydroxy-4-methoxybenzaldehyde and 5-Bromo-2-hydroxy-4-methoxybenzaldehyde
3.2.1. Synthesis of 3-Bromo-2-hydroxy-4-methoxybenzaldehyde
3.2.2. Synthesis of 5-Bromo-2-hydroxy-4-methoxybenzaldehyde
3.2.3. Synthesis of 8-Bromo-7-methoxy-2H-chromen-2-one-3a
3.2.4. Synthesis of 6-Bromo-7-methoxy-2H-chromen-2-one-3b
3.3. General Procedure for Table 1, Table 2, Table 3 and Table 4
3.3.1. Synthesis of trans-Dehydroosthol (1): Table 2, Entry 6–1 (Gram Scale)
3.3.2. Synthesis of Citrubuntin (2): Table 4, Entry 1–2 (Gram Scale)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Zhang, J.Y.; Song, Z.J.; Zhu, G.L.; Liu, M.M.; Dai, H.Q.; Hsiang, T.; Liu, X.T.; Zhang, L.X.; Quinn, R.J.; et al. Genome-based mining of new antimicrobial meroterpenoids from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2020, 104, 3835–3846. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Gan, Y.T.; Peng, X.G.; Ouyang, Q.X.; Pei, J.; Ruan, H.L. Peniandranoids A–E: Meroterpenoids with antiviral and immunosuppressive activity from a Penicillium sp. J. Nat. Prod. 2023, 86, 66–75. [Google Scholar] [CrossRef]
- Zbakh, H.; Zubía, E.; Reyes, C.; Calderón-Montaño, J.M.; López-Lázaro, M.; Motilva, V. Meroterpenoids from the brown alga Cystoseira usneoides as potential anti-inflammatory and lung anticancer agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Guo, H.; Wu, Q.L.; Lu, X.; Zou, Y.T.; Fu, Q.Y.; Chen, S.H.; Liu, L.; Peng, B.; Chen, S.H. Anti-inflammatory acetylenic meroterpenoids from the ascidian-derived fungus Amphichorda felina SYSU-MS7 908. Bioorg. Chem. 2023, 139, 106715. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Liu, X.M.; Lian, C.L.; Ke, J.Y.; Liu, J.Q. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Nishida, H.; Kim, Y.K.; Obata, R.; Sunazuka, T.; Omura, S.; Bordner, J.; Guadliana, M.; Dormer, P.G.; Smith, A.B., III. Relative and Absolute Stereochemistry of Pyripyropene A, A Potent, Bioavailable Inhibitor of Acyl-CoA:Cholesterol Acyltransferase (ACAT). J. Am. Chem. Soc. 1994, 116, 12097–12098. [Google Scholar] [CrossRef]
- Kuno, F.; Otoguro, K.; Shiomi, K.; Iwai, Y.; Omura, S. Arisugacins A and B, novel and selective acetylcholinesterase inhibitors from Penicillium sp. FO-4259. J. Antibiot. 1996, 49, 742–751. [Google Scholar] [CrossRef]
- Ito, C.; Furukawa, H. Constituents of Murraya exotica L. structure elucidation of new coumarins. Chem. Pharm. Bull. 1987, 35, 4277–4285. [Google Scholar] [CrossRef]
- Ito, C.; Ono, T.; Tanaka, E.; Takemura, Y.; Nakata, T.; Uchida, H.; Ju-Ichi, M.; Omura, M.; Furukawa, H. Structure of Pummeloquinoe, a new coumarin-Naphthoquinone dimer isolated from Citrus Plants. Chem. Pharm. Bull. 1993, 41, 205–207. [Google Scholar] [CrossRef]
- Wang, X.T.; Liang, H.Z.; Zeng, K.W.; Zhao, M.B.; Tu, P.F.; Li, J.; Jiang, Y. Panitins A-G: Coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry 2019, 162, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.R.; Senior, R.G.; Taylor, W.C. The chemical constituents of Australian Zanthoxylum species. VII. Some transformation products of suberosin. Aust. J. Chem. 1980, 33, 395–411. [Google Scholar] [CrossRef]
- Wu, T.S. Alkaloids and coumarins of Citrus grandis. Phytochemistry 1988, 27, 3717–3718. [Google Scholar] [CrossRef]
- Huang, S.; Luo, X.M.; Wang, J.H. Study on the chemical constituents from ethyl acetate extract of Micromelum falcatum. J. Chin. Med. Mater. 2013, 36, 744–746. [Google Scholar]
- Suthiwong, J.; Sriphana, U.; Thongsri, Y.; Promsuwan, P.; Prariyachatigul, C.; Yenjai, C. Coumarinoids from the fruits of Micromelum falcatum. Fitoterapia 2014, 94, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Asghar, H.; Asghar, H.; Asghar, T. A Review on anti-urease potential of Coumarins. Curr. Drug Targets 2021, 22, 1926–1943. [Google Scholar] [CrossRef] [PubMed]
- Reisch, J.; Bathe, A. Naturstoffchemie, 1181) synthese der cumarine 6- und 8-naphthoherniarin, dehydrogeijerin und Murraol. Liebigs Ann. Chem. 1988, 1988, 543–547. [Google Scholar] [CrossRef]
- Murray, R.D.H.; Zeghdi, S. Synthesis of the natural coumarins, murraol (CM-c2), trans-dehydroosthol and swietenocoumarin G. Phytochemistry 1989, 28, 227–230. [Google Scholar]
- Lepovitz, L.T.; Martin, S.F. Biomimetically inspired synthesis of exotine A. Org. Lett. 2018, 20, 7875–7878. [Google Scholar] [CrossRef] [PubMed]
- Lepovitz, L.T.; Martin, S.F. Biomimetically inspired, one-step synthesis of exotine A and exotine B. J. Org. Chem. 2021, 86, 10946–10953. [Google Scholar] [CrossRef] [PubMed]
- Reisch, J.; Herath, H.M.T.B.; Kumar, N.S. ChemInform abstract: Natural product chemistry. Part 139. Synthesis of the natural coumarins (E)-suberenol, cyclobisuberodiene and two other related new coumarins. Liebigs. Ann. Chem. 1990, 1990, 931–933. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhang, C.; Zeng, K.W.; Li, J.; Guo, X.Y.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Exotines A and B, two heterodimers of isopentenyl-substituted indole and coumarin derivatives from Murraya exotica. Org. Lett. 2015, 17, 4380–4383. [Google Scholar] [CrossRef] [PubMed]
- Su, F.Y.; Zhao, Z.; Ma, S.G.; Wang, R.B.; Li, Y.; Liu, Y.B.; Li, Y.H.; Li, L.; Qu, J.; Yu, S.S. Cnidimonins A–C, three types of hybrid dimer from Cnidium monnieri: Structural elucidation and semisynthesis. Org. Lett. 2017, 19, 4920–4923. [Google Scholar] [CrossRef] [PubMed]
- Rozsa, Z.; Mester, I.; Reisch, J.; Szendrei, K. Naphthoherniarin: An unusual coumarin derivative from Ruta graveolens. Planta Med. 1989, 55, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Guise, G.B.; Ritchie, E.; Senior, R.G.; Taylor, W.C. The chemical constituents of Australian Zanthoxylum species. IV. Two new coumarins from Z. suberosum C. T. White (syn. Z. dominianum Merr. & Perry; Z. ovalifolium Wight). Aust. J. Chem. 1967, 20, 2429–2439. [Google Scholar]
- Furukawa, H.; Ito, C.; Mizuno, T.; Ju-ichi, M.; Inoue, M.; Kajiura, I.; Omura, M. Spectrometric elucidation of acrimarines, the first naturally occurring acridone–coumarin dimers. J. Chem. Soc. Perkin Trans. 1990, 1, 1593–1599. [Google Scholar] [CrossRef]
- Guthertz, A.; Leutzsch, M.; Wolf, L.M.; Gupta, P.; Rummelt, S.M.; Goddard, R.; Farès, C.; Thiel, W.; Fürstner, A. Half-sandwich ruthenium carbene complexes link trans-hydrogenation and gem-hydrogenation of internal alkynes. J. Am. Chem. Soc. 2018, 140, 3156–3169. [Google Scholar] [CrossRef]
- Jeffery, T. Advances in Metal-Organic Chemistry; Liebeskind, L.S., Ed.; JAI: London, UK, 1996. [Google Scholar]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Wang, L.H.; Lei, T.; Wang, F.S.; Jiang, S.Z.; Yan, G.Y. Total synthesis of indiacen A using a practical one-pot reaction: Promoted by a key waste product, and its utility in natural products synthesis. Tetrahedron Lett. 2021, 66, 152822. [Google Scholar] [CrossRef]
- Li, T.; Song, J.-J.; Wang, L.H.; Lei, T.; Jiang, S.Z.; Wang, F. A simple and efficient total synthesis of anticancer indole alkaloids TMC-205 and its analogues. Tetrahedron Lett. 2021, 74, 153137. [Google Scholar] [CrossRef]
- Song, J.J.; Lu, G.D.; Yang, B.Q.; Bai, M.J.; Li, J.J.; Wang, F.S.; Lei, T.; Jiang, S.Z. A concise first total synthesis of luteoride A and luteoride B. Tetrahedron 2022, 122, 132933. [Google Scholar] [CrossRef]
- Gong, X.S.; Bai, M.J.; Lu, G.D.; Yang, B.Q.; Lei, T.; Jiang, S.Z. Total synthesis of murraol, (E)-suberenol and toward the collective total synthesis of exotines A, cnidimonins A-Cetc. Tetrahedron 2022, 126, 133061. [Google Scholar] [CrossRef]




| Entry | Variable | t[h] | 3a (%) a | 6 (%) a | 1 (%) a |
|---|---|---|---|---|---|
| 1 b | None | 3 b/31 | - | 97/32 | 2/67 |
| 2 b | 120 °C | 10 | - | 63 | 35 |
| 3 | Et3N (0.9) c | 18 | - | 31 | 68 |
| 4 | (n-C3H7)3N (1.0/0.9) c | 21/29 | -/- | 31/22 | 69/77 |
| 5 | (n-C8H17)3N (1.0/0.9) c | 29/22 | -/- | 22/4 | 75/84 |
| 6 | DIPEA | 9 | - | 25 | 67 |
| 7 | (Cy)2NMe d | 27 | - | 89 | 10 |
| 8 | DABCO | 13 | - | 67 | 32 |
| 9 | DMAN e (0.85) c | 31 | 4 | 39 | 56 |
| 10 | N,N-Diethylaniline | 5 | 64 | - | 28 |
| 11 | 2,6-Lutidine | 10 | 71 | - | 27 |
| Entry | Variable | t[h] | 3a (%) a | 6 (%) a | 1 (%) a |
|---|---|---|---|---|---|
| 1 | Xylene | 27 | - | 78 | 20 |
| 2 | Chlorobenzene | 6 | 9 | 5 | 70 |
| 3 | 2,4-DCT b | 22 | 36 | 21 | 27 |
| 4 c | DCE (1.0/0.9) d | 21/8 | -/- | 4/- | 82/86 |
| 5 c | 1,2-DCP e | 30 | - | 31 | 61 |
| 6 | 1,3-DCP f (1.0/0.9) d | 10(10 g)/14 | -(-)/11 | 3(2)/16 | 90(94)/69 |
| 7 c | CH3CN | 21 | - | 61 | 36 |
| 8 | DMF | 17 | - | 47 | 48 |
| 9 | DMAC | 18 | - | 47 | 47 |
| 10 | NMP | 18 | - | 47 | 39 |
| 11 | DMSO | 19 | - | 12 | 26 |
| 12 c | THF | 21 | - | 33 | 66 |
| 13 c | 2-MeTHF h | 22 | - | 38.1 | 51.8 |
| 14 c | 1,4-Dioxane | 15 | - | 15 | 77 |
| 15 c | DME | 22 | - | 60.7 | 35.3 |
| 16 | DEE i | 20 | - | 58 | 30 |
| 17 | n-BuOAc j | 21 | - | 74 | 25 |

| Entry | Variable | t[h] | 3a (%) a | 6 (%) a | 1 (%) a |
|---|---|---|---|---|---|
| 1 b | DCE, (n-C8H17)3N (1.0/0.9) c | 18/7 | -/- | 10/2 | 84/89 |
| 2 b | DCE, (n-C3H7)3N (1.0/0.9) c | 14(14 d)/11 | -(-)/- | 4(-)/7 | 91(93)/83 |
| 3 | 1,3-DCP, (n-C8H17)3N (1.0/0.9) c | 21.5/21 | -/- | 35/28 | 64/70 |
| 4 | 1,3-DCP, (n-C3H7)3N (1.0/0.9) c | 26/16 | -/- | 15/23 | 78/67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ge, B.; Gong, X.; Wang, F.; Lei, T.; Jiang, S. Novel One-Step Total Synthesis of trans-Dehydroosthol and Citrubuntin. Molecules 2025, 30, 1067. https://doi.org/10.3390/molecules30051067
Liu Z, Ge B, Gong X, Wang F, Lei T, Jiang S. Novel One-Step Total Synthesis of trans-Dehydroosthol and Citrubuntin. Molecules. 2025; 30(5):1067. https://doi.org/10.3390/molecules30051067
Chicago/Turabian StyleLiu, Zhiwen, Baoyue Ge, Xushun Gong, Fusheng Wang, Ting Lei, and Shizhi Jiang. 2025. "Novel One-Step Total Synthesis of trans-Dehydroosthol and Citrubuntin" Molecules 30, no. 5: 1067. https://doi.org/10.3390/molecules30051067
APA StyleLiu, Z., Ge, B., Gong, X., Wang, F., Lei, T., & Jiang, S. (2025). Novel One-Step Total Synthesis of trans-Dehydroosthol and Citrubuntin. Molecules, 30(5), 1067. https://doi.org/10.3390/molecules30051067