Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells
Abstract
1. Introduction
2. Results
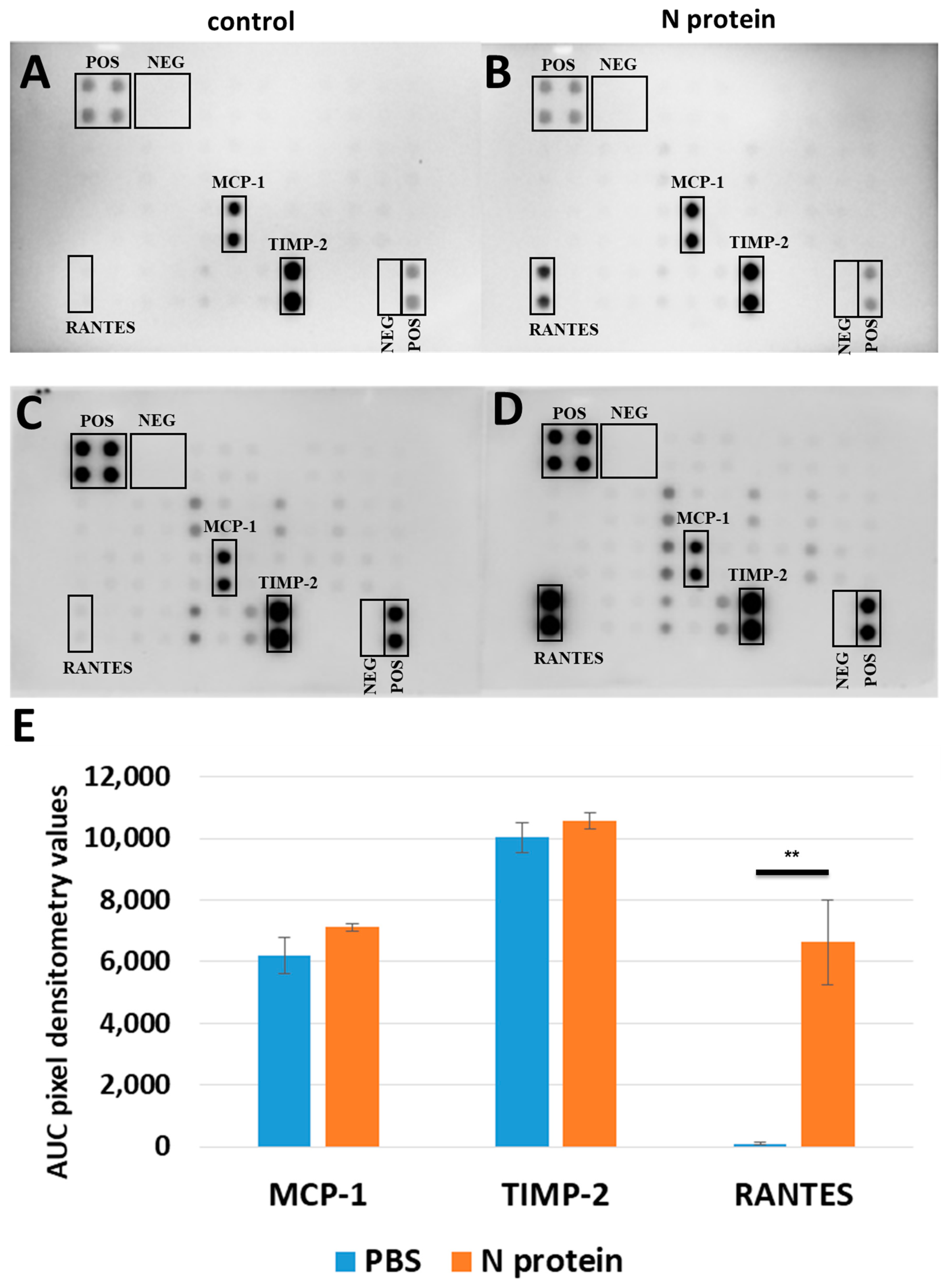
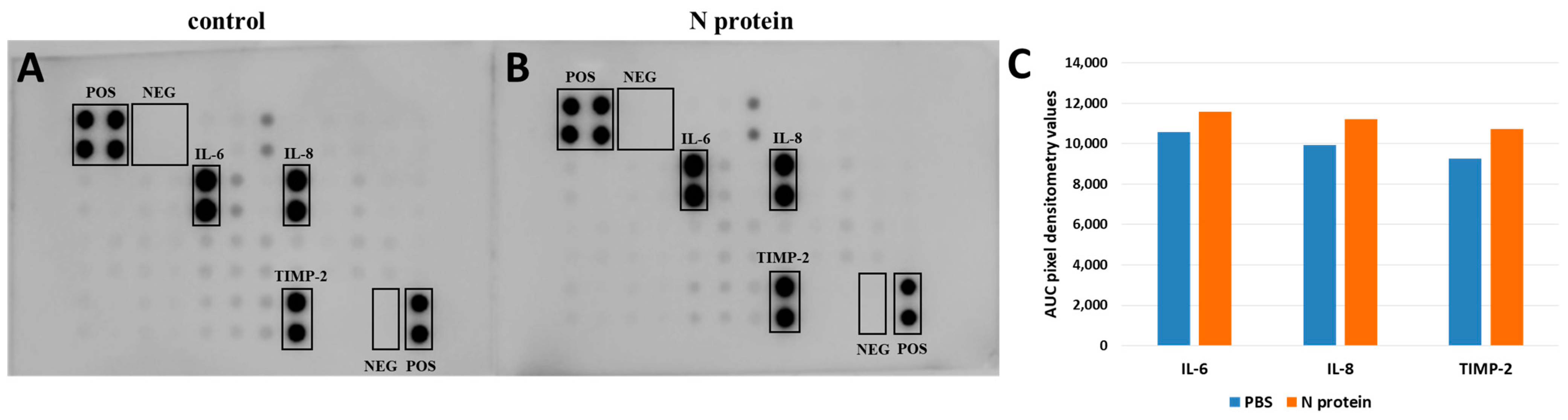
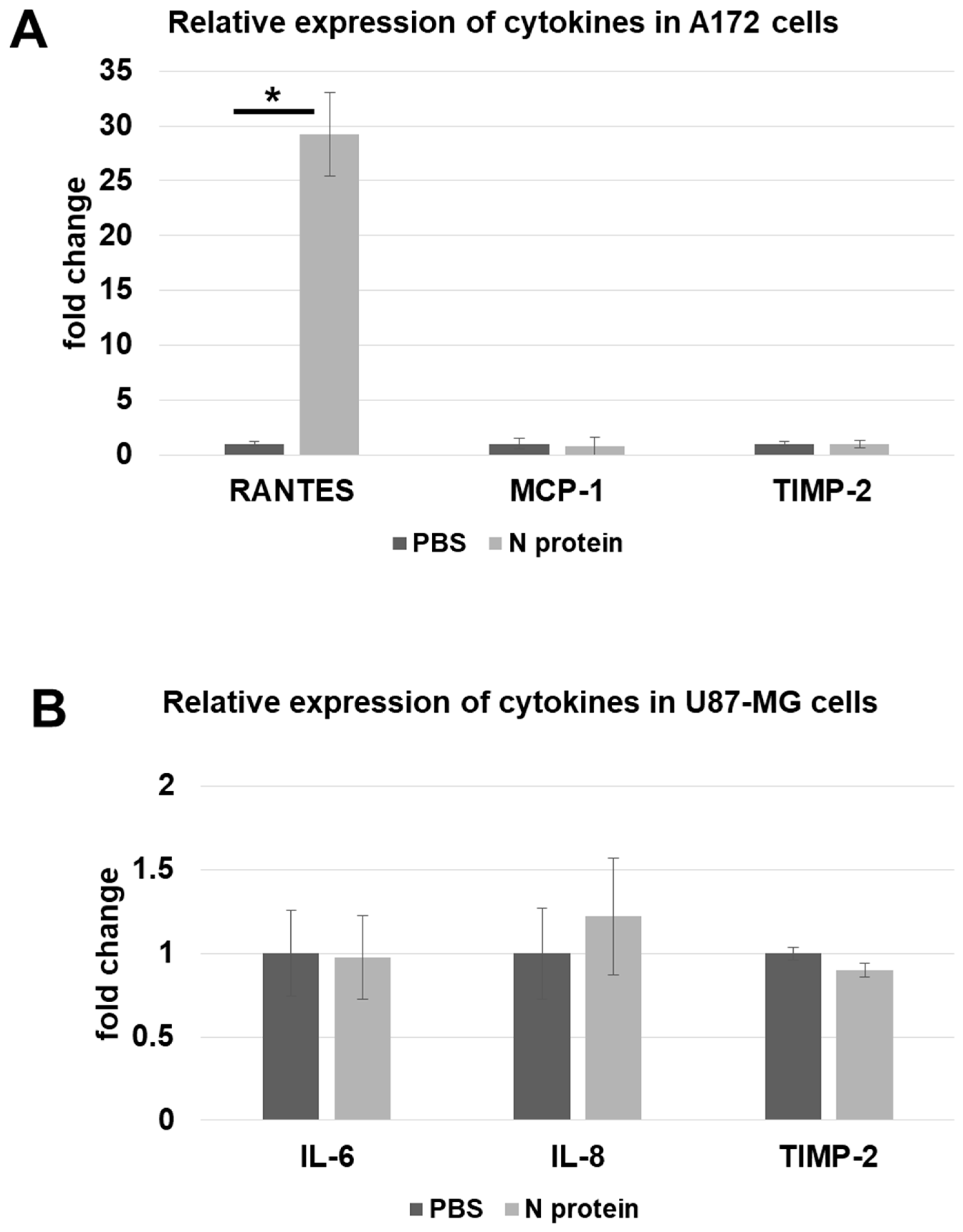
2.1. N Protein Upregulates RANTES Expression
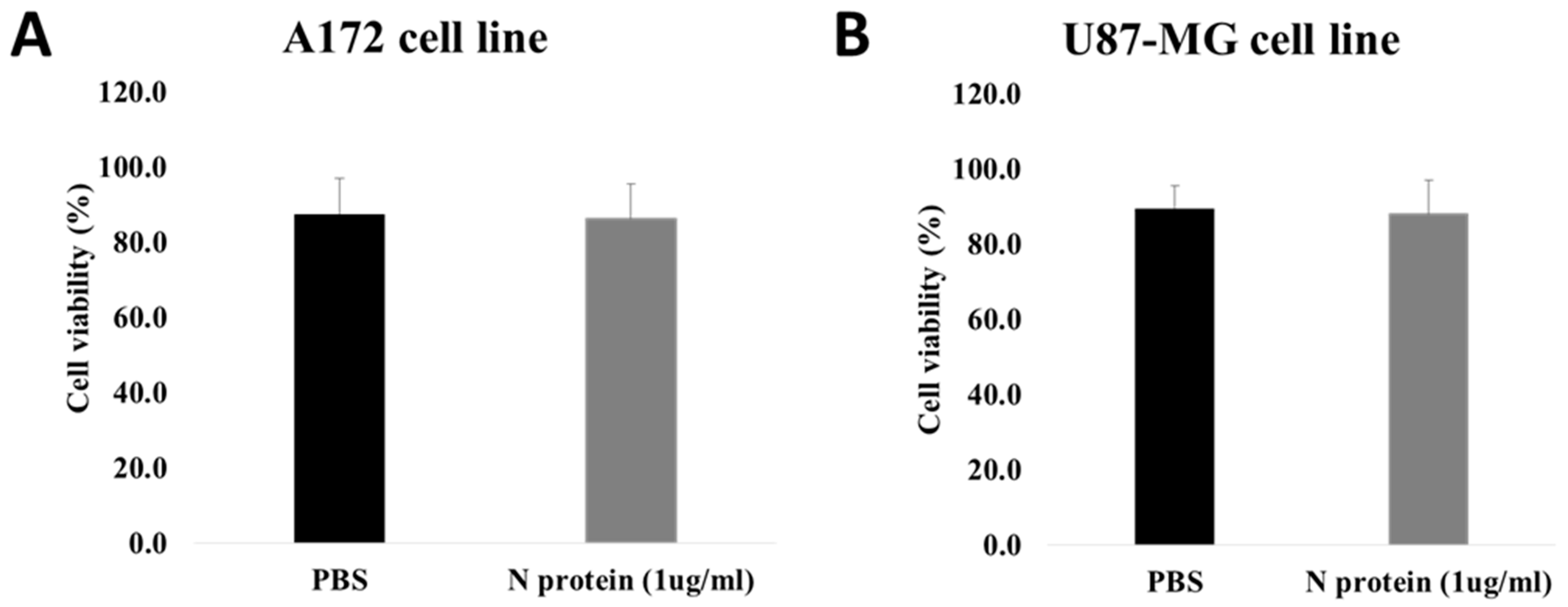
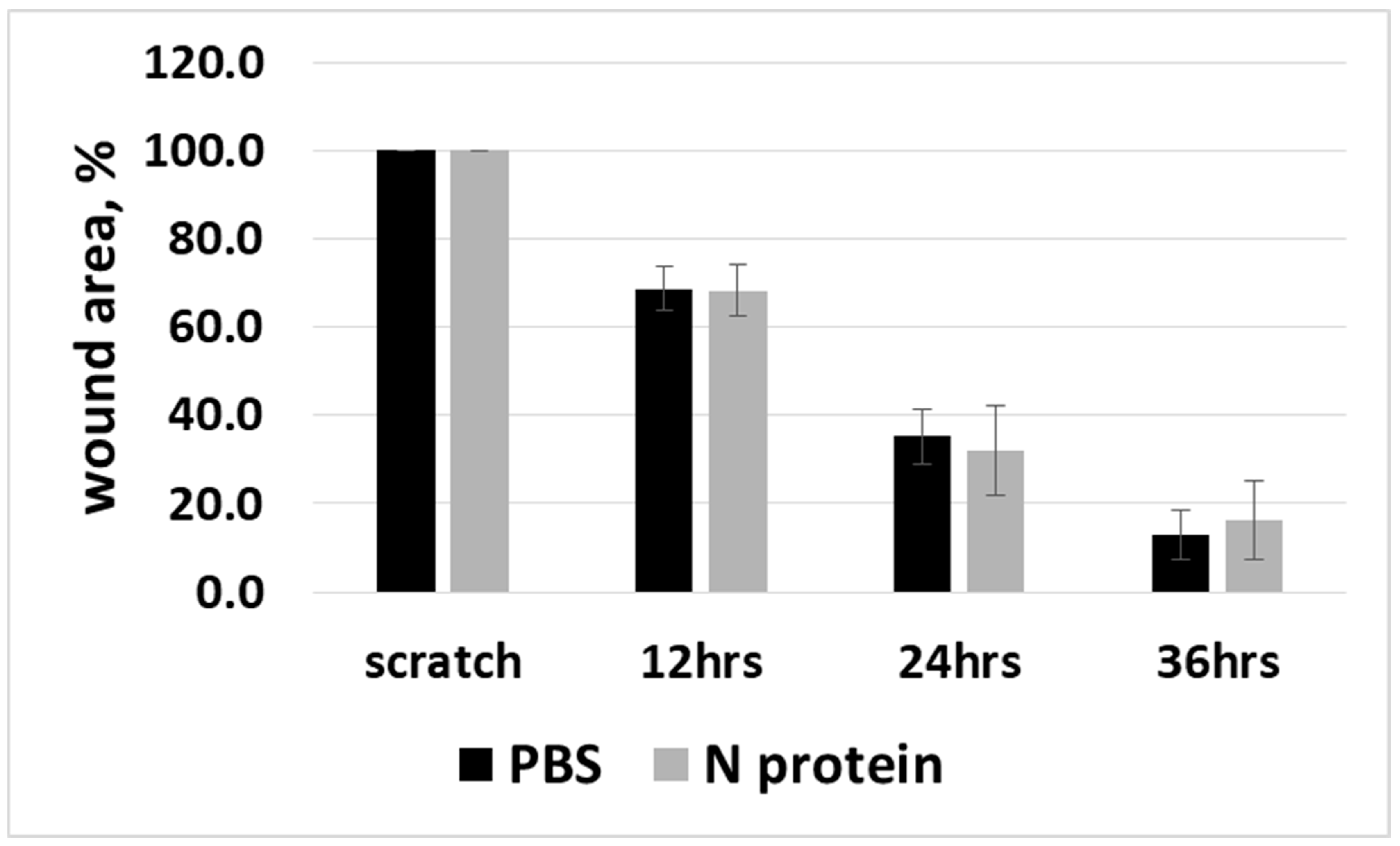
2.2. N Protein Has No Effect on Cell Viability and Cell Migration
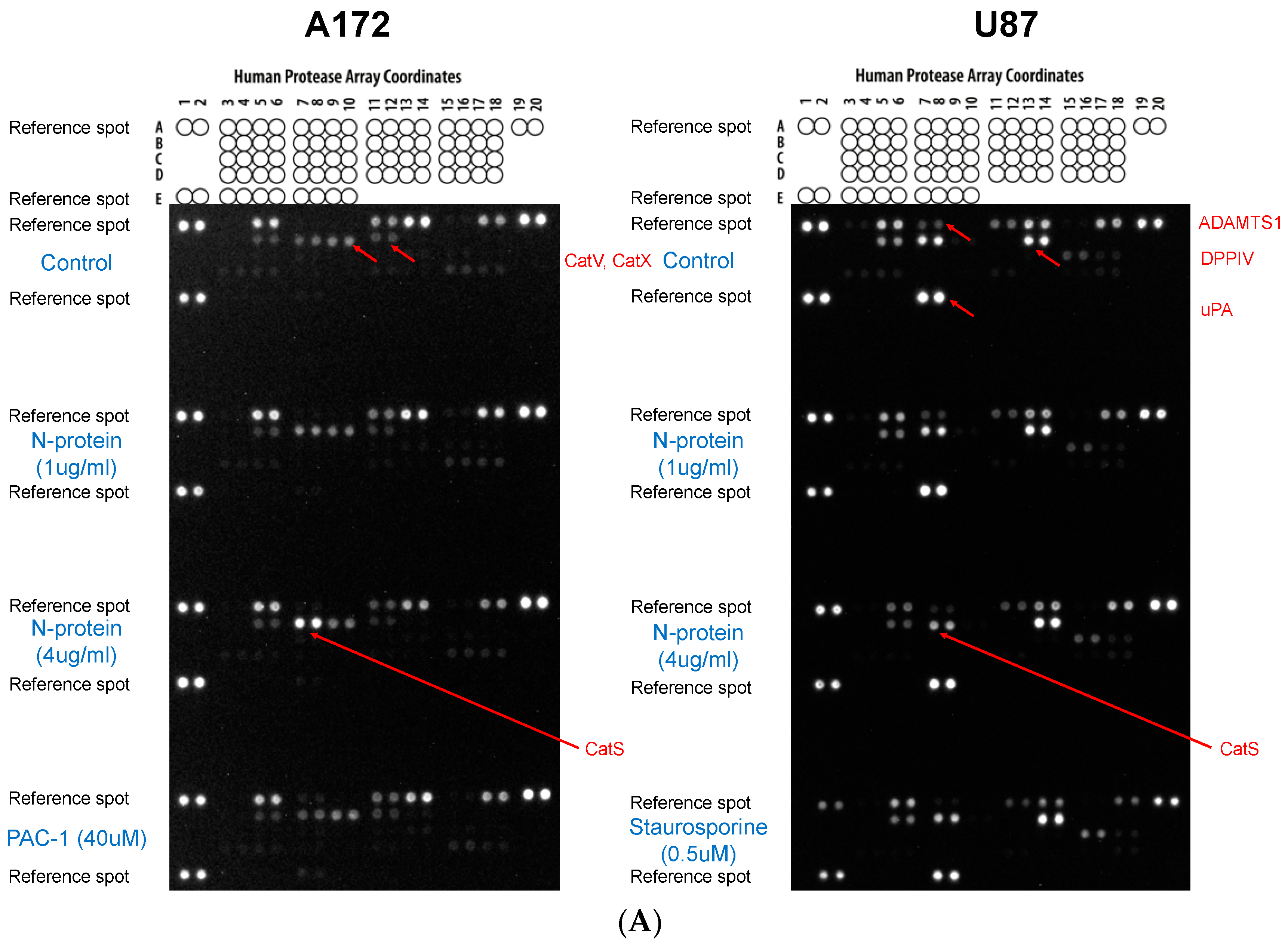
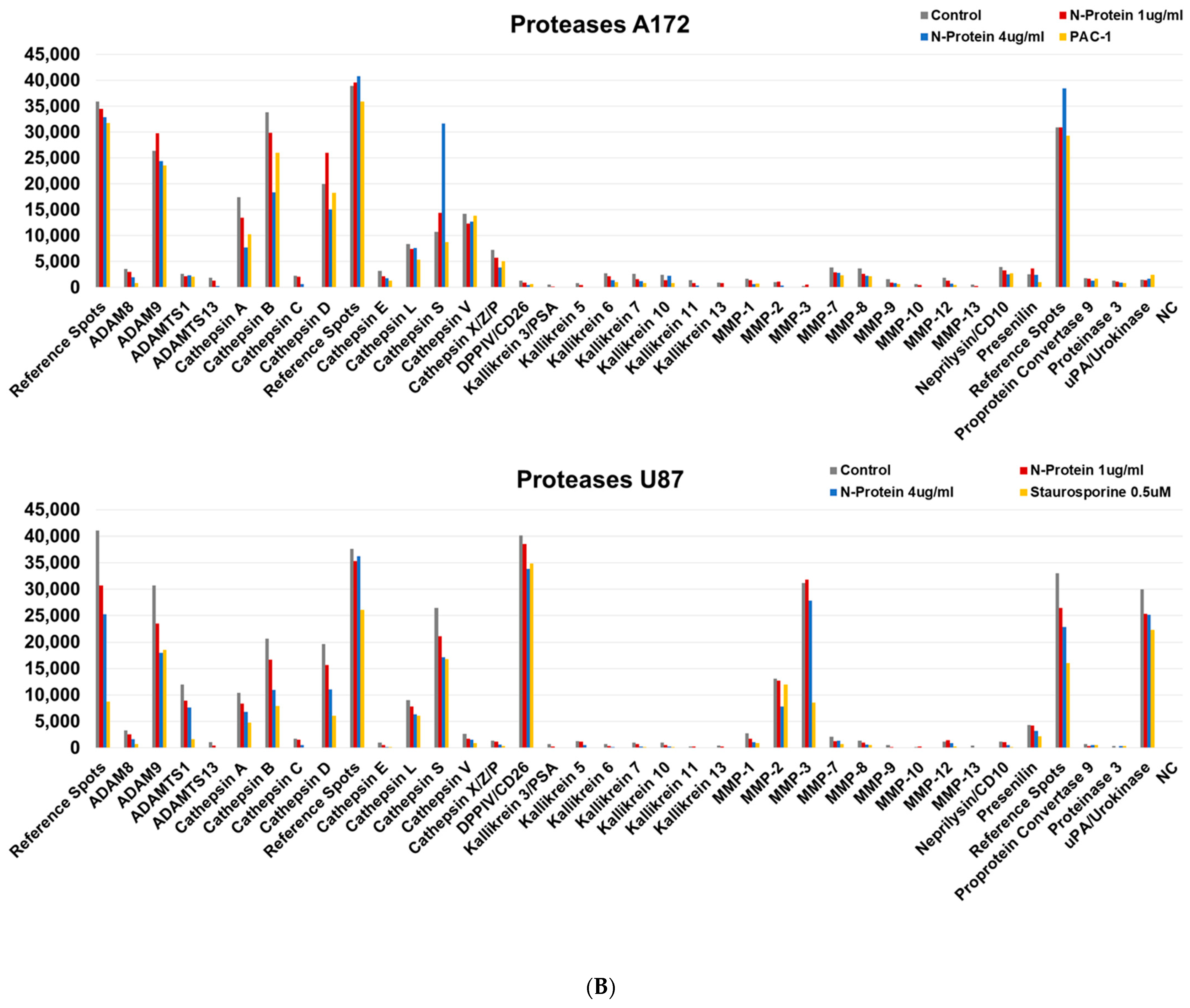
2.3. N Protein Provokes an Increase in Cathepsin S in A172 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cytokine Array Assay
4.3. RT-qPCR
4.4. Cell Viability Assay by Flow Cytometry
4.5. Scratch Assay
4.6. The Protease Profiler Array
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 Genome, Structure, Evolution, Pathogenesis and Therapies: Structural Genomics Approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Wang, X.; Tang, G.; Liu, Y.; Zhang, L.; Chen, B.; Han, Y.; Fu, Z.; Wang, L.; Hu, G.; Ma, Q.; et al. The Role of IL-6 in Coronavirus, Especially in COVID-19. Front. Pharmacol. 2022, 13, 1033674. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, K.; Wang, D.; Yue, X.; Song, D.; Zhu, Y.; Wu, J. Nucleocapsid Protein of SARS-CoV Activates Interleukin-6 Expression through Cellular Transcription Factor NF-κB. Virology 2007, 365, 324–335. [Google Scholar] [CrossRef]
- Karwaciak, I.; Sałkowska, A.; Karaś, K.; Dastych, J.; Ratajewski, M. Nucleocapsid and Spike Proteins of the Coronavirus SARS-CoV-2 Induce IL6 in Monocytes and Macrophages-Potential Implications for Cytokine Storm Syndrome. Vaccines 2021, 9, 54. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Brola, W.; Wilski, M. Neurological Consequences of COVID-19. Pharmacol. Rep. PR 2022, 74, 1208–1222. [Google Scholar] [CrossRef]
- Tsukahara, T.; Brann, D.H.; Datta, S.R. Mechanisms of SARS-CoV-2-Associated Anosmia. Physiol. Rev. 2023, 103, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A Versatile and Controversial Chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Palesch, D.; Wagner, J.; Meid, A.; Molenda, N.; Sienczyk, M.; Burkhardt, J.; Münch, J.; Prokop, L.; Stevanovic, S.; Westhoff, M.-A.; et al. Cathepsin G-Mediated Proteolytic Degradation of MHC Class I Molecules to Facilitate Immune Detection of Human Glioblastoma Cells. Cancer Immunol. Immunother. CII 2016, 65, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 Infection: An Overview on Cytokine Storm and Related Interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, H.M.; Khattab, A.; El-Agamy, D.S.; Eltahir, H.M.; Alhaddad, A.; Aljohani, F.D.; Almuzaini, T.M.; Abouzied, M.M.; Aldhafiri, A. IL-6 at the Center of Cytokine Storm: Circulating Inflammation Mediators as Biomarkers in Hospitalized COVID-19 Patients. J. Clin. Lab. Anal. 2023, 37, e24881. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 Induces Cytokine Storm with High Mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated with Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, J.; Zhang, Q.; Xie, Y.; Wang, K.; Qiu, S.; Lu, K.; Liu, Y. Comparing the Nucleocapsid Proteins of Human Coronaviruses: Structure, Immunoregulation, Vaccine, and Targeted Drug. Front. Mol. Biosci. 2022, 9, 761173. [Google Scholar] [CrossRef]
- Chen, C.J.; Liao, S.L.; Kuo, M.D.; Wang, Y.M. Astrocytic Alteration Induced by Japanese Encephalitis Virus Infection. Neuroreport 2000, 11, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Liu, C.C.; Chuang, J.I.; Lei, H.Y.; Yeh, T.M.; Lin, Y.S.; Huang, Y.H.; Liu, H.S. Involvement of Oxidative Stress, NF-IL-6, and RANTES Expression in Dengue-2-Virus-Infected Human Liver Cells. Virology 2000, 276, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Casola, A.; Burger, N.; Liu, T.; Jamaluddin, M.; Brasier, A.R.; Garofalo, R.P. Oxidant Tone Regulates RANTES Gene Expression in Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Role in Viral-Induced Interferon Regulatory Factor Activation. J. Biol. Chem. 2001, 276, 19715–19722. [Google Scholar] [CrossRef] [PubMed]
- Melchjorsen, J.; Paludan, S.R. Induction of RANTES/CCL5 by Herpes Simplex Virus Is Regulated by Nuclear Factor Kappa B and Interferon Regulatory Factor 3. J. Gen. Virol. 2003, 84, 2491–2495. [Google Scholar] [CrossRef]
- Crawford, A.; Angelosanto, J.M.; Nadwodny, K.L.; Blackburn, S.D.; Wherry, E.J. A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection. PLoS Pathog. 2011, 7, e1002098. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 Axis in Human Diseases and Related Treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Schwenk, U.; Maune, S.; Bartels, J.; Küpper, M.; Fichtner, I.; Schröder, J.-M.; Schadendorf, D. The Chemokine RANTES Is Secreted by Human Melanoma Cells and Is Associated with Enhanced Tumour Formation in Nude Mice. Br. J. Cancer 1999, 79, 1025–1031. [Google Scholar] [CrossRef]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective Attraction of Monocytes and T Lymphocytes of the Memory Phenotype by Cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef]
- Gross, E.; Amella, C.A.; Pompucci, L.; Franchin, G.; Sherry, B.; Schmidtmayerova, H. Macrophages and Lymphocytes Differentially Modulate the Ability of RANTES to Inhibit HIV-1 Infection. J. Leukoc. Biol. 2003, 74, 781–790. [Google Scholar] [CrossRef]
- Carrion, A.W.; Shah, A.C.; Kotch, C. Neurofibromatosis Type 1-Associated Gliomas and Other Tumors: A New Pathway Forward? Pediatr. Hematol. Oncol. J. 2023, 8, 129–135. [Google Scholar] [CrossRef]
- Tassano, E.; Giacomini, T.; Severino, M.; Gamucci, A.; Fiorio, P.; Gimelli, G.; Ronchetto, P. Characterization of the Phenotype Associated with Microduplication Reciprocal to NF1 Microdeletion Syndrome. Cytogenet. Genome Res. 2017, 152, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Kuan, I.-I.; Guo, J.-Y.; Hsu, W.-C.; Tang, W.-C.; Chan, H.-J.; Chen, Y.-J.; Chen, B.-C.; Wu, H.-C.; Liao, J.C. SARS-CoV-2 N Protein Mediates Intercellular Nucleic Acid Dispersion, a Feature Reduced in Omicron. iScience 2023, 26, 105995. [Google Scholar] [CrossRef] [PubMed]
- Montalvo Villalba, M.C.; Valdés Ramírez, O.; Muné Jiménez, M.; Arencibia Garcia, A.; Martinez Alfonso, J.; González Baéz, G.; Roque Arrieta, R.; Rosell Simón, D.; Alvárez Gainza, D.; Sierra Vázquez, B.; et al. Interferon Gamma, TGF-Β1 and RANTES Expression in Upper Airway Samples from SARS-CoV-2 Infected Patients. Clin. Immunol. 2020, 220, 108576. [Google Scholar] [CrossRef]
- Patterson, B.K.; Seethamraju, H.; Dhody, K.; Corley, M.J.; Kazempour, K.; Lalezari, J.; Pang, A.P.S.; Sugai, C.; Mahyari, E.; Francisco, E.B.; et al. CCR5 Inhibition in Critical COVID-19 Patients Decreases Inflammatory Cytokines, Increases CD8 T-Cells, and Decreases SARS-CoV2 RNA in Plasma by Day 14. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 103, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Adam, A.P.; Chopra, A.; Chieng, H.C.; Drake, L.A.; Martino, N.; Bossardi Ramos, R.; Feustel, P.J.; Overmyer, K.A.; Shishkova, E.; et al. Unique Inflammatory Profile Is Associated with Higher SARS-CoV-2 Acute Respiratory Distress Syndrome (ARDS) Mortality. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 320, R250–R257. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, F.; Martin-Vicente, M.; Rojas-García, R.L.; Castilla-García, L.; Muñoz-Gomez, M.J.; Hervás Fernández, I.; González Ventosa, V.; Vidal-Alcántara, E.J.; Cuadros-González, J.; Bermejo-Martin, J.F.; et al. High SARS-CoV-2 Viral Load and Low CCL5 Expression Levels in the Upper Respiratory Tract Are Associated with COVID-19 Severity. J. Infect. Dis. 2022, 225, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Génin, P.; Algarté, M.; Roof, P.; Lin, R.; Hiscott, J. Regulation of RANTES Chemokine Gene Expression Requires Cooperativity Between NF-κB and IFN-Regulatory Factor Transcription Factors. J. Immunol. 2000, 164, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Ge, W.; Lei, Z.; Luo, W.; Liu, Y.; Guan, Z.; Chen, L.; Yu, Z.; Shen, M.; Hu, D.; et al. SARS-CoV-2 N Protein Enhances the Anti-Apoptotic Activity of MCL-1 to Promote Viral Replication. Signal Transduct. Target. Ther. 2023, 8, 194. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Smyth, P.; Sasiwachirangkul, J.; Williams, R.; Scott, C.J. Cathepsin S (CTSS) Activity in Health and Disease—A Treasure Trove of Untapped Clinical Potential. Mol. Aspects Med. 2022, 88, 101106. [Google Scholar] [CrossRef]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef] [PubMed]
- Bollavaram, K.; Leeman, T.H.; Lee, M.W.; Kulkarni, A.; Upshaw, S.G.; Yang, J.; Song, H.; Platt, M.O. Multiple Sites on SARS-CoV-2 Spike Protein Are Susceptible to Proteolysis by Cathepsins B, K, L, S, and V. Protein Sci. 2021, 30, 1131–1143. [Google Scholar] [CrossRef]
- Carlin, S.M.; Resink, T.J.; Tamm, M.; Roth, M. Urokinase Signal Transduction and Its Role in Cell Migration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 195–202. [Google Scholar] [CrossRef]
- Kaphle, P.; Li, Y.; Yao, L. The Mechanical and Pharmacological Regulation of Glioblastoma Cell Migration in 3D Matrices. J. Cell. Physiol. 2019, 234, 3948–3960. [Google Scholar] [CrossRef]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C.; et al. Cathepsin L Plays a Key Role in SARS-CoV-2 Infection in Humans and Humanized Mice and Is a Promising Target for New Drug Development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
| Primers | Forward | Reverse |
|---|---|---|
| RANTES | CGAAAGAACCGCCAAGTGTG | GCCTCCCAAGCTAGGACAAG |
| TIMP-2 | ACCCTCTGTGACTTCATCGTGC | GGAGATGTAGCACGGGATCATG |
| MCP-1 | CAGCAGGTGTCCCAAAGAAGCTGT | CCATTCCTTATTGGGGTCAGCACAGA |
| IL-6 | AGCAAAGAGGCACTGGCAGAAAACA | AGAAGAAGGAATGCCCATTAACAAC |
| IL-8 | GAGAGTGATTGAGAGTGGACCAC | CACAACCCTCTGCACCCAGTTT |
| GAPD | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadilgereyeva, B.; Kunushpayeva, Z.; Abdrakhmanova, M.; Khassenova, A.; Minigulov, N.; Burster, T.; Filchakova, O. Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells. Molecules 2025, 30, 1066. https://doi.org/10.3390/molecules30051066
Gadilgereyeva B, Kunushpayeva Z, Abdrakhmanova M, Khassenova A, Minigulov N, Burster T, Filchakova O. Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells. Molecules. 2025; 30(5):1066. https://doi.org/10.3390/molecules30051066
Chicago/Turabian StyleGadilgereyeva, Bakhytgul, Zhanar Kunushpayeva, Mira Abdrakhmanova, Aizere Khassenova, Nail Minigulov, Timo Burster, and Olena Filchakova. 2025. "Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells" Molecules 30, no. 5: 1066. https://doi.org/10.3390/molecules30051066
APA StyleGadilgereyeva, B., Kunushpayeva, Z., Abdrakhmanova, M., Khassenova, A., Minigulov, N., Burster, T., & Filchakova, O. (2025). Nucleocapsid Protein of SARS-CoV-2 Upregulates RANTES Expression in A172 Glioblastoma Cells. Molecules, 30(5), 1066. https://doi.org/10.3390/molecules30051066






