Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives
Abstract
1. Introduction
2. Methods
3. Obesity Treating Natural Products
3.1. Single Compound
3.2. Foods
3.3. Teas
3.4. Fruits
3.5. Herbal Medicines
3.5.1. Herbal Medicines—Single Extracts
3.5.2. Herbal Medicines—Decoctions
3.5.3. Herbal Medicines—External Preparations
4. Discussion
4.1. Antiobesity Mechanism
4.1.1. Lipid Metabolism
4.1.2. Anti-Inflammation
4.1.3. Antioxidant
4.1.4. Appetite Loss
4.1.5. Thermogenesis
4.2. Limitations
4.3. Well-Designed Studies in Antiobesity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 22 November 2022).
- Kim, K.-S.; Yang, H.J.; Choi, E.-K.; Shin, M.H.; Kim, K.-H.; Um, J.Y.; Lee, B.-C.; Jang, H.-J. The effects of complex herbal medicine composed of Cornus fructus, Dioscoreae rhizoma, Aurantii fructus, and Mori folium in obese type-2 diabetes mice model. Orient. Pharm. Exp. Med. 2013, 13, 69–75. [Google Scholar] [CrossRef]
- World Health Organization. World Obesity Day 2022—Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 22 November 2022).
- Centers for Disease Control and Preservation. Health Effects of Overweight and Obesity. Available online: https://www.cdc.gov/healthyweight/effects/index.html (accessed on 22 November 2022).
- National Institute of Diabetes and Digestive and Kidney Diseases. Prescription Medications to Treat Overweight & Obesity. Available online: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 22 November 2022).
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Lee, S.E.; Lim, C.; Lim, S.; Lee, B.; Cho, S. Effect of Ephedrae Herba methanol extract on high-fat diet-induced hyperlipidaemic mice. Pharm. Biol. 2019, 57, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Streeper, R.T.; Louden, C.; Izbicka, E. Oral Azelaic Acid Ester Decreases Markers of Insulin Resistance in Overweight Human Male Subjects. In Vivo 2020, 34, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mathews, A.E.; Rodrigues, C.; Eudy, B.J.; Rowe, C.A.; O’Donoughue, A.; Percival, S.S. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2018, 24, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Hamzah, S.; Hajifaraji, M.; Radzi, C.W.; Cordell, G.A. Slimming and Appetite-Suppressing Effects of Caraway Aqueous Extract as a Natural Therapy in Physically Active Women. Phytother. Res. 2016, 30, 981–987. [Google Scholar] [CrossRef]
- Izaola, O.; Primo, D.; Rico Bargués, D.; Martín-Diana, A.B.; Martínez Villaluenga, C.; Miranda, J.; de Luis Román, D.A. Effects of a snack enriched with carob and Undaria pinnatifida (wakame) on metabolic parameters in a double blind, randomized clinical trial in obese patients. Nutr. Hosp. 2020, 34, 465–473. [Google Scholar]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Bernardinelli, L.; Fazia, T.; Peroni, G.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; et al. The Metabolic Effects of Cynara Supplementation in Overweight and Obese Class I Subjects with Newly Detected Impaired Fasting Glycemia: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2020, 12, 3298. [Google Scholar] [CrossRef]
- Szulińska, M.; Kręgielska-Narożna, M.; Świątek, J.; Styś, P.; Kuźnar-Kamińska, B.; Jakubowski, H.; Walkowiak, J.; Bogdański, P. Garlic extract favorably modifies markers of endothelial function in obese patients—Randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Parandoosh, M.; Yousefi, R.; Khorsandi, H.; Nikpayam, O.; Saidpour, A.; Babaei, H. The effects of grape seed extract (Vitis vinifera) supplement on inflammatory markers, neuropeptide Y, anthropometric measures, and appetite in obese or overweight individuals: A randomized clinical trial. Phytother. Res. 2020, 34, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Xu, T.; Bai, J.; Xia, S.; Liu, Q.; Li, J.; Xiao, X.; Dong, Y. Effect of Lactobacillus plantarum fermented barley on plasma glycolipids and insulin sensitivity in subjects with metabolic syndrome. J. Food Biochem. 2020, 44, e13471. [Google Scholar] [CrossRef] [PubMed]
- Boix-Castejón, M.; Herranz-López, M.; Pérez Gago, A.; Olivares-Vicente, M.; Caturla, N.; Roche, E.; Micol, V. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: A randomized controlled trial. Food Funct. 2018, 9, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Morimoto-Kobayashi, Y.; Ohara, K.; Ashigai, H.; Kanaya, T.; Koizumi, K.; Manabe, F.; Kaneko, Y.; Taniguchi, Y.; Katayama, M.; Kowatari, Y.; et al. Matured hop extract reduces body fat in healthy overweight humans: A randomized, double-blind, placebo-controlled parallel group study. Nutr. J. 2016, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Oniki, K.; Kawakami, T.; Nakashima, A.; Miyata, K.; Watanabe, T.; Fujikawa, H.; Nakashima, R.; Nasu, A.; Eto, Y.; Takahashi, N.; et al. Melinjo seed extract increases adiponectin multimerization in physiological and pathological conditions. Sci. Rep. 2020, 10, 4313. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Hegde, S.; Pacioretty, L.M.; DeBenedetto, J.; Babish, J.G. Nigella sativa and Trigonella foenum-graecum Supplemented Chapatis Safely Improve HbA1c, Body Weight, Waist Circumference, Blood Lipids, and Fatty Liver in Overweight and Diabetic Subjects: A Twelve-Week Safety and Efficacy Study. J. Med. Food 2020, 23, 905–919. [Google Scholar] [CrossRef]
- Choi, H.N.; Choue, R.; Park, Y.; Yim, J.E. Onion Peel Extract Increases Erythrocyte Membrane n-3 Fatty Acids in Overweight and Obese Korean Subjects. J. Med. Food 2020, 23, 37–42. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, E.Y.; Kim, J.W.; Lee, Y.; Ryu, R.; Yun, J.; Kim, M.; Choi, M.S. Intervention Study on the Efficacy and Safety of Platycodon grandiflorus Ethanol Extract in Overweight or Moderately Obese Adults: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2445. [Google Scholar] [CrossRef]
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 12, 91. [Google Scholar] [CrossRef]
- Amini, L.; Mojab, F.; Jahanfar, S.; Sepidarkish, M.; Raoofi, Z.; Maleki-Hajiagha, A. Efficacy of Salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: A double-blinded placebo-controlled clinical trial. Complement. Ther. Med. 2020, 48, 102245. [Google Scholar] [CrossRef]
- Maia-Landim, A.; Ramírez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement. Altern. Med. 2018, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Berset, V.; Moore, L. Effects of Stevia Extract on Postprandial Glucose Response, Satiety and Energy Intake: A Three-Arm Crossover Trial. Nutrients 2019, 11, 3036. [Google Scholar] [CrossRef]
- Leverrier, A.; Daguet, D.; Calame, W.; Dhoye, P.; Kodimule, S.P. Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Nurkolis, F.; Hardinsyah, H.; Taslim, N.A.; Sabrina, N.; Ibrahim, F.M.; Visnu, J.; Kumalawati, D.A.; Febriana, S.A.; Sudargo, T.; et al. Metabolomic Assay, Computational Screening, and Pharmacological Evaluation of Caulerpa racemosa as an Anti-obesity With Anti-aging by Altering Lipid Profile and Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-α Levels. Front. Nutr. 2022, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Bhat, B.; Ansari, M.; Pandey, A.; Bani, S.; Mundkur, L. The Anti-Obesity Potential of Cyperus rotundus Extract Containing Piceatannol, Scirpusin A and Scirpusin B from Rhizomes: Preclinical and Clinical Evaluations. Diabetes. Metab. Syndr. Obes. 2022, 15, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.; Rao, R.P.; Dorairaj, P.; Koshta, A.; Suresh, S.; Rafiq, M.; Kumawat, R.; Paramesh, R.; Bu, V.; Venkatesh, K.V. A clinical and computational study on anti-obesity effects of hydroxycitric acid. RSC Adv. 2019, 9, 18578–18588, Erratum in RSC Adv. 2019, 9, 22288. [Google Scholar] [CrossRef]
- Han, H.-S.; Chung, K.-S.; Shin, Y.-K.; Yu, J.-S.; Kang, S.-H.; Lee, S.-H.; Lee, K.-T. Effect of Standardized Hydrangea serrata (Thunb.) Ser. Leaves Extract on Body Weight and Body Fat Reduction in Overweight or Obese Humans: A Randomized Double-Blind Placebo-Controlled Study. Nutrients 2022, 14, 208. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chung, Y.M.; Yang, H.T.; Lin, Y.H.; Lin, Y.H.; Hu, W.C.; Chiang, C.F. The potential of immature poken (Citrus reticulata) extract in the weight management, lipid and glucose metabolism. J. Complement. Integr. Med. 2021, 19, 279–285. [Google Scholar] [CrossRef]
- Yonekura, Y.; Terauchi, M.; Hirose, A.; Odai, T.; Kato, K.; Miyasaka, N. Daily Coffee and Green Tea Consumption Is Inversely Associated with Body Mass Index, Body Fat Percentage, and Cardio-Ankle Vascular Index in Middle-Aged Japanese Women: A Cross-Sectional Study. Nutrients 2020, 12, 1370. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Afzalpour, M.E.; Nayebifar, S. Combined high-intensity interval training and green tea supplementation enhance metabolic and antioxidant status in response to acute exercise in overweight women. J. Physiol. Sci. 2020, 70, 31. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawano, T.; Ukawa, Y.; Sagesaka, Y.M.; Fukuhara, I. Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: A randomized double-blind placebo-controlled trial. Food Funct. 2016, 7, 498–507. [Google Scholar] [CrossRef]
- Koyama, T.; Maekawa, M.; Ozaki, E.; Kuriyama, N.; Uehara, R. Daily Consumption of Coffee and Eating Bread at Breakfast Time Is Associated with Lower Visceral Adipose Tissue and with Lower Prevalence of Both Visceral Obesity and Metabolic Syndrome in Japanese Populations: A Cross-Sectional Study. Nutrients 2020, 12, 3090. [Google Scholar] [CrossRef]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef]
- Haidari, F.; Samadi, M.; Mohammadshahi, M.; Jalali, M.T.; Engali, K.A. Energy restriction combined with green coffee bean extract affects serum adipocytokines and the body composition in obese women. Asia Pac. J. Clin. Nutr. 2017, 26, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Miyazaki, Y.; Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Shimizu, Y.; Wada, H.; Hasegawa, K.; et al. Kosen-cha, a Polymerized Catechin-Rich Green Tea, as a Potential Functional Beverage for the Reduction of Body Weight and Cardiovascular Risk Factors: A Pilot Study in Obese Patients. Biol. Pharm. Bull. 2020, 43, 675–681. [Google Scholar] [CrossRef]
- Zhang, S.; Takano, J.; Murayama, N.; Tominaga, M.; Abe, T.; Park, I.; Seol, J.; Ishihara, A.; Tanaka, Y.; Yajima, K.; et al. Subacute Ingestion of Caffeine and Oolong Tea Increases Fat Oxidation without Affecting Energy Expenditure and Sleep Architecture: A Randomized, Placebo-Controlled, Double-Blinded Cross-Over Trial. Nutrients 2020, 12, 3671. [Google Scholar] [CrossRef]
- Jensen, G.S.; Beaman, J.L.; He, Y.; Guo, Z.; Sun, H. Reduction of body fat and improved lipid profile associated with daily consumption of a Puer tea extract in a hyperlipidemic population: A randomized placebo-controlled trial. Clin. Interv. Aging 2016, 11, 367–376. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Ziobro, A.; Rapacka, E.; Koter-Michalak, M.; Bukowska, B. Changes in Cholinesterase Activity in Blood of Adolescent with Metabolic Syndrome after Supplementation with Extract from Aronia melanocarpa. Biomed. Res. Int. 2018, 2018, 5670145. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Peroni, G.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Naso, M.; Faliva, M.A.; Tartara, A.; et al. Bergamot phytosome improved visceral fat and plasma lipid profiles in overweight and obese class I subject with mild hypercholesterolemia: A randomized placebo controlled trial. Phytother. Res. 2021, 35, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Capomolla, A.S.; Janda, E.; Paone, S.; Parafati, M.; Sawicki, T.; Mollace, R.; Ragusa, S.; Mollace, V. Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients 2019, 11, 1271. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, G.; Shen, T.; Zhang, S.; Rao, J.; Shi, R. Effects of 12-week supplementation of Citrus bergamia extracts-based formulation CitriCholess on cholesterol and body weight in older adults with dyslipidemia: A randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2017, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Jung, U.J.; Kim, H.J.; Cho, S.J.; Kim, A.H.; Han, Y.; Choi, M.S. Combined Supplementation with Grape Pomace and Omija Fruit Ethanol Extracts Dose-Dependently Improves Body Composition, Plasma Lipid Profiles, Inflammatory Status, and Antioxidant Capacity in Overweight and Obese Subjects. J. Med. Food. 2016, 19, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Santamarina, A.B.; Flygare, A.C.; Gagliardi, A.; de Rosso, V.V.; Dourado, V.Z.; Pisani, L.P. Effects of the juçara fruit supplementation on metabolic parameters in individuals with obesity: A double-blind randomized controlled trial. J. Nutr. Biochem. 2020, 83, 108430. [Google Scholar] [CrossRef]
- Watanabe, M.; Gangitano, E.; Francomano, D.; Addessi, E.; Toscano, R.; Costantini, D.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; et al. Mangosteen Extract Shows a Potent Insulin Sensitizing Effect in Obese Female Patients: A Prospective Randomized Controlled Pilot Study. Nutrients 2018, 10, 586. [Google Scholar] [CrossRef]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar]
- Yousefi, R.; Mottaghi, A.; Saidpour, A. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: A randomized controlled trial. Complement. Ther. Med. 2018, 40, 106–112. [Google Scholar] [CrossRef]
- Abolghasemi, J.; Farboodniay Jahromi, M.A.; Hossein Sharifi, M.; Mazloom, Z.; Hosseini, L.; Zamani, N.; Nimrouzi, M. Effects of Zataria oxymel on obesity, insulin resistance and lipid profile: A randomized, controlled, triple-blind trial. J. Integr. Med. 2020, 18, 401–408. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Choung, S.Y.; Hwang, Y.C.; Ahn, K.J.; Chung, H.Y.; Jeong, I.K. Aster spathulifolius Maxim extract reduces body weight and fat mass in obese humans. Nutr. Res. 2016, 36, 671–678. [Google Scholar] [CrossRef]
- Valero-Pérez, M.; Bermejo, L.M.; López-Plaza, B.; García, M.A.; Palma-Milla, S.; Gómez-Candela, C. Regular Consumption of Lipigo(®) Promotes the Reduction of Body Weight and Improves the Rebound Effect of Obese People Undergo a Comprehensive Weight Loss Program. Nutrients 2020, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Hajmohammadi, Z.; Heydari, M.; Nimrouzi, M.; Faridi, P.; Zibaeenezhad, M.J.; Omrani, G.R.; Shams, M. Rhus coriaria L. increases serum apolipoprotein-A1 and high-density lipoprotein cholesterol levels: A double-blind placebo-controlled randomized clinical trial. J. Integr. Med. 2018, 16, 45–50. [Google Scholar] [CrossRef]
- Cheon, C.; Song, Y.K.; Ko, S.G. Efficacy and safety of Euiiyin-tang in Korean women with obesity: A randomized, double-blind, placebo-controlled, multicenter trial. Complement. Ther. Med. 2020, 51, 102423. [Google Scholar] [CrossRef]
- Cho, Y.G.; Jung, J.H.; Kang, J.H.; Kwon, J.S.; Yu, S.P.; Baik, T.G. Effect of a herbal extract powder (YY-312) from Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode on body fat mass in overweight adults: A 12-week, randomized, double-blind, placebo-controlled, parallel-group clinical trial. BMC Complement. Altern. Med. 2017, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Olivares-Vicente, M.; Boix-Castejón, M.; Caturla, N.; Roche, E.; Micol, V. Differential effects of a combination of Hibiscus sabdariffa and Lippia citriodora polyphenols in overweight/obese subjects: A randomized controlled trial. Sci. Rep. 2019, 9, 2999. [Google Scholar] [CrossRef]
- Kudiganti, V.; Kodur, R.R.; Kodur, S.R.; Halemane, M.; Deep, D.K. Efficacy and tolerability of Meratrim for weight management: A randomized, double-blind, placebo-controlled study in healthy overweight human subjects. Lipids Health Dis. 2016, 15, 136. [Google Scholar] [CrossRef][Green Version]
- Dixit, K.; Kamath, D.V.; Alluri, K.V.; Davis, B.A. Efficacy of a novel herbal formulation for weight loss demonstrated in a 16-week randomized, double-blind, placebo-controlled clinical trial with healthy overweight adults. Diabetes Obes. Metab. 2018, 20, 2633–2641. [Google Scholar] [CrossRef]
- Chung, W.; Ryu, J.; Chung, S.; Kim, S. Effect of Qingxue Dan on obesity and metabolic biomarker: A double-blind randomized-controlled pilot study. J. Tradit. Chin. Med. 2016, 36, 291–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamska-Patruno, E.; Billing-Marczak, K.; Orlowski, M.; Gorska, M.; Krotkiewski, M.; Kretowski, A. A Synergistic Formulation of Plant Extracts Decreases Postprandial Glucose and Insulin Peaks: Results from Two Randomized, Controlled, Cross-Over Studies Using Real-World Meals. Nutrients 2018, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Lee, S.; Lee, J.D. Effects of Gambisan in overweight adults and adults with obesity: A retrospective chart review. Medicine 2019, 98, e18060. [Google Scholar] [CrossRef]
- Moszak, M.; Zawada, A.; Juchacz, A.; Grzymisławski, M.; Bogdański, P. Comparison of the effect of rapeseed oil or amaranth seed oil supplementation on weight loss, body composition, and changes in the metabolic profile of obese patients following 3-week body mass reduction program: A randomized clinical trial. Lipids Health Dis. 2020, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Escalante, G.; Bryan, P.; Rodriguez, J. Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J. Cosmet. Dermatol. 2019, 18, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Galvão Cândido, F.; Xavier Valente, F.; da Silva, L.E.; Gonçalves Leão Coelho, O.; Gouveia Peluzio, M.D.C.; Gonçalves Alfenas, R.C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2018, 57, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Sasani, M.R.; Akhlaghi, M.; Kohanmoo, A. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: A randomised double-blind controlled trial. Br. J. Nutr. 2020, 123, 994–1002. [Google Scholar] [CrossRef]
- Lima, R.P.A.; do Nascimento, R.A.F.; Luna, R.C.P.; Persuhn, D.C.; da Silva, A.S.; da Conceição Rodrigues Gonçalves, M.; de Almeida, A.T.C.; de Moraes, R.M.; Junior, E.V.; Fouilloux-Meugnier, E.; et al. Effect of a diet containing folate and hazelnut oil capsule on the methylation level of the ADRB3 gene, lipid profile and oxidative stress in overweight or obese women. Clin. Epigenetics 2017, 9, 110. [Google Scholar] [CrossRef]
- Liu, X.; Kris-Etherton, P.M.; West, S.G.; Lamarche, B.; Jenkins, D.J.; Fleming, J.A.; McCrea, C.E.; Pu, S.; Couture, P.; Connelly, P.W.; et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity 2016, 24, 2261–2268. [Google Scholar] [CrossRef]
- Oliveira-de-Lira, L.; Santos, E.M.C.; de Souza, R.F.; Matos, R.J.B.; Silva, M.C.D.; Oliveira, L.D.S.; Nascimento, T.G.D.; Schemly, P.; Souza, S.L. Supplementation-Dependent Effects of Vegetable Oils with Varying Fatty Acid Compositions on Anthropometric and Biochemical Parameters in Obese Women. Nutrients 2018, 10, 932. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef]

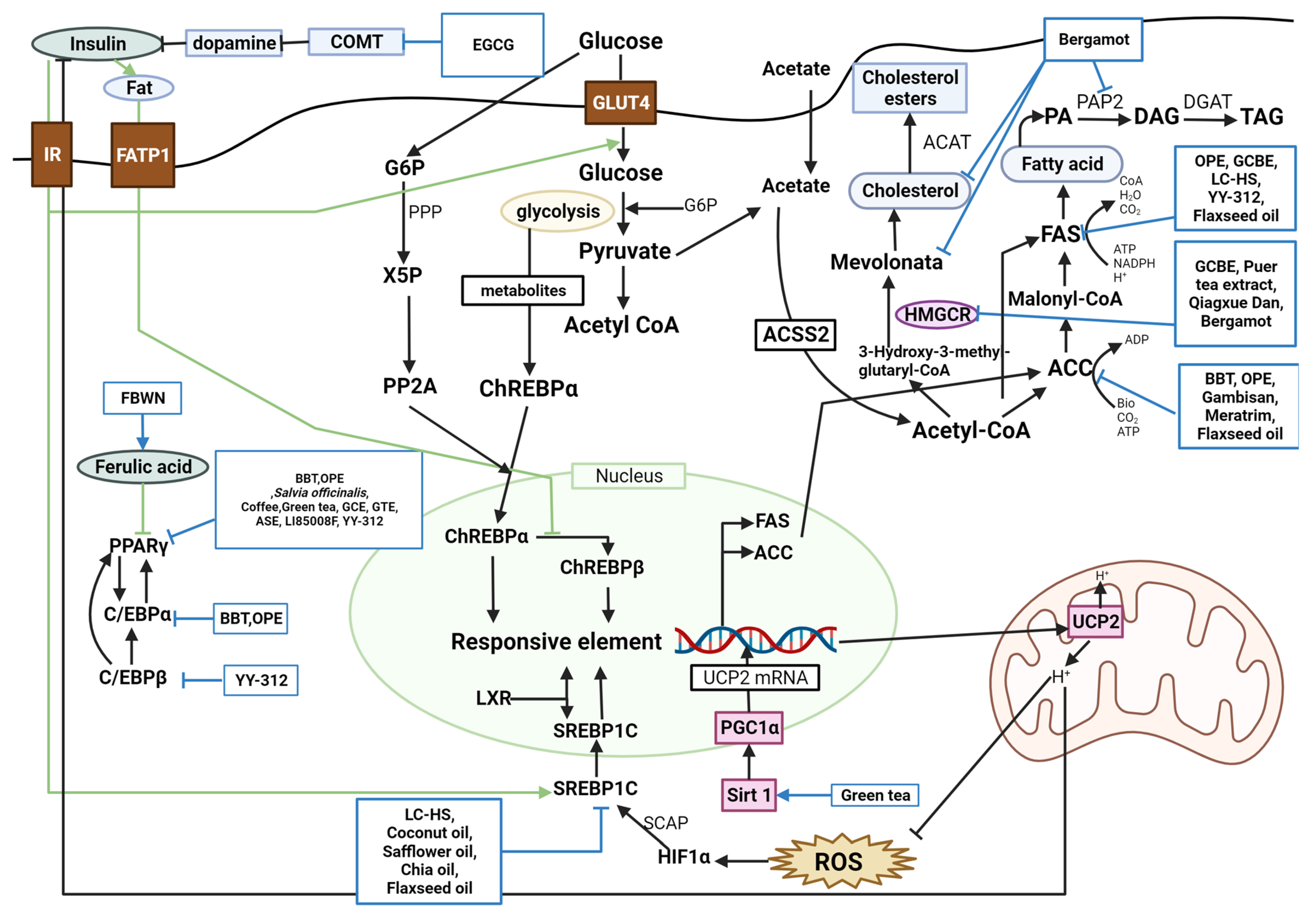
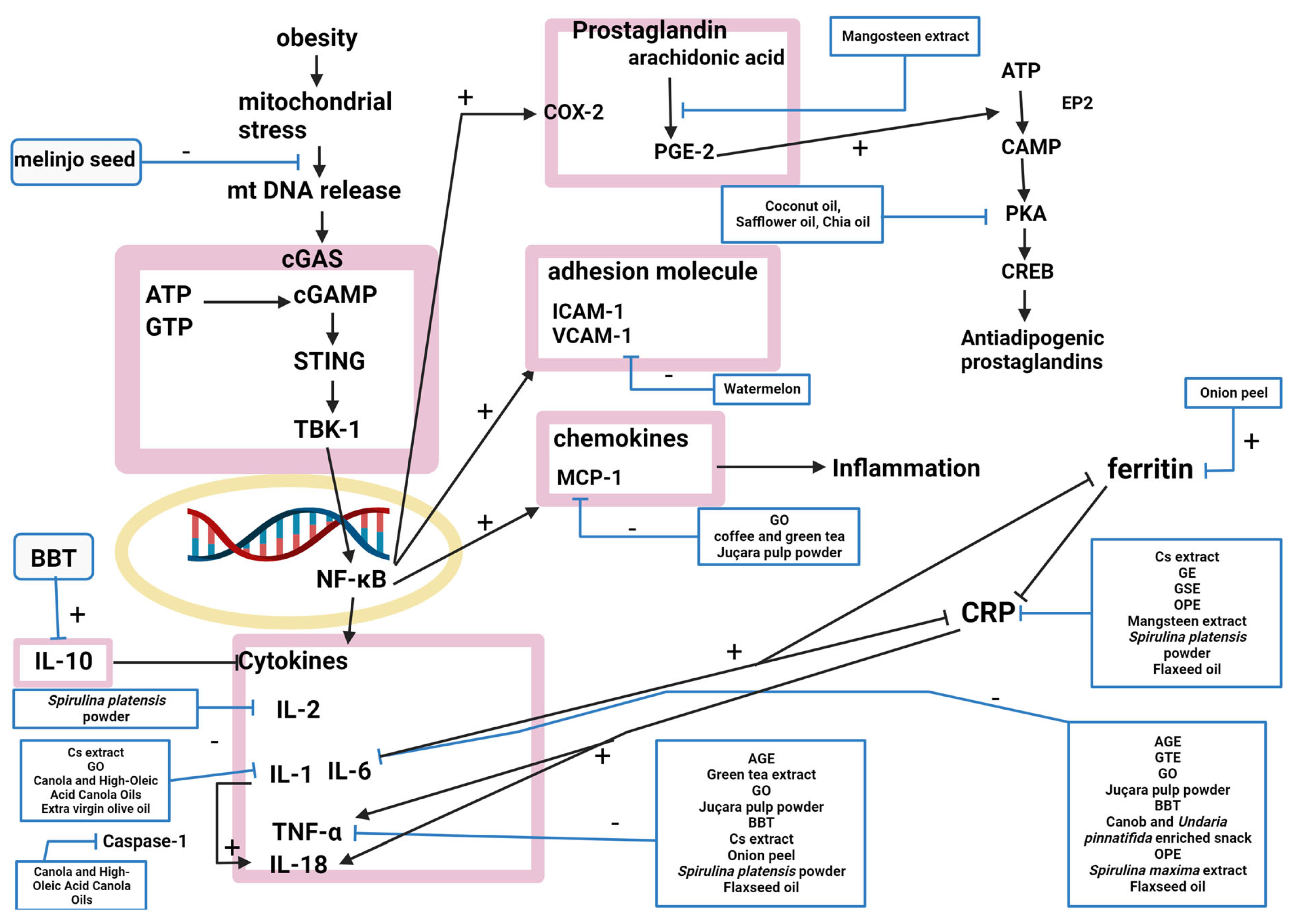
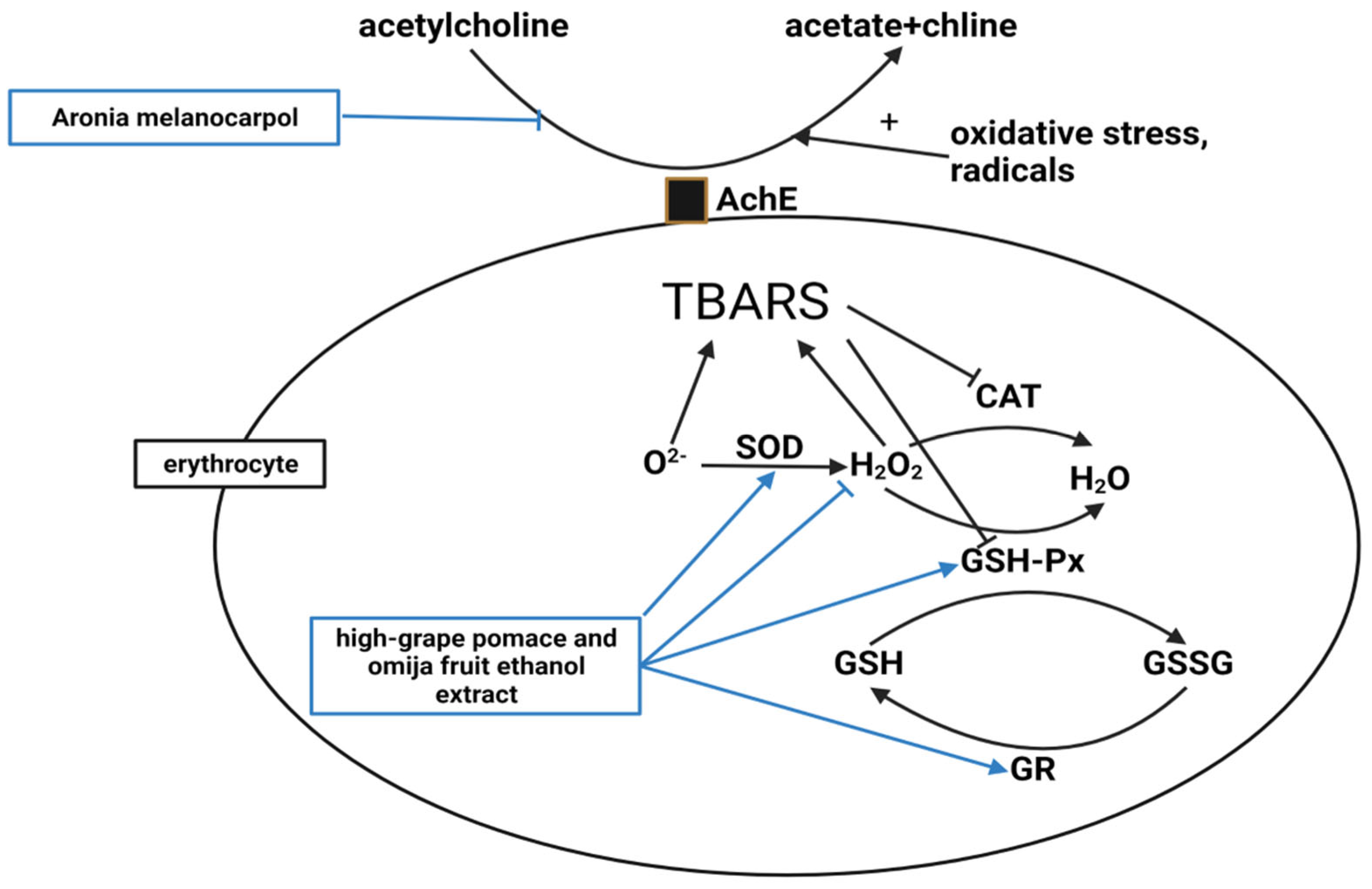
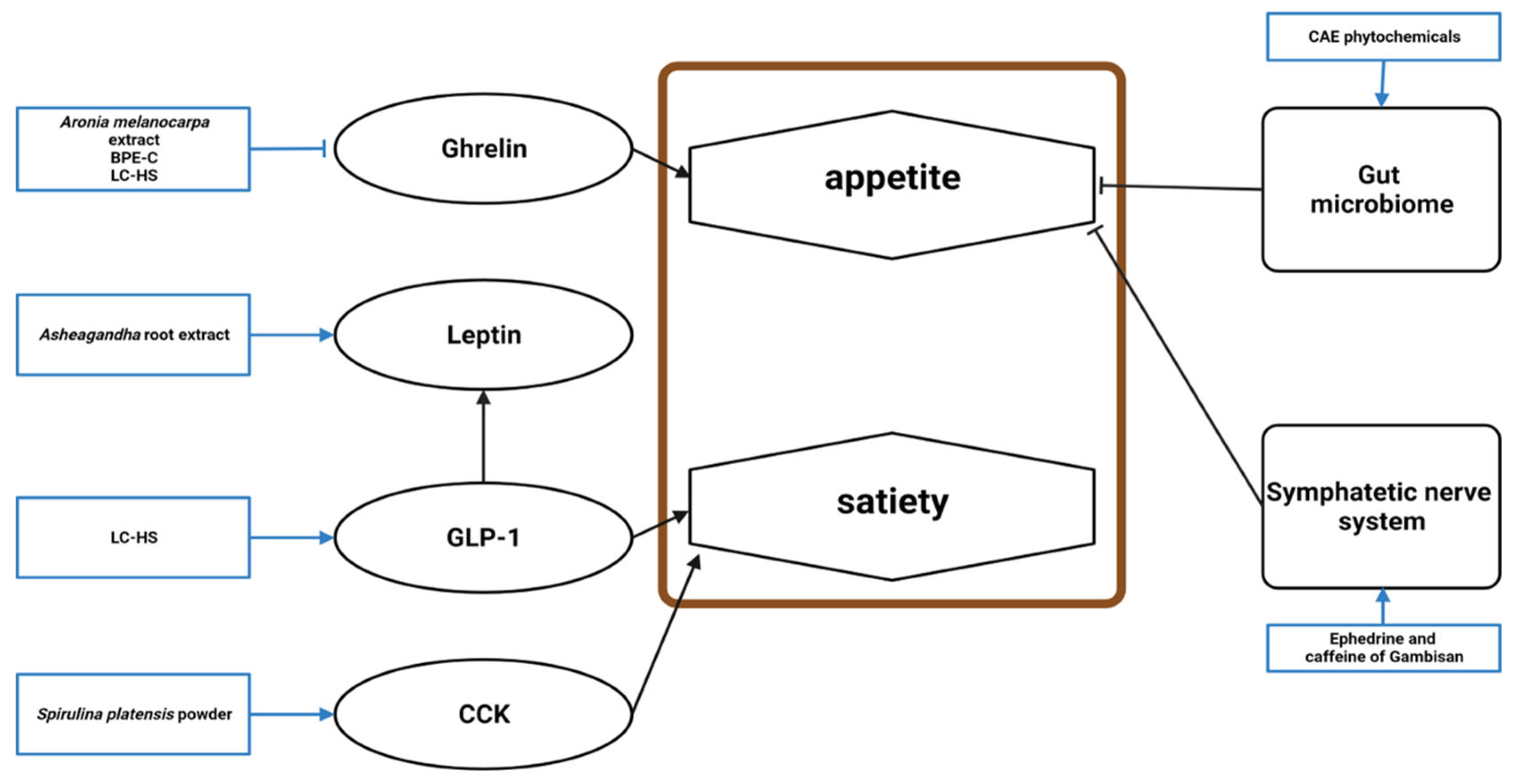

| Compound | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Diethyl azelate | 21 days prospective, before–after | 17 | Completed | Decreased obesity | ↓ TC/HDL ratio, LDL/HDL ratio, noncholesterol HDL/HDL ratio | [8] |
| Extract | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Allium sativum (aged garlic extract) | Double-blind, randomized, placebo-controlled clinical trial | 48 | Completed | NCT01959646 | Decreased obesity | ↓ LDL | [9] |
| Citrus bergamia (bergamot) and Cynara cardunculus | Double-blind placebo-controlled clinical trial | 86 | Completed | ISRCTN12833814 | Decreased BW | ↓ LDL-C, HDL-C, non-HDL-C, TC | [10] |
| Glycine max (L.) Merr (black soybean testa extract) | 8-week planned, randomized, double-blind, placebo-controlled clinical trial | 63 | Completed | NCT02108691 | Decreased obesity | ↓ TG, LDL, non-HDL | [11] |
| Carum carvi L. (caraway aqueous extract) | Triple-blind, placebo-controlled clinical trial | 60 | Completed | NCT01833377 | Decreased obesity, appetite | [12] | |
| Ceratonia siliqua (carob) and Undaria pinnatifida (wakame) enriched snack | 8-week, randomized, placebo-controlled clinical trial | 32 | Completed | NCT03420989 | Decreased obesity | ↓ TC, resistin levels, LDL-C | [13] |
| Cynara scolymus (artichoke) extract | Double-blind, placebo-controlled, randomized clinical trial | 54 | Decreased obesity, decreased BW and BMI | ↑ HDL; ↓TC, TC/HDL, LDL, LDL/HDL, ApoB, ApoB/ApoA | [14] | ||
| Allium sativum (garlic extract) | Randomized double-blind placebo-controlled nutritional intervention clinical trial with two parallel arms | 92 | DRKS00010533 | Decreased obesity | ↓ LDL-C | [15] | |
| Vitis vinifera L. (grape) seed extract | Randomized, double-blind, placebo-controlled clinical trial | 40 | Completed | IRCT2015073015968N3 | Decreased obesity | ↓ NPY | [16] |
| Lactobacillus plantarum fermented Hordeum vulgare-Triticum aestivum (barley-wheat) flour compound noodle | Single-blinded, controlled, parallel clinical trial | 30 | Completed | ChiCTR1800019614 | Decreased obesity | ↓ TG | [17] |
| Lippia citriodora (lemon beebrush) and Hibiscus sabdariffa (roselle) | 8-week, randomized, double-blind, placebo-controlled clinical trial | 54 | Completed | Decreased obesity, appetite | ↓ Leptin, resistin | [18] | |
| Matured Humulus lupulus L. (hops) | Randomized, double-blind, placebo-controlled parallel-arm clinical trial | 178 | Completed | UMIN000014185 | Decreased BF | [19] | |
| Gnetum gnemon Linn (melinjo) seed | Prospective, randomized, parallel, double-blind, placebo-controlled clinical trial | 42 | Completed | UMIN000025643 | Increased APN multimerization | ↑ HMW/total APN ratio | [20] |
| Nigella sativa (black seed or jintan hitam) and Trigonella foenum-graecum (fenugreek) supplemented chapatis | 12-week prospective, before–after clinical trial | 40 | Completed | Decreased obesity | ↓ TC, non-↑ HDL-C, VLDL, TG, ↓ HbA1C, FPG | [21] | |
| Allium cepa L. (onion) peel | Randomized, double-blind, placebo-controlled clinical trial | 61 | Decreased obesity | ↑ PUFA n-6 ↓ PUFA n-3 | [22] | ||
| Platycodon grandiflorus (balloon flower) ethanol extract | Single-center, randomized, double-blind, placebo-controlled clinical trial | 72 | Completed | Decreased obesity | PGE571: ↓ leptin. PGE2855: ↓ L:A ratio | [23] | |
| Quercetin-rich Allium cepa L. (onion) powder | Randomized, double-blind, placebo-controlled, parallel-group clinical trial | 54 | Completed | UMIN000033410 | Subjects with lower HDL-C: decreased VFA. | [24] | |
| Salvia officinalis (common sage) | Randomized triple-blinded placebo-controlled clinical trial | 60 | Completed | IRCT201504146917N2 | Decreased obesity | [25] | |
| Garcinia cambogia (Malabar tamarind) and Amorphophallus konjac (konjac) | Prospective, nonrandomized controlled intervention clinical trial | 214 | Completed | Decreased weight | ↓ Cholesterol, TG | [26] | |
| Stevia rebaudiana (stevia) | Randomized, three-arm, single-blinded crossover clinical trial | 30 | Completed | NCT01115088 | Decreased energy intake | [27] | |
| Helianthus annuus (sunflower) seed extract | Randomized, placebo-controlled, double-blind, parallel-group clinical pilot study | 46 | Completed | Decreased obesity | ↓ Cholesterol, long-lasting LDL | [28] | |
| Citrullus lanatus (watermelon) | Randomized 2-arm design with a single 6-week intervention period | 45 | Completed | NCT04015544 | Decreased obesity | [29] | |
| Caulerpa racemosa (green algae) | Randomized, double-blind, placebo-controlled clinical trial | 74 | Completed | NCT05037591 | Decreased obesity | ↑ HDL, proliferator-activated receptor-γ coactivator α (PGC-1α); ↓ TC, TG | [30] |
| Cyperus rotundus rhizome extract | Randomized, double-blind, parallel-group, placebo-controlled pilot study | 30 | Completed | CTRI/2014/05/004633 | Decreased waist circumference and BMI | ↓ TC, TG, LDL, VLDL; ↑ HDL | [31] |
| Garcinia cambogia (Malabar tamarind) extract | Open-label clinical study | 100 | Completed | Improved anthropometric and metabolic state | ↓ LDL; ↑ HDL | [32] | |
| Hydrangea serrata (Thunb.) Ser. leaf extract | Randomized, double-blind, placebo-controlled clinical trial | 93 | Completed | KCT0005594 | Decreased overweight | ↓ LDL, TG | [33] |
| Citrus reticulata (immature poken) extract | Randomized, placebo-controlled clinical trial | 20 | Completed | CMUH103-REC2-040 | Decreased weight and fat metabolism by suppressing adipogenesis | ↓ LDL, TG, TC | [34] |
| Tea | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Coffea arabica (coffee), Camellia sinensis (green tea) | Cross-sectional, brief-type self-administered diet history questionnaire | 232 | Completed | Decreased BW and BMI | [35] | ||
| Coffee, green tea | Cross-sectional, Japan multi-institutional collaborative cohort study | 3539 | Completed | Coffee: decreased VAT, metabolic syndrome | [38] | ||
| Decaffeinated green coffee bean extract | Randomized, double-blind, placebo-controlled trial | 43 | Completed | NCT02764957 | Decreased obesity and appetite | [39] | |
| Green coffee bean extract | Randomized, double-blind, placebo-controlled clinical trial | 64 | Completed | Decreased obesity | ↑ Serum adiponectin; ↓ total serum cholesterol, LDL, FFA, leptin | [40] | |
| Green tea | 10-week randomized, placebo-controlled trial | 30 | Completed | NCT04950062 | Increased metabolic status | ↑ PGC-1α | [36] |
| Green tea | Randomized, double-blind, placebo-controlled clinical trial | 124 | Completed | Decreased BF | [37] | ||
| Green tea extract | Double-blinded placebo-controlled trial | 45 | Completed | IRCT20151025024699N3 | Decreased obesity | ↑ Adiponectin, irisin | [41] |
| High-dose green tea extract (epigallocatechin gallate) | Randomized, single-center, placebo-controlled, double-blind study | 77 | Unknown | NCT02147041 | Decreased weight | ↑ Adiponectin; ↓ cholesterol, LDL, ghrelin | [42] |
| Kosen-cha | 12-week, prospective, before–after study | 6 | Completed | Decreased obesity | ↓TG, ↑insulin sensitivity | [43] | |
| Oolong tea | 14-day, placebo-controlled, double-blind, crossover intervention trial | 12 | Completed | Increased FO | [44] | ||
| Puer tea extract | Randomized, double-blind, placebo-controlled clinical trial | 59 | Completed | NCT03613688 | Decreased obesity | ↓ Cholesterol | [45] |
| Extract | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Aronia melanocarpa extract | Placebo-controlled trial | 77 | Completed | Decreased cholinesterase activity | ↑ HDL, cholesterol, TAC “fast” parameter; ↓ TC, LDL, TG, TAC “slow” parameter, lipid peroxidation, cholesterol in the erythrocyte membranes | [46] | |
| Citrus bergamia (bergamot) phytosome | Randomized, double-blind, placebo-controlled trial | 64 | Completed | Decreased VAT | ↓ TC, LDL, ApoB, LDL/HDL; ↑ ApoA/HDL | [47] | |
| Citrus bergamia (bergamot) polyphenol extract-complex | Randomized, double-blind, placebo-controlled trial | 45 | Completed | UNICZ Trial No. 182/2016 | Decreased weight | ↓ TC, LDL, TAG, serum leptin, serum ghrelin; ↑ HDL, serum adiponectin | [48] |
| Citrus bergamia (bergamot) | Randomized, double-blind, placebo-controlled trial | 98 | Completed | Decreased cholesterol and BW | ↓ LDL | [49] | |
| Grape pomace and Schisandra chinensis (omija) fruit ethanol extract | Randomized, double-blind, placebo-controlled trial | 76 | Completed | Decreased obesity-related dyslipidemia | High GO: ↑ ApoA-1; ↓ TC, non-HDL-C, LDL-C, plasma ApoB, Apo B/ApoA-1 ratio, plasma Lp(a) | [50] | |
| Euterpe edulis (juçara) pulp powder | Randomized, double-blind trial | 35 | Completed | RBR-5RXR2B | Decreased obesity | ↑ HDL-C, serum adiponectin; ↓ TC, LDL, TAG, L:A ratio | [51] |
| Garcinia mangostana (mangosteen) extract | 26-week prospective randomized, controlled, parallel-group study | 20 | Completed | NCT02823561 | Decreased weight | ↓ HDL | [52] |
| Extract | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Withania somnifera (ashwagandha) root extract | Double-blind, randomized, placebo-controlled trial | 50 | Completed | Decreased BW | ↓ Mean FCQ scores, mean TFEQ score | [56] | |
| Aster spathulifolius Maxim | Randomized, double-blind, placebo-controlled clinical trial | 41 | Completed | Decreased BW and FM | ↑ LDL | [57] | |
| Lipigo® | Randomized, double-blinded, placebo-controlled clinical trial | WLP: 98 P-WLP: 73 | Completed | NCT03554525 | Decreased BW, rebound effect | [58] | |
| Rhus coriaria L. powder ethanolic extract | Randomized, double-blind, placebo-controlled clinical trial with two arms | 70 | Completed | NCT02295293 | Increased ApoA-1 and HDL | ↑ HDL, serum Apo-A1 | [59] |
| Spirulina maxima extract | Randomized Double-Blind Placebo-Controlled Trial | 50 | Completed | NCT02575690 | Decreased obesity | ↓ LDL | [53] |
| Spirulina platensis powder | Randomized, double-blinded, placebo-controlled clinical trial | 38 | Completed | NCT02993627 | Decreased obesity | ↓ TG | [54] |
| Zataria multiflora Boiss with or without oxymel | Randomized, controlled, triple-blind Trial | 92 | Completed | IRCT20171220037976N1 | Decreased obesity | [55] |
| Drug | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Euiiyin-tang | Randomized, double-blind, placebo-controlled, multicenter trial | 149 | Completed | NCT01724099 | Decreased obesity | [60] | |
| Gambisan | Double-blinded, randomized, placebo-controlled, phase 2 trial | 205 | Completed | Decreased obesity and appetite | [67] | ||
| Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode (YY-312) | Randomized, double-blind, placebo-controlled, parallel-group clinical trial | 60 | Completed | KCT0001225 | Decreased BFM | [61] | |
| Lippia citriodora (lemon beebrush) and Hibiscus sabdariffa (roselle; LC-HS) | Double-blind, placebo-controlled, randomized trial | 56 | Completed | P201731147 | Decreased obesity | [62] | |
| Meratrim | Randomized, double-blind, placebo-controlled trial | 57 | Completed | CTRI/2014/07/004727 | Decreased obesity and appetite | ↑ Glycerol production, AMPK, ACC phosphorylation, HDL; ↓ TG, TC, LDL | [63] |
| Moringa oleifera leaf aqueous ethanol extract, Murraya koenigii (L.) Spreng. leaf aqueous ethanol extract, and Curcuma longa L. extract (LI85008F) | Randomized, double-blind, placebo-controlled trial | 140 | Completed | C007185 | Decreased weight | ↑ HDL; ↓ LDL, VLDL, TC, TG | [64] |
| Qingxue Dan | Randomized, double-blinded, placebo-controlled trial with parallel arms | 26 | Completed | Decreased obesity | ↓ TG | [65] | |
| White mulberry, white bean extract, and green coffee (IP-A and IP-B) | Randomized, double-blind, placebo-controlled, crossover trial | Study 1: 32 Study 2: 150 | Completed | PCT/IB2015/052650 | Decreased obesity | [66] |
| Drug | |
|---|---|
| Euiiyin-tang | Ephedra sinica Stapf, Angelica gigantis Radi, Atractylodis rhizoma Alba, Coicis semen, Cinnamomi cortex, Paeonia lactiflora, and Glycyrrhiza uralensis. |
| Gambisan | The herbal part of Ephedra intermedia Schrenk, Gypsum Fibrosum, the rhizome part of Atractylodes lancea DC, and the leaf part of Thea sinensis L. |
| LC-HS | Combination of polyphenolic extracts from Lippia citriodora L. and Hibiscus sabdariffa L. |
| YY-312 | Herbal extract powder from Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode. |
| Meratrim | A blend of two plant extracts obtained from Sphaeranthus indicus flower heads and Garcinia mangostana fruit rinds. |
| LI85008F | Six parts Moringa oleifera leaf aqueous ethanol extract, three parts Murraya koenigii (L.) Spreng. leaf aqueous ethanol extract, and 1 part Curcuma longa L. extract. |
| Qingxue Dan | Herbal formula consisting of radix of Scutellaria baicalensis Georgi, rhizoma of Coptis japonica Makino, cortex of Phellodendron amurense Ruprecht, fructus of Gardenia jasminoides Ellis, and rhizoma of Rheum palmatum Linne. |
| IP-A and IP-B | IP-A: A mixture of Morus alba (white mulberry), Phaseolus vulgaris (white bean) extract, and Coffea arabica (green coffee). IP-B: A mixture of white mulberry, white bean extract, and green coffee supplemented with inulin and glucomannan. |
| Drug | Study Design | Population | Status | Number | Outcome | Lab Test | Reference |
|---|---|---|---|---|---|---|---|
| Amaranthus cruentus (amaranth) seed oil and Brassica napus (rapeseed) oil | Randomized, double-blind, controlled trial with three parallel arms | 81 | Completed | Decreased obesity | [68] | ||
| Aminophylline, caffeine, Yohimbe, l-carnitine, and Centella asiatica (gotu kola; Lipoxyderm) | 28-day, double-blind, placebo-controlled, within-group study | 7 | Completed | Decreased thigh circumference, skinfold thickness, and FM | [69] | ||
| Canola oil, oleic, and DHA; Zea mays (corn)/Carthamus tinctorius (safflower) oil; and Linum usitatissimum (flax)/safflower oil | Randomized, crossover, controlled feeding study | 101 | Completed | NCT01351012 | Decreased abdominal FM | ↑ Plasma oleic acid; ↓ android FM, android-to-gynoid FM ratio; canola oleic oil: ↓ TG | [73] |
| Cocos nucifera (coconut) oil, Carthamus tinctorius (safflower) oil, Salvia hispanica (chia) oil | Randomized, double-blind, placebo-controlled clinical trial | 75 | Completed | RBR-36bjsc | Decreased obesity | Chia oil: ↑ HDL-C; ↓ cholesterol, LDL-C, and TG | [74] |
| Extra virgin Olea europaea (olive) oil | 9-week, randomized, double-blind, placebo-controlled clinical trial | 54 | Completed | Decreased obesity. | [70] | ||
| Linum usitatissimum (flax) seed oil | Randomized, double-blind, placebo-controlled clinical trial | 68 | Completed | IRCT 2016011125957 N1 | Decreased weight | [71] | |
| Folate and Corylus (hazelnut) oil capsules | Double-blind, placebo-controlled intervention study | 40 | Completed | NCT02846025 | Decreased obesity | ↑ HDL; ↓ LDL. Group 1: ↑ HDL. Group 3: ↓ WHtR, LDL, and total fat intake. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Nurkolis, F.; Won, H.; Yang, J.; Oh, D.; Jo, H.; Choi, J.; Chung, S.; Kurniawan, R.; Kim, B. Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives. Molecules 2023, 28, 6604. https://doi.org/10.3390/molecules28186604
Park J, Nurkolis F, Won H, Yang J, Oh D, Jo H, Choi J, Chung S, Kurniawan R, Kim B. Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives. Molecules. 2023; 28(18):6604. https://doi.org/10.3390/molecules28186604
Chicago/Turabian StylePark, Jiwon, Fahrul Nurkolis, Hyunji Won, Jiye Yang, Dayeon Oh, Hyunkyung Jo, Jinwon Choi, Sanghyun Chung, Rudy Kurniawan, and Bonglee Kim. 2023. "Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives" Molecules 28, no. 18: 6604. https://doi.org/10.3390/molecules28186604
APA StylePark, J., Nurkolis, F., Won, H., Yang, J., Oh, D., Jo, H., Choi, J., Chung, S., Kurniawan, R., & Kim, B. (2023). Could Natural Products Help in the Control of Obesity? Current Insights and Future Perspectives. Molecules, 28(18), 6604. https://doi.org/10.3390/molecules28186604









