Possible Side Effects of Polyphenols and Their Interactions with Medicines
Abstract
1. Introduction
2. Role of Polyphenols as Antioxidants
3. Polyphenols in Disease Prevention and Treatment
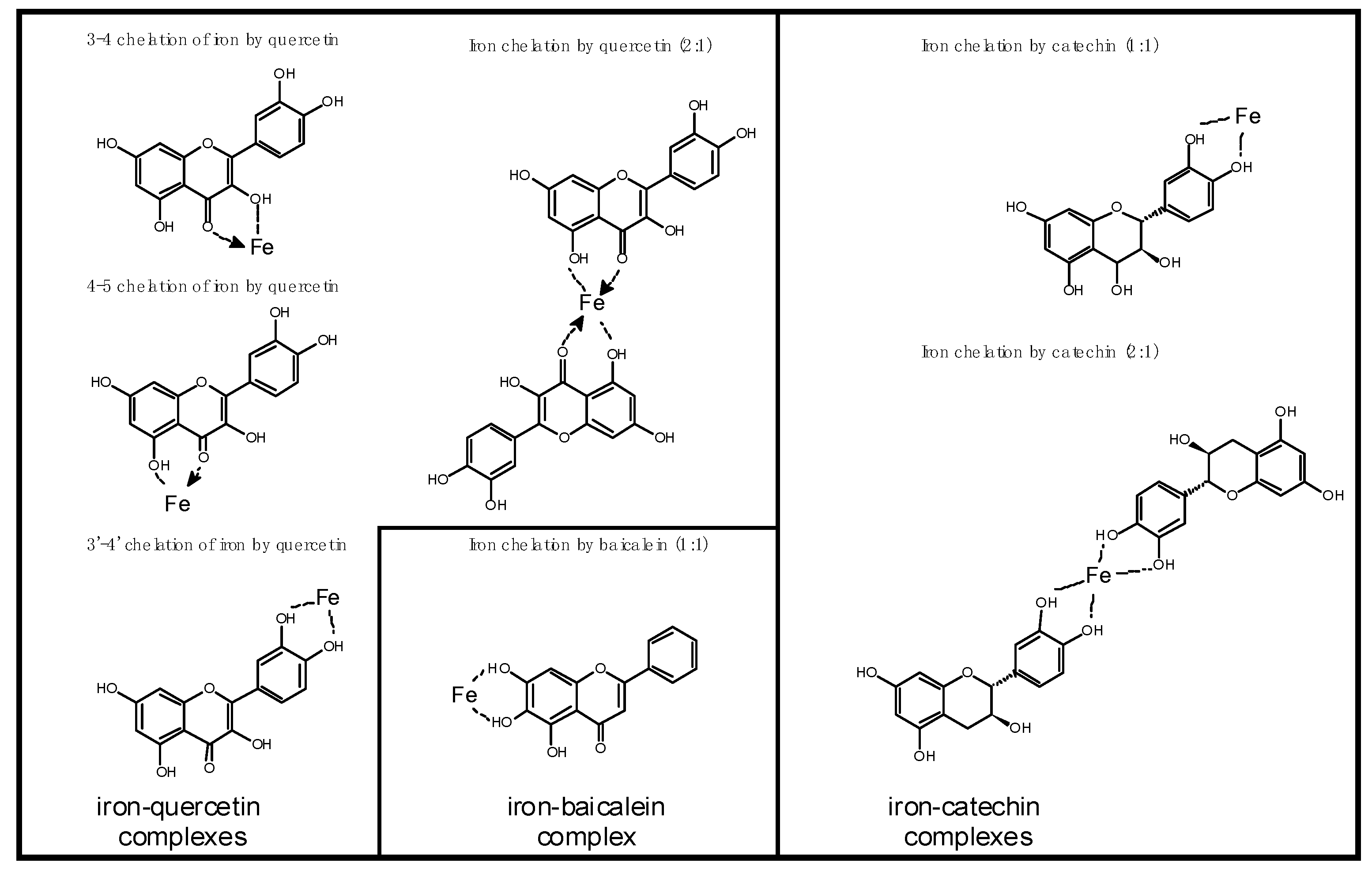
4. Possible Negative Consequences of Blocking Iron Uptake
5. The Inhibition of Digestive Enzymes by Polyphenolic Compounds
6. Possibility of the Intestinal Microbiota Inhibition and Consequences
7. Interactions of Polyphenolic Compounds with Drug Disposition and Metabolism
8. Can Polyphenols Induce a Hormonal Imbalance?
9. Prooxidant Activity of Polyphenols and the Consequences
10. Mutagenic, Cancerogenic and Genotoxic Effects
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Soares, S.; Sousa, C.F.; Dias, R.; Gameiro, P.; Soares, S.; de Freitas, V. Interaction of polyphenols with model membranes: Putative implications to mouthfeel perception. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183133. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S112–S120. [Google Scholar] [CrossRef]
- Bongiorno, D.; Di Stefano, V.; Indelicato, S.; Avellone, G.; Ceraulo, L. Bio-phenols determination in olive oils: Recent mass spectrometry approaches. Mass Spectrom Rev. 2021, e21744. [Google Scholar] [CrossRef]
- Indelicato, S.; Houmanat, K.; Bongiorno, D.; Ejjilani, A.; Hssaini, L.; Razouk, R.; Charafi, J.; Ennahli, S.; Hanine, H. Freeze dried pomegranate juices of Moroccan fruits: Main representative phenolic compounds. J. Sci. Food Agric. 2023, 103, 1355–1365. [Google Scholar] [CrossRef]
- Lyu, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic compounds profiling and their antioxidant capacity in the peel, pulp, and seed of Australian grown avocado. Antioxidants 2023, 12, 185. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019, 49 (Suppl. S1), 3–23. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.-A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New mesoporous silica materials loaded with polyphenols: Caffeic acid, ferulic acid and p-coumaric acid as dietary supplements for oral administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44 (Suppl. S1), S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2010, 2, 1–12. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Datla, S.R.; Griendling, K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 7–20. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, B.A.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Jopkiewicz, S. Oxidative stress Part I. Oxidative stress as a factor in the development of civilization diseases. Med. Srod. 2018, 21, 48–52. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and anti-inflammatory effects of coenzyme Q10 supplementation on infectious diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Alov, P.; Tsakovska, I.; Pajeva, I. Computational studies of free radical-scavenging properties of phenolic compounds. Curr. Top. Med. Chem. 2015, 15, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; Al-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Truscott, T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids—A review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Al-Sehemi, A.G.; Irfan, A. Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arab. J. Chem. 2017, 10, S1703–S1710. [Google Scholar] [CrossRef]
- Thbayh, D.K.; Reizer, E.; Kahaly, M.U.; Viskolcz, B.; Fiser, B. Antioxidant potential of santowhite as synthetic and ascorbic acid as natural polymer additives. Polymers 2022, 14, 3518. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RCS Adv. 2015, 35, 27986. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Rad. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Kouvari, M.; D’Cunha, N.M.; Travica, N.; Sergi, D.; Zec, M.; Marx, W.; Naumovski, N. Metabolic syndrome, cognitive impairment and the role of diet: A narrative review. Nutrients 2022, 14, 333. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxid. Med. Cell Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The action of polyphenols in diabetes mellitus and Alzheimer’s disease: A common agent for overlapping pathologies. Curr. Neuropharmacol. 2019, 17, 590–613. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; McIver, I.E.; Johnston, C.S. Hypertensive crisis associated with high dose soy isoflavone supplementation in a post-menopausal woman: A case report [ISRCTN98074661]. BMC Women Health 2005, 5, 9. [Google Scholar] [CrossRef]
- Shi, M.; Lu, Y.; Wu, J.; Zheng, Z.; Lv, C.; Ye, J.; Qin, S.; Zeng, C. Beneficial effects of theaflavins on metabolic syndrome: From molecular evidence to gut microbiome. Int. J. Mol. Sci. 2022, 23, 7595. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 292S–297S. [Google Scholar] [CrossRef]
- Ponzo, V.; Goitre, I.; Fadda, M.; Gambino, R.; De Francesco, A.; Soldati, L.; Gentile, L.; Magistroni, P.; Cassader, M.; Bo, S. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J. Transl. Med. 2015, 13, 218. [Google Scholar] [CrossRef]
- Dalgaard, F.; Bondonno, N.P.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Kyrø, C.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Associations between habitual flavonoid intake and hospital admissions for atherosclerotic cardiovascular disease: A prospective cohort study. Lancet Planet. Health 2019, 3, e450–e459. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.L. The role of flavonoids in the prevention and management of cardiovascular complications: A narrative review. Ann. Palliat. Med. 2021, 10, 8254–8263. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Gross, M. Flavonoids and cardiovascular disease. Pharm. Biol. 2004, 42 (Suppl. 1), 21–35. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Mizuno, T.; Aida, K.; Uchino, K. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci. Biotechnol. Biochem. 1997, 61, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Alagawadi, K.R. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm. Biol. 2013, 51, 607–613. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review. Evid.-Based Complement. Altern. Med. 2015, 2015, 973143. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Ding, J.; Chen, G. Effect of green tea supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Medicine 2020, 99, e19047. [Google Scholar] [CrossRef]
- Negishi, H.; Xu, J.W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004, 134, 38–42. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, R.; Wang, B.; Yu, X.; Yang, X.; Liu, K.; Mi, M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014, 4, 6251. [Google Scholar] [CrossRef]
- Schaffer, S.; Asseburg, H.; Kuntz, S.; Muller, W.E.; Eckert, G.P. Effects of polyphenols on brain ageing and Alzheimer’s disease: Focus on mitochondria. Mol. Neurobiol. 2012, 46, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; Glenn, J.M.; Bott, N.T.; Müller, N.; Chtourou, H.; Driss, T.; et al. Effects of polyphenol-rich interventions on cognition and brain health in healthy young and middle-aged adults: Systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural phenols. Expert Rev. Neurother. 2015, 15, 41–52. [Google Scholar] [CrossRef]
- Maulik, M.; Mitra, S.; Sweeney, M.; Lu, B.; Taylor, B.E.; Bult-Ito, A. Complex interaction of dietary fat and Alaskan bog blueberry supplementation influences manganese mediated neurotoxicity and behavioral impairments. J. Funct. Foods. 2019, 53, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, S.R. Beneficial effects of flavonoids against Parkinson’s disease. J. Med. Food. 2018, 21, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Mangrulkar, S.; Chaple, D. Pharmacological assessments of polyphenolic extract of Cymbopogon citratus leaves in rodent model of Parkinson’s disease. J. Drug. Deliv. Ther. 2019, 9, 311–315. [Google Scholar] [CrossRef]
- Maqbool, M.; Zehravi, M. Neuroprotective role of polyphenols in treatment of neurological disorders: A review. Interv. Pain Med. Neuromod. 2021, 1, e117170. [Google Scholar] [CrossRef]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 284S–291S. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M. The potential roles of epigallocatechin-3-gallate in the treatment of ovarian cancer: Current state of knowledge. Drug. Des. Devel. Ther. 2020, 14, 4245–4250. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Lin, Q.; Wang, Y.; Sun, H.; Wang, J.; Cui, G.; Cai, L.; Dong, X. Tea polyphenols induced apoptosis of breast cancer cells by suppressing the expression of survivin. Sci. Rep. 2014, 4, 4416. [Google Scholar] [CrossRef]
- Wang, S.T.; Cui, W.Q.; Pan, D.; Jiang, M.; Chang, B.; Sang, L.X. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 2020, 26, 562–597. [Google Scholar] [CrossRef]
- Cui, G.; zhang, Y.; Liu, C.; Skinner, S.; Qin, Y. Pancreatic cancer suppression by natural polyphenols. Sch. Res. Exch. 2008, 2008, 540872. [Google Scholar] [CrossRef]
- Weh, K.M.; Zhang, Y.; Howard, C.L.; Howell, A.B.; Clarke, J.L.; Kresty, L.A. Cranberry polyphenols in esophageal cancer inhibition: New insights. Nutrients 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shi, A.; Wang, N.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Tao, Y.; Wang, Q.; et al. Polyphenolic proanthocyanidin-B2 suppresses proliferation of liver cancer cells and hepatocellular carcinogenesis through directly binding and inhibiting AKT activity. Redox Biol. 2020, 37, 101701. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, S.; Ding, H.; Shao, Y.; Zhou, S.; Pu, W.; Han, L.; Wang, T.; Yu, H. Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention. Cell Prolif. 2023, 56, e13346. [Google Scholar] [CrossRef]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and their metabolites in renal diseases: An overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Ahmad, N.; Feyes, D.K.; Agarwal, R.; Mukhtar, H.; Nieminen, A.-L. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Nat. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef]
- Han, D.H.; Jeong, J.H.; Kim, J.H. Anti-proliferative and apoptosis induction activity of green tea polyphenols on human promyelocytic leukemia HL-60 cells. Anticancer Res. 2009, 29, 1417–1421. [Google Scholar]
- Gupta, K.; Thakur, V.S.; Bhaskaran, N.; Nawab, A.; Babcook, M.A.; Jackson, M.W.; Gupta, S. Green tea polyphenols induce p53-dependent and p53-independent apoptosis in prostate cancer cells through two distinct mechanisms. PLoS ONE 2012, 7, e52572. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; Bonanno, G.; De Vincenzo, R.; Ferrandina, G.; Rumi, C.; Larocca, L.M.; Mancuso, S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br. J. Cancer 1990, 62, 942–946. [Google Scholar] [CrossRef]
- Dou, C.Z.; Liu, Y.F.; Zhang, L.L.; Chen, S.H.; Hu, C.Y.; Liu, Y.; Zhao, Y.T. Polyphenols from Broussonetia papyrifera induce apoptosis of HepG2 cells via inactivation of ERK and AKT signaling pathways. Evid.-Based Complement. Alternat. Med. 2021, 2021, 8841706. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Gascón, S.; Luquin, A.; Laguna, M.; Ancin-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef] [PubMed]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int. J. Cancer 2002, 98, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Alsamri, H.; El Hasasna, H.; Al Dhaheri, Y.; Eid, A.H.; Attoub, S.; Iratni, R. Carnosol, a natural polyphenol, inhibits migration, metastasis, and tumor growth of breast cancer via a ROS-dependent proteasome degradation of STAT3. Front. Oncol. 2019, 9, 743. [Google Scholar] [CrossRef]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014, 164, 363–370. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Not, C.; Guinó, E.; Luján-Barroso, L.; García, R.M.; Biondo, S.; Salazar, R.; Moreno, V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case-control study (the Bellvitge Colorectal Cancer Study). Cancer Causes Control 2013, 24, 549–557. [Google Scholar] [CrossRef]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef]
- Woo, H.D.; Lee, J.; Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kwon, O.; Kim, J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef] [PubMed]
- Geybels, M.S.; Verhage, B.A.; Arts, I.C.; van Schooten, F.J.; Goldbohm, R.A.; van den Brandt, P.A. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.Y.; Naidu, A.; Parent, M.É.; Pintos, J.; Abrahamowicz, M.; Siemiatycki, J.; Koushik, A. The risk of lung cancer related to dietary intake of flavonoids. Nutr. Cancer 2012, 64, 964–974. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An efficient way to improve antitumoral activity and reduce resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef] [PubMed]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xiao, X.; Chen, D.; Yu, B.; He, J. Tea and its components prevent cancer: A review of the redox-related mechanism. Int. J. Mol. Sci. 2019, 20, 5249. [Google Scholar] [CrossRef] [PubMed]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and dietary polyphenols. Oxid. Med. Cell Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Gundala, S.R.; Yang, C.; Donthamsetty, S.; Cantuaria, G.; Jadhav, G.R.; Vangala, S.; Reid, M.D.; Aneja, R. Modulation of cytochrome P450 metabolism and transport across intestinal epithelial barrier by ginger biophenolics. PLoS ONE 2014, 9, e108386. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as potent epigenetics agents for cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Palacio, C.; Noriega, L.G.; Mercado, A. Potential of polyphenols to restore SIRT1 and NAD+ metabolism in renal disease. Nutrients 2022, 14, 653. [Google Scholar] [CrossRef]
- Jayasena, T.; Poljak, A.; Smythe, G.; Braidy, N.; Münch, G.; Sachdev, P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 867–883. [Google Scholar] [CrossRef]
- de Boer, V.C.; de Goffau, M.C.; Arts, I.C.; Hollman, P.C.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef]
- Testai, L.; Piraginem, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The citrus flavonoid naringenin protects the myocardium from ageing-dependent dysfunction: Potential role of SIRT1. Oxid. Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Utian, W.H.; Jones, M.; Setchell, K.D.R. S-equol: A potential nonhormonal agent for menopause-related symptom relief. J. Women Health 2015, 24, 200–208. [Google Scholar] [CrossRef]
- Mace, T.A.; Ware, M.B.; King, S.A.; Loftus, S.; Farren, M.R.; McMichael, E.; Scoville, S.; Geraghty, C.; Young, G.; Carson, W.E., 3rd; et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci. Rep. 2019, 9, 5068. [Google Scholar] [CrossRef]
- Ankolekar, C.; Johnson, D.; da Silva Pinto, M.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Lina, Y.-T.; Ghaedianb, R.; Shetty, K. Cranberry synergies for dietary management of Helicobacter pylori infec-tions. Process Biochem. 2005, 40, 1583–1592. [Google Scholar] [CrossRef]
- Xiao, Z.-P.; Shi, D.-H.; Li, H.-Q.; Zhang, L.N.; Xua, C.; Zhua, H.-L. Polyphenols based on isoflavones as inhibitors of Helicobacter pylori urease. Bioorg. Med. Chem. 2007, 15, 3703–3710. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Mahmud, R.; Ismail, S. Mechanism of action of isolated caffeic acid and epicatechin 3-gallate from Euphorbia hirta against Pseudomonas aeruginosa. Pharmacogn Mag. 2017, 13 (Suppl. 2), S311–S315. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Sci. Biotechnol. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin reduces Streptococcus mutans biofilm formation by inhibiting sortase A activity. Arch. Oral Biol. 2013, 58, 1343–1348. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mix-tures of the main polyphenolic compounds found in chilean propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrynogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Joung, D.-K.; Kim, S.-B.; Park, S.-J.; Seo, Y.-S.; Gong, R.; Choi, J.-G.; Shin, D.-W.; Rho, J.-R.; Kang, O.-H.; et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Hitoshi Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Sal-monella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.d.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Fu, Y.; Zhang, W.; Chen, X.; Zhao, J.; Song, W.; Li, Y.; Huang, Y.; Wu, Z.; Sun, R.; et al. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumonia through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016, 16, 181. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Kohda, C.; Yanagawa, Y.; Shimamura, T. Epigallocatechin gallate inhibits intracellular survival of Listeria monocytogenes in mac-rophages. Biochem. Biophys. Res. Commun. 2008, 365, 310–315. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.-R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Summanen, P.H.; Lee, R.-P.; Yang, J.; Finegold, S.M.; Heber, D.; Li, Z. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe 2017, 43, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Chen, M.-I.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut mi-crobiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Le, Y.S.G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S44–S55. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry pow-der drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Volstatova, T.; Marsik, P.; Rada, V.; Geigerova, M.; Havlik, J. Effect of apple extracts and selective polyphenols on the adhesion of potential probiotic strains of Lactobacillus gasseri R and Lactobacillus casei FMP. J. Funct. Foods 2017, 35, 391–397. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; de Benoist, B., McLean, E., Egli, I., Cogswell, M., Eds.; World Health Organization Press: Geneva, Switzerland, 2008; pp. 1–40. Available online: https://apps.who.int/iris/handle/10665/43894 (accessed on 2 February 2023).
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F.; Wessling-Resnick, M.; Knutson, M.D. Hepcidin regulation of iron transport. J. Nutr. 2008, 138, 2284–2288. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and its role in regulating systemic iron metabolism. Hematol. Am. Soc. Hematol. Educ. Program. 2006, 1, 29–35. [Google Scholar] [CrossRef]
- Dasa, F.; Abera, T. Factors affecting iron absorption and mitigation mechanisms: A review. Int. J. Agric. Sci. Food Technol. 2018, 4, 24–31. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C. Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 2021, 9, 685364. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef]
- Lesjak, M.; Srai, S.K.S. Role of dietary flavonoids in iron homeostasis. Pharmaceuticals 2019, 12, 119. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ham, S.K.; Shigenaga, M.K.; Han, O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kim, E.Y.; Han, O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kim, E.Y.; Lindsay, E.A.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
- Kumar, A.; Brookes, M.J. Iron therapy in inflammatory bowel disease. Nutrients 2020, 12, 3478. [Google Scholar] [CrossRef]
- Silva, B.; Faustino, P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim. Biophys. Acta 2015, 1852, 1347–1359. [Google Scholar] [CrossRef]
- Baccan, M.M.; Chiarelli-Neto, O.; Pereira, R.M.; Espósito, B.P. Quercetin as a shuttle for labile iron. J. Inorg. Biochem. 2012, 107, 34–39. [Google Scholar] [CrossRef]
- Mazhar, M.; Faizi, S.; Gul, A.; Kabir, N.; Simjee, S. Effects of naturally occurring flavonoids on ferroportin expression in the spleen in iron deficiency anemia in vivo. RSC Adv. 2017, 7, 23238–23245. [Google Scholar] [CrossRef]
- Pasdar, Y.; Oubari, F.; Zarif, M.N.; Abbasi, M.; Pourmahmoudi, A.; Hosseinikia, M. Effects of quercetin supplementation on hematological parameters in non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Clin. Nutr. Res. 2020, 9, 11–19. [Google Scholar] [CrossRef]
- Al-aboud, N.M. Effect of Red Raisins (Vitis vinifera L.) Intake on the Level of Some Hematological Tests in a Group of Female Volunteers. Biomed. J. Sci. Tech. Res. 2018, 2, 2659–2665. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C.; Campbell, R.; Manoharan, A. Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism. Molecules 2022, 27, 6417. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Mascitelli, L.; Goldstein, M.R. Inhibition of iron absorption by polyphenols as an anti-cancer mechanism. QJM 2011, 104, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Maldonado-Alvarez, L. Polyphenol-protein complexes and their consequences for the redox activity, structure and function of honey. A current view and new hypothesis—A review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran. 2017, 31, 134. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Mohanram Ramkumar, K.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Heinonen, I.; Kalliokoski, K.K.; Hannukainen, J.C.; Duncker, D.J.; Nuutila, P.; Knuuti, J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology 2014, 29, 421–436. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Training the gut for athletes. Sports Med. 2017, 47 (Suppl. 1), 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Fukazawa, A.; Karasawa, T.; Terada, S. Effects of long-term exercise training for different durations on pancreatic amylase activity and intestinal glucose transporter content in rats. Physiol. Rep. 2019, 7, e14255. [Google Scholar] [CrossRef] [PubMed]
- Oben, J.; Kothari, S.C.; Anderson, M.L. An open label study to determine the effects of an oral proteolytic enzyme system on whey protein concentrate metabolism in healthy males. J. Int. Soc. Sports Nutr. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.C.; Bailey, S.P.; Barnes, M.E.; Derr, S.J.; Hall, E.E. The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. J. Sports Sci. 2004, 22, 365–372. [Google Scholar] [CrossRef]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food allergy and intolerance: A narrative review on nutritional concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Allergy and aging: An old/new emerging health issue. Aging Dis. 2017, 8, 162–175. [Google Scholar] [CrossRef]
- Onyimba, F.; Crowe, S.E.; Johnson, S.; Leung, J. Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to food. Clin. Gastroenterol. Hepatol. 2021, 19, 2230–2240.e1. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019, 157, 109–118.e5. [Google Scholar] [CrossRef]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive enzyme supplementation in gastrointestinal diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Marini, E.; Ivaldi, L. A pilot trial on subjects with lactose and/or oligosaccharides intolerance treated with a fixed mixture of pure and enteric-coated alpha-and beta-galactosidase. Clin. Exp. Gastroenterol. 2015, 8, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corazza, G.R.; Benati, G.; Sorge, M.; Strocchi, A.; Calza, G.; Gasbarrini, G. β-Galactosidase from Aspergillus niger in adult lactose malabsorption: A double-blind crossover study. Aliment. Pharmacol. Ther. 1992, 6, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Somaraju, U.R.R.; Solis-Moya, A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst. Rev. 2020, 8, CD008227. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Holster, S.; Bruins, M.J.; Brummer, R.J. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci. Rep. 2017, 7, 13100. [Google Scholar] [CrossRef]
- Gass, J.; Bethune, M.T.; Siegel, M.; Spencer, A.; Khosla, C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology 2007, 133, 472–480. [Google Scholar] [CrossRef]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar] [CrossRef]
- Amarya, S.; Singh, K.; Sabharwal, M. Changes during aging and their association with malnutrition. J. Clin. Gerontol Geriatr. 2015, 6, 78–84. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences (Review). J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef]
- Karajibani, M.; Montazerifar, F.; Hosseini, R.; Suni, F.; Dashipour, A.R.; Fadaaeimokhtarkanlo, M. The relationship between malnutrition and liver enzymes in hospitalized children in Zahedan: A Case-control Study. Zahedan J. Res. Med. Sci. 2021, 23, e102994. [Google Scholar] [CrossRef]
- Cristina, N.M.; Lucia, D. Nutrition and healthy aging: Prevention and treatment of gastrointestinal diseases. Nutrients 2021, 13, 4337. [Google Scholar] [CrossRef]
- Russell, R.M. Factors in aging that effect the bioavailability of nutrients. J. Nutr. 2001, 131 (Suppl. 4), 1359S–1361S. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, T.; Thomson, A.B. Nutrient absorption and intestinal adaptation with ageing. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.W.; Connett, G.; Hall, R.I.; Charnley, R.M. Review article: Enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment Pharmacol Ther. 2010, 32 (Suppl. 1), 1–25. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Koch, S.Y.; Koch, K.L. Lipase supplementation before a high-fat meal reduces perceptions of fullness in healthy subjects. Gut Liver 2015, 9, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.F.; Wang, W.J.; Yin, Z.P.; Zheng, G.D.; Chen, J.G.; Li, J.E.; Chen, L.L.; Zhang, Q.F. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021, 43, 101248. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Netzel, G.; Gidley, M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. J. Funct. Food 2016, 26, 144–156. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Lu, Q.; Liu, R.; Tian, J.; Huang, Y. Study on interaction between human salivary α-amylase and sorghum procyanidin tetramer: Binding characteristics and structural analysis. Int. J. Biol. Macromol. 2018, 118 Pt A, 1136–1141. [Google Scholar] [CrossRef]
- Sun, L.; Gidley, M.J.; Warren, F.J. Tea polyphenols enhance binding of porcine pancreatic α-amylase with starch granules but reduce catalytic activity. Food Chem. 2018, 258, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, Y.; Zhang, S.; Wang, Y.; Zhang, J.; Cao, J.; Sun, L. Caffeoyl substitution changes the inhibition mode of tartaric acid against α-amylase: Analysis of the enzyme inhibition by four caffeic and tartaric acid derivates. LWT 2020, 133, 109942. [Google Scholar] [CrossRef]
- Li, H.; Tanaka, T.; Zhang, Y.J.; Yang, C.R.; Kouno, I. Rubusuaviins A-F, monomeric and oligomeric ellagitannins from Chinese sweet tea and their alpha-amylase inhibitory activity. Chem. Pharm. Bull. 2007, 55, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Li, H.; Shi, S.; Peng, X. Mechanistic study and synergistic effect on inhibition of α-amylase by structurally similar flavonoids. J. Molec. Liquids 2022, 360, 119485. [Google Scholar] [CrossRef]
- Wu, X.; Ding, H.; Hu, X.; Pan, J.; Liao, Y.; Gong, D.; Zhang, G. Exploring inhibitory mechanism of gallocatechin gallate on α-amylase and α-glucosidase relevant to postprandial hyperglycemia. J. Funct. Foods 2018, 48, 200–209. [Google Scholar] [CrossRef]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Molec. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.-J.; Song, H.-C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Ehiakhamen, E.O. Effect of processing methods on the antioxidant properties and inhibition of α-amylase and α-glucosidase by African pear (Dacryodes edulis) fruit. Nutrafoods 2015, 14, 19–26. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar]
- Yang, X.; Kong, F. Effects of tea polyphenols and different teas on pancreatic α-amylase activity in vitro. LWT 2016, 48, 200–209. [Google Scholar] [CrossRef]
- Tian, J.L.; Si, X.; Wang, Y.H.; Gong, E.S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341 Pt 1, 128149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of human and rat sucrase and maltase activities to assess antiglycemic potential: Optimization of the assay using acarbose and polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef]
- Li, W.-T.; Chuang, Y.-H.; Hsieh, J.-F. Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata. Foods 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Siddiqi, R.; Dew, T.P.; Williamson, G. Epigallocatechin-3-gallate inhibits lactase but is alleviated by salivary proline-rich proteins. J. Agric. Food Chem. 2011, 59, 2734–2738. [Google Scholar] [CrossRef]
- Hashemi-Shahraki, F.; Shareghi, B.; Farhadian, S.; Yadollahi, E. A comprehensive insight into the effects of caffeic acid (CA) on pepsin: Multi-spectroscopy and MD simulations methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 289, 122240. [Google Scholar] [CrossRef]
- Zeng, H.J.; Yang, R.; Liang, H.; Qu, L.B. Molecular interactions of flavonoids to pepsin: Insights from spectroscopic and molecular docking studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 576–590. [Google Scholar] [CrossRef]
- Li, Q.; Wwei, Q.; Yuan, E.; Yang, J.; Ning, Z. Interaction between four flavonoids and trypsin: Effect on the characteristics of trypsin and antioxidant activity of flavonoids. Int. J. Food Sci. Technol. 2014, 49, 1063–1069. [Google Scholar] [CrossRef]
- Maliar, T.; Jedinák, A.; Kadrabová, J.; Sturdík, E. Structural aspects of flavonoids as trypsin inhibitors. Eur. J. Med. Chem. 2004, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. A novel approach to trypsin inhibition by flavonoids. J. Food Bioact. 2021, 14, 102–113. [Google Scholar] [CrossRef]
- Lee, E.M.; Lee, S.S.; Chung, B.Y.; Cho, J.-Y.; Lee, I.C.; Ahn, S.R.; Jang, S.J.; Kim, T.H. Pancreatic lipase inhibition by C-glycosidic flavones isolated from Eremochloa ophiuroides. Molecules 2010, 15, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J. Funct. Foods 2017, 38, 644–655. [Google Scholar] [CrossRef]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Shapiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Boath, A.S.; Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit digestive enzymes: A source of potential health benefits? Food Dig. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Darwiche, G.; Björgell, O.; Almér, L.O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef]
- Inoue, Y.; Cormanes, L.; Yoshimura, K.; Sano, A.; Hori, Y.; Suzuki, R.; Kanamoto, I. Effect of apple consumption on postprandial blood glucose levels in normal glucose tolerance people versus those with impaired glucose tolerance. Foods 2022, 11, 1803. [Google Scholar] [CrossRef]
- Coe, S.; Ryan, L. Impact of polyphenol-rich sources on acute postprandial glycaemia: A systematic review. J. Nutr. Sci. 2016, 5, e24. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2009, 35, 390–406. [Google Scholar] [CrossRef]
- Sato, Y.; Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015, 8, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Al-Bayati, F.A.; Sulaiman, K.D. In vitro antimicrobial activity of Salvadora persica L. extracts against some isolated oral pathogens in Iraq. Turk. J. Biol. 2008, 32, 57–62. [Google Scholar]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolome, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Bustos, I.; Garcia-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martinez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat. Commun. 2019, 10, 2012. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef] [PubMed]
- Chałupnik Chałupnik, A.; Chilimoniuk, Z.; Sobstyl, A.; Dobosz, M.; Borkowska, A.; Wieteska, M. Role of the gut microbiota in human health. J. Educ. Health Sport 2020, 10, 458–469. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts imbalance imbalances the brain: A review of gut microbiota association with neurological and psychiatric disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Role of the gut–brain axis, gut microbial composition, diet, and probiotic intervention in Parkinson’s disease. Microorganisms 2022, 10, 1544. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The microbiota–gut–brain axis in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Angoorani, P.; Ejtahed, H.S.; Siadat, S.D.; Sharifi, F.; Larijani, B. Is there any link between cognitive impairment and gut microbiota? A systematic review. Gerontology 2022, 68, 1201–1213. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Fukami, T.; Yokoi, T.; Nakajima, M. Non-P450 drug-metabolizing enzymes: Contribution to drug disposition, toxicity, and development. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 405–425. [Google Scholar] [CrossRef]
- Sheweita, S.A. Drug-metabolizing enzymes: Mechanisms and functions. Curr. Drug Metab. 2000, 1, 107–132. [Google Scholar] [CrossRef]
- Pey, A.L.; Megarity, C.F.; Timson, D.J. NAD(P)H quinone oxidoreductase (NQO1): An enzyme which needs just enough mobility, in just the right places. Biosci. Rep. 2019, 39, BSR20180459. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Bhamre Vaibhav, G.; Deore Pranjal, D.; Amrutkar Rakesh, D.; Patil Vinod, R. Polyphenols: The interactions with CYP 450 isoenzymes and effect on pharmacokinetics of drugs. Curr. Trends Pharm. Pharm. Chem. 2022, 4, 13–23. [Google Scholar] [CrossRef]
- Mohos, V.; Bencsik, T.; Boda, G.; Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interactions of casticin, ipriflavone, and resveratrol with serum albumin and their inhibitory effects on CYP2C9 and CYP3A4 enzymes. Biomed. Pharmacother. 2018, 107, 777–784. [Google Scholar] [CrossRef]
- Munro, A.W.; Girvan, H.M.; McLean, K.J. Cytochrome P450--redox partner fusion enzymes. Biochim. Biophys. Acta 2007, 1770, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Isin, E.M.; Guengerich, F.P. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta 2007, 1770, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Bibi, Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. 2008, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Role of cytochrome P450 enzymes in drug-drug interactions. Adv. Pharmacol. 1997, 43, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Ondieki, G.; Nyagblordzro, M.; Kikete, S.; Liang, R.; Wang, L.; He, X. Cytochrome P450 and P-glycoprotein-mediated interactions involving African herbs indicated for common noncommunicable diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 2582463. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yoon, I.S. Pharmacokinetic interactions of herbs with cytochrome p450 and P-glycoprotein. Evid. Based Complement. Alternat. Med. 2015, 2015, 736431. [Google Scholar] [CrossRef]
- Showande, S.J.; Fakeye, T.O.; Kajula, M.; Hokkanen, J.; Tolonen, A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs-Implication for herb-drug interactions. Food Sci. Nutr. 2018, 7, 44–55. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Manda, V.K.; Dale, O.R.; Egiebor, N.O.; Walker, L.A.; Khan, S.I. Modulation of cytochrome P450, P-glycoprotein and Pregnane X receptor by selected antimalarial herbs—Implication for herb-drug interaction. Molecules 2017, 22, 2049. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Prachayasittikul, V. Cytochrome P450 enzyme mediated herbal drug interactions (Part 1). EXCLI J. 2014, 13, 347–391. [Google Scholar]
- Mueller, S.C.; Majcher-Peszynska, J.; Uehleke, B.; Klammt, S.; Mundkowski, R.G.; Miekisch, W.; Sievers, H.; Bauer, S.; Frank, B.; Kundt, G.; et al. The extent of induction of CYP3A by St. John’s wort varies among products and is linked to hyperforin dose. Eur. J. Clin. Pharmacol. 2006, 62, 29–36. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Müller, F.; Glaeser, H.; König, J.; Fromm, M.F. Inhibitory effects of green tea and (–)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS ONE 2015, 10, e0139370. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tarapcsák, S.; Gyöngy, Z.; Ritter, Z.; Batta, G.; Bosire, R.; Remenyik, J.; Goda, K. Effects of polyphenols on P-glycoprotein (ABCB1) activity. Pharmaceutics 2021, 13, 2062. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential risks resulting from fruit/vegetable-drug interactions: Effects on drug-metabolizing enzymes and drug transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef]
- Taylor, J.R.; Wilt, V.M. Probable antagonism of warfarin by green tea. Ann. Pharmacother. 1999, 33, 426–428. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yu, C.P.; Hsieh, Y.W.; Lin, S.P.; Hou, Y.C. Resveratrol stereoselectively affected (±)warfarin pharmacokinetics and enhanced the anticoagulation effect. Sci. Rep. 2020, 10, 15910. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.A.; Camilo, M.A.; Marques, L.G.; de Oliveira, C.M.; Figueiredo, S.A.; Santos, L.B.; de Oliveira Paula, R.A.; de Araújo Paula, F.B.; Rodrigues, M.R.; da Silveira Duarte, S.M. Increased warfarin anticoagulant activity and its potential interaction with aqueous extract of goji berry (Lycium barbarum L.) in Wistar rats. Res. Soc. Dev. 2020, 9, e29591211070. [Google Scholar] [CrossRef]
- Yu, C.P.; Yang, M.S.; Hsu, P.W.; Lin, S.P.; Hou, Y.C. Bidirectional influences of cranberry on the pharmacokinetics and pharmacodynamics of warfarin with mechanism elucidation. Nutrients 2021, 13, 3219. [Google Scholar] [CrossRef]
- Mohammed Abdul, M.I.; Jiang, X.; Williams, K.M.; Day, R.O.; Roufogalis, B.D.; Liauw, W.S.; Xu, H.; McLachlan, A.J. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br. J Pharmacol. 2008, 154, 1691–1700. [Google Scholar] [CrossRef]
- Aston, J.L.; Lodolce, A.E.; Shapiro, N.L. Interaction between warfarin and cranberry juice. Pharmacotherapy 2006, 26, 1314–1319. [Google Scholar] [CrossRef]
- Paeng, C.H.; Sprague, M.; Jackevicius, C.A. Interaction between warfarin and cranberry juice. Clin. Ther. 2007, 29, 1730–1735. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, L.; Xie, B. Bleeding due to a probable interaction between warfarin and Gouqizi (Lycium barbarum L.). Toxicol. Rep. 2015, 2, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Ferro, C.L.; Bursua, A.J.; Gerber, B.S. Probable interaction between Lycium barbarum (goji) and warfarin. Pharmacotherapy 2012, 32, e50–e53. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.Y.; Elmer, G.W.; Mohutsky, M.A. Possible interaction between warfarin and Lycium barbarum L. Ann. Pharmacother. 2001, 35, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.F.; Zhao, F.L.; Chen, H.; Zhou, Q.; Geng, P.W.; Zhou, Y.F.; Wu, H.L.; Chong, J.; Wang, F.; Dai, D.P.; et al. Naringenin has an inhibitory effect on rivaroxaban in rats both in vitro and in vivo. Pharmacol. Res. Perspect. 2021, 9, e00782. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Burstein, A.H.; Welden, N.; Gallicano, K.D.; Falloon, J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin. Infect. Dis. 2002, 34, 234–238. [Google Scholar] [CrossRef]
- Hussain, S.A.; Jaccob, A.A. Effects of long-term use of polyphenols on the absorption and tissue distribution of orally administered metformin and atenolol in rats. J. Intercult. Ethnopharmacol. 2013, 2, 147–154. [Google Scholar] [CrossRef]
- Kim, T.E.; Shin, K.H.; Park, J.E.; Kim, M.G.; Yun, Y.M.; Choi, D.H.; Kwon, K.J.; Lee, J. Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des. Devel. Ther. 2018, 12, 2139–2147. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ariyoshi, N.; Kotani, A.; Ishii, I.; Nakamura, H.; Nakasa, H.; Ida, M.; Nakamura, H.; Kimura, N.; Kimura, M.; et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab. Pharmacokinet. 2004, 19, 280–289. [Google Scholar] [CrossRef]
- Kupferschmidt, H.H.; Ha, H.R.; Ziegler, W.H.; Meier, P.J.; Krähenbühl, S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995, 58, 20–28. [Google Scholar] [CrossRef]
- Hegazy, S. The effect of green tea on sildenafil pharmacokinetics in Egyptian healthy volunteers. Br. J. Pharm. Res. 2014, 4, 289–300. [Google Scholar] [CrossRef]
- Misaka, S.; Abe, O.; Sato, H.; Ono, T.; Shikama, Y.; Onoue, S.; Yabe, H.; Kimura, J. Lack of pharmacokinetic interaction between fluvastatin and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Abdelkawy, K.S.; Abdelaziz, R.M.; Abdelmageed, A.M.; Donia, A.M.; El-Khodary, N.M. Effects of green tea extract on atorvastatin pharmacokinetics in healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hu, M.; Lee, H.K.; Wat, E.; Lau, C.B.S.; Ho, C.S.; Wong, C.K.; Tomlinson, B. Effects of soy isoflavones and green tea extract on simvastatin pharmacokinetics and influence of the SLCO1B1 521T > C polymorphism. Front. Nutr. 2022, 9, 868126. [Google Scholar] [CrossRef]
- Kim, T.E.; Ha, N.; Kim, Y.; Kim, H.; Lee, J.W.; Jeon, J.Y.; Kim, M.G. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des. Devel. Ther. 2017, 11, 1409–1416. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Q.; Liu, L.; Bian, Y.; Zhang, S.; Huang, C.; Miao, L. Effect of green tea and (-)-epigallocatechin gallate on the pharmacokinetics of rosuvastatin. Curr. Drug Metab. 2020, 21, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, L.; Ramos Mandíková, J.; Pávek, P.; Mladěnka, P.; Trejtnar, F. Honey flavonoids inhibit hOATP2B1 and hOATP1A2 transporters and hOATP-mediated rosuvastatin cell uptake in vitro. Xenobiotica 2018, 48, 745–755. [Google Scholar] [CrossRef]
- Zeng, W.; Hu, M.; Lee, H.K.; Wat, E.; Lau, C.B.S.; Ho, C.S.; Wong, C.K.; Tomlinson, B. Effect of green tea extract and soy isoflavones on the pharmacokinetics of rosuvastatin in healthy volunteers. Front. Nutr. 2022, 9, 850318. [Google Scholar] [CrossRef]
- Misaka, S.; Miyazaki, N.; Fukushima, T.; Yamada, S.; Kimura, J. Effects of green tea extract and (-)-epigallocatechin-3-gallate on pharmacokinetics of nadolol in rats. Phytomedicine 2013, 20, 1247–1250. [Google Scholar] [CrossRef]
- Misaka, S.; Yatabe, J.; Müller, F.; Takano, K.; Kawabe, K.; Glaeser, H.; Yatabe, M.S.; Onoue, S.; Werba, J.P.; Watanabe, H.; et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin. Pharmacol. Ther. 2014, 95, 432–438. [Google Scholar] [CrossRef]
- Tan, H.J.; Ling, W.C.; Chua, A.L.; Lee, S.K. Oral epigallocatechin gallate reduces intestinal nadolol absorption via modulation of Oatp1a5 and Oct1 transcriptional levels in spontaneously hypertensive rats. Phytomedicine 2021, 90, 153623. [Google Scholar] [CrossRef]
- Abe, O.; Ono, T.; Sato, H.; Müller, F.; Ogata, H.; Miura, I.; Shikama, Y.; Yabe, H.; Onoue, S.; Fromm, M.F.; et al. Role of (-)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Abe, O.; Ono, T.; Ono, Y.; Ogata, H.; Miura, I.; Shikama, Y.; Fromm, M.F.; Yabe, H.; Shimomura, K. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br. J. Clin. Pharmacol. 2020, 86, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Ono, Y.; Uchida, A.; Ono, T.; Abe, O.; Ogata, H.; Sato, H.; Suzuki, M.; Onoue, S.; Shikama, Y.; et al. Impact of green tea catechin ingestion on the pharmacokinetics of lisinopril in healthy volunteers. Clin. Transl. Sci. 2021, 14, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Han, H.K. Pharmacokinetic interaction between diltiazem and morin, a flavonoid, in rats. Pharmacol. Res. 2005, 52, 386–391. [Google Scholar] [CrossRef]
- Hong, S.P.; Choi, D.H.; Choi, J.S. Effects of resveratrol on the pharmacokinetics of diltiazem and its major metabolite, desacetyldiltiazem, in rats. Cardiovasc. Ther. 2008, 26, 269–275. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Hao, H.; Li, H.; Guo, X.; Zhang, D. Effect of epigallocatechin-3-gallate on the pharmacokinetics of amlodipine in rats. Xenobiotica 2019, 49, 970–974. [Google Scholar] [CrossRef]
- Chung, J.H.; Choi, D.H. Effects of oral epigallocatechin gallate on the oral pharmacokinetics of verapamil in rats. Biopharm. Drug Dispos. 2009, 30, 90–93. [Google Scholar] [CrossRef]
- Lown, K.S.; Bailey, D.G.; Fontana, R.J.; Janardan, S.K.; Adair, C.H.; Fortlage, L.A.; Brown, M.B.; Guo, W.; Watkins, P.B. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J. Clin. Investig. 1997, 99, 2545–2553. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory effects of quercetin and its main methyl, sulfate, and glucuronic acid conjugates on cytochrome P450 enzymes, and on OATP, BCRP and MRP2 transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef]
- Gufford, B.T.; Chen, G.; Lazarus, P.; Graf, T.N.; Oberlies, N.H.; Paine, M.F. Identification of diet-derived constituents as potent inhibitors of intestinal glucuronidation. Drug Metab. Dispos. 2014, 42, 1675–1683. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Bakos, É.; Kuffa, K.; Bencsik, T.; Zsidó, B.Z.; Hetényi, C.; Telbisz, Á.; Özvegy-Laczka, C.; et al. Effects of chrysin and its major conjugated metabolites chrysin-7-sulfate and chrysin-7-glucuronide on cytochrome P450 enzymes and on OATP, P-gp, BCRP, and MRP2 transporters. Drug Metab. Dispos. 2020, 48, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Ferlito, V.; Xu, C.; Flockhart, D.A.; Caccamese, S. Enantiomers of naringenin as pleiotropic, stereoselective inhibitors of cytochrome P450 isoforms. Chirality 2011, 23, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Steuck, M.; Hellhake, S.; Schebb, N.H. Food polyphenol apigenin inhibits the cytochrome P450 monoxygenase branch of the arachidonic acid cascade. J. Agric. Food Chem. 2016, 64, 8973–8976. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef]
- Šarić Mustapić, D.; Debeljak, Ž.; Maleš, Ž.; Bojić, M. The inhibitory effect of flavonoid aglycones on the metabolic activity of CYP3A4 enzyme. Molecules 2018, 23, 2553. [Google Scholar] [CrossRef]
- Quintieri, L.; Palatini, P.; Nassi, A.; Ruzza, P.; Floreani, M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochem. Pharmacol. 2008, 75, 1426–1437. [Google Scholar] [CrossRef]
- Jaikang, C.; Niwatananun, K.; Narongchai, P.; Narongchai, S.; Chaiyasut, C. Inhibitory effect of caffeic acid and its derivatives on human liver cytochrome P450 3A4 activity. J. Med. Plants Res. 2011, 5, 3530–3536. [Google Scholar]
- Stupans, L.; Tan, H.W.; Kirlich, A.; Tuck, K.; Hayball, P.; Murray, M. Inhibition of CYP3A-mediated oxidation in human hepatic microsomes by the dietary derived complex phenol, gallic acid. J. Pharm. Pharmacol. 2002, 54, 269–275. [Google Scholar] [CrossRef]
- Tan, C.S.S.; Lee, S.W.H. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br. J. Clin. Pharmacol. 2021, 87, 352–374. [Google Scholar] [CrossRef]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current view on green tea catechins formulations, their interactions with selected drugs, and prospective applications for various health conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.; Glover, V.; Elsworth, J.; Sandler, M. Equol and other compounds from bovine urine as monoamine oxidase inhibitors. J. Neural. Transm. 1986, 65, 147–150. [Google Scholar] [CrossRef]
- Gao, G.Y.; Li, D.J.; Keung, W.M. Synthesis of potential antidipsotropic isoflavones: Inhibitors of the mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway. J. Med. Chem. 2001, 44, 3320–3328. [Google Scholar] [CrossRef]
- Dzau, V.J.; Herrmann, H.C. Hormonal control of angiotensinogen production. Life Sci. 1982, 30, 577–584. [Google Scholar] [CrossRef]
- Weber, K.S.; Setchell, K.D.; Stocco, D.M.; Lephart, E.D. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J. Endocrinol. 2001, 170, 591–599. [Google Scholar] [CrossRef]
- Martinez, J.; Lewi, J.E. An unusual case of gynecomastia associated with soy product consumption. Endocr. Pract. 2008, 14, 415–418. [Google Scholar] [CrossRef]
- Reed, K.E.; Camargo, J.; Hamilton-Reeves, J.; Kurzer, M.; Messina, M. Neither soy nor isoflavone intake affects male reproductive hormones An expanded and updated meta-analysis of clinical studies. Reprod. Toxicol. 2021, 100, 60–67. [Google Scholar] [CrossRef]
- Messina, M. Soybean isoflavone exposure does not have feminizing effects on men: A critical examination of the clinical evidence. Fertil. Steril. 2010, 93, 2095–2104. [Google Scholar] [CrossRef]
- Chang, H.C.; Doerge, D.R. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 2000, 168, 244–252. [Google Scholar] [CrossRef]
- Šošić-Jurjević, B.; Filipović, B.; Ajdžanović, V.; Savin, S.; Nestorović, N.; Milosević, V.; Sekulić, M. Suppressive effects of genistein and daidzein on pituitary–thyroid axis in orchidectomized middle-aged rats. Exp. Biol. Med. 2010, 235, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Divi, R.L.; Doerge, D.R. Inhibition of thyroid peroxidase by dietary flavonoids. Chem. Res. Toxicol. 1996, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Divi, R.L.; Chang, H.C.; Doerge, D.R. Anti-thyroid isoflavones from soybean: Isolation, characterization, and mechanisms of action. Biochem. Pharmacol. 1997, 54, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Manuchehri, A.M.; Thatcher, N.J.; Rigby, A.S.; Chapman, T.; Kilpatrick, E.S.; Atkin, S.L. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 2011, 96, 1442–1449. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhang, D.; Li, G.; Ho, S.C.; Chen, Y.M.; Ma, J.; Huang, Q.; Li, S.; Ling, W.H. The 6-month effect of whole soy and purified isoflavones daidzein on thyroid function-A double-blind, randomized, placebo controlled trial among Chinese equol-producing postmenopausal women. Phytother Res. 2021, 35, 5838–5846. [Google Scholar] [CrossRef]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef]
- Radović, B.; Mentrup, B.; Köhrle, J. Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid. Br. J. Nutr. 2006, 95, 1171–1176. [Google Scholar] [CrossRef]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Yu, L.; Takeda, S.; Koibuchi, N. A Possible novel mechanism of action of genistein and daidzein for activating thyroid hormone receptor-mediated transcription. Toxicol. Sci. 2018, 164, 417–427. [Google Scholar] [CrossRef]
- Fort, P.; Moses, N.; Fasano, M.; Goldberg, T.; Lifshitz, F. Breast and soy-formula feedings in early infancy and the prevalence of autoimmune thyroid disease in children. J. Am. Coll. Nutr. 1990, 9, 164–167. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. 1994, 54 (Suppl. 7), 1895s–1898s. [Google Scholar] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Zheng, L.F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell. Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Zheng, L.F.; Wei, Q.Y.; Cai, Y.J.; Fang, J.G.; Zhou, B.; Yang, L.; Liu, Z.L. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: Mechanism and structure-activity relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. prooxidant properties of the flavonoid, kaempferol, in the presence of Cu(II) ions: A ROS-scavenging activity, Fenton reaction and DNA damage study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: Inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.X.; Long, Y.; Wu, W.M.; Wang, R. DNA damage induced by caffeic acid phenyl ester in the presence of Cu(II) ions: Potential mechanism of its anticancer properties. Cancer Lett. 2008, 263, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.J.; Jin, X.L.; Qian, Y.P.; Wang, Q.; Yang, R.T.; Dai, F.; Tang, J.J.; Shang, Y.J.; Cheng, L.X.; Yang, J.; et al. Hydroxycinnamic acids as DNA-cleaving agents in the presence of Cu(II) ions: Mechanism, structure-activity relationship, and biological implications. Chemistry 2009, 15, 12889–12899. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, F.; Hua, Y.; Huang, S.; Lin, S.; Wen, L.; Jiang, Y. Prooxidant activities of quercetin, p-coumaric acid and their derivatives analyzed by quantitative structure–activity relationship. Food Chem. 2012, 131, 508–512. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A role of gallic acid in oxidative damage diseases: A comprehensive review. Nat. Prod. Commun. 2019, 14, 8. [Google Scholar] [CrossRef]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Caro, A.A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and pro-oxidant mechanisms of (+)catechin in microsomal CYP2E1-dependent oxidative stress. Toxicol. In Vitro 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Yamanaka, N.; Oda, O.; Nagao, S. Green tea catechins such as (-)-epicatechin and (-)-epigallocatechin accelerate Cu2+-induced low density lipoprotein oxidation in propagation phase. FEBS Lett. 1997, 401, 230–234. [Google Scholar] [CrossRef]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration dependence of anti- and pro-oxidant activity of polyphenols as evaluated with a light-emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, B.; Zhu, L. DNA binding and oxidative DNA damage induced by a quercetin copper(II) complex: Potential mechanism of its antitumor properties. J. Biol. Inorg. Chem. 2009, 14, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhu, L.; Wang, B. DNA binding and cleavage activity of quercetin nickel(II) complex. Dalton Trans. 2009, 24, 4722–4728. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.; Bochu, W.; Liancai, Z. Hydrolytic cleavage of DNA by quercetin zinc(II) complex. Bioorg. Med. Chem. Lett. 2007, 17, 1197–1199. [Google Scholar] [CrossRef]
- Jun, T.; Bochu, W.; Liancai, Z. Hydrolytic cleavage of DNA by quercetin manganese(II) complexes. Colloids Surf. B Biointerfaces 2007, 55, 149–152. [Google Scholar] [CrossRef]
- Spissu, Y.; Gil, K.A.; Dore, A.; Sanna, G.; Palmieri, G.; Sanna, A.; Cossu, M.; Belhadj, F.; Gharbi, B.; Pinna, M.B.; et al. Anti- and pro-oxidant activity of polyphenols extracts of Syrah and Chardonnay grapevine pomaces on melanoma cancer cells. Antioxidants 2023, 12, 80. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In vitro and in vivo evaluation of the antioxidant and prooxidant activity of phenolic compounds obtained from grape (Vitis vinifera) pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef]
- Garjonyte, R.; Budiene, J.; Labanauskas, L.; Judzentiene, A. In vitro antioxidant and prooxidant activities of red raspberry (Rubus idaeus L.) stem extracts. Molecules 2022, 27, 4073. [Google Scholar] [CrossRef]
- Tsukada, M.; Nakashima, T.; Kamachi, T.; Niwano, Y. Prooxidative potential of photo-irradiated aqueous extracts of grape pomace, a recyclable resource from winemaking process. PLoS ONE 2016, 11, e0158197. [Google Scholar] [CrossRef]
- Bandele, O.J.; Osheroff, N. Bioflavonoids as poisons of human topoisomerase II alpha and II beta. Biochemistry 2007, 46, 6097–6108. [Google Scholar] [CrossRef] [PubMed]
- Bandele, O.J.; Osheroff, N. (–)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem. Res. Toxicol. 2008, 21, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S.; Alfaiz, F.A.; Paramasivam, A. Efficacy and mechanisms of flavonoids against the emerging opportunistic nontuberculous Mycobacteria. Antibiotics 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Oblak, M.; Kotnik, M.; Solmajer, T. Discovery and development of ATPase inhibitors of DNA gyrase as antibacterial agents. Curr. Med. Chem. 2007, 14, 2033–2047. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Podlogar, B.L.; Nguyen, V.N.; Bernstein, J.I.; Krause, H.M.; Hilliard, J.J.; Barrett, J.F. DNA gyrase inhibitory and antimicrobial activities of some diphenic acid monohydroxamides. J. Med. Chem. 1997, 40, 3292–3296. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Levine, C.; Hiasa, H.; Marians, K.J. DNA gyrase and topoisomerase IV: Biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta. 1998, 1400, 29–43. [Google Scholar] [CrossRef]
- Spada, P.D.; de Souza, G.G.; Bortolini, G.V.; Henriques, J.A.; Salvador, M. Antioxidant, mutagenic, and antimutagenic activity of frozen fruits. J. Med. Food. 2008, 11, 144–151. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Ramesh, V.; Nagaraja, V.; Parish, J.H.; Hadi, S.M. Mode of binding of quercetin to DNA. Mutagenesis 1994, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Johnson, W.; Dobson, V.L. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat. Res. 1997, 390, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Goodenow, D.; Emmanuel, F.; Berman, C.; Sahyouni, M.; Richardson, C. Bioflavonoids cause DNA double-strand breaks and chromosomal translocations through topoisomerase II-dependent and -independent mechanisms. Mutat Res Genet Toxicol Environ Mutagen. 2020, 849, 503144. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Kawanishi, S. Distinct mechanisms of DNA damage in apoptosis induced by quercetin and luteolin. Free Rad. Res. 2000, 33, 623–633. [Google Scholar] [CrossRef]
- Popp, R.; Schimmer, O. Induction of sister-chromatid exchanges (SCE), polyploidy, and micronuclei by plant flavonoids in human lymphocyte cultures. A comparative study of 19 flavonoids. Mutat. Res. 1991, 246, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Alvi, N.K.; Rizvi, R.Y.; Hadi, S.M. Interaction of quercetin with DNA. Biosci Rep. 1986, 6, 861–868. [Google Scholar] [CrossRef]
- Nafisi, S.; Hashemi, M.; Rajabi, M.; Tajmir-Riahi, H.A. DNA adducts with antioxidant flavonoids: Morin, apigenin, and naringin. DNA Cell. Biol. 2008, 27, 433–442. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Diamantoglou, S.; Tajmir-Riahi, H.A. DNA interaction with naturally occurring antioxidant flavonoids quercetin, kaempferol, and delphinidin. J. Biomol. Struct. Dyn. 2005, 22, 719–724. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Interaction of antioxidant flavonoids with tRNA: Intercalation or external binding and comparison with flavonoid-DNA adducts. DNA Cell. Biol. 2006, 25, 116–123. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gray, G.C. Interactions of flavonoids, trace metals, and oxygen: Nuclear DNA damage and lipid peroxidation induced by myricetin. Cancer Lett. 1993, 70, 73–79. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gray, G.C. Kaempferol-induced nuclear DNA damage and lipid peroxidation. Cancer Lett. 1994, 85, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Ainley, K.; Parish, J.H.; Hadi, S.M. Free radical-induced fragmentation of proteins by quercetin. Carcinogenesis 1994, 15, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- van Waalwijk van Doorn-Khosrovani, S.B.; Janssen, J.; Maas, L.M.; Godschalk, R.W.; Nijhuis, J.G.; van Schooten, F.J. Dietary flavonoids induce MLL translocations in primary human CD34+ cells. Carcinogenesis 2007, 28, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; de Bock, L.; Godschalk, R.W.; van Schooten, F.J.; van Waalwijk van Doorn-Khosrovani, S.B. Prenatal exposure to flavonoids: Implication for cancer risk. Toxicol. Sci. 2011, 120, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ou, N.; Lu, Q.B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef]
- Rody, H.V.S.; Gontijo, D.D.C.; Coelho, V.P.M.; Ventrella, M.C.; Pádua, R.M.; Fietto, L.G.; Leite, J.P.V. Mutagenic activity and chemical composition of phenolic-rich extracts of leaves from two species of Ficus medicinal plants. J. Toxicol. Environ. Health A 2018, 81, 861–872. [Google Scholar] [CrossRef]
- Viau, C.M.; Moura, D.J.; Pflüger, P.; Facundo, V.A.; Saffi, J. Structural aspects of antioxidant and genotoxic activities of two flavonoids obtained from ethanolic extract of Combretum leprosum. Evid. Based Complement. Alternat. Med. 2016, 2016, 9849134. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of flavonoid–biomembrane interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef]
- Altunayar-Unsalan, C.; Unsalan, O.; Mavromoustakos, T. Insights into molecular mechanism of action of citrus flavonoids hesperidin and naringin on lipid bilayers using spectroscopic, calorimetric, microscopic and theoretical studies. J. Mol. Liq. 2022, 347, 118411. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
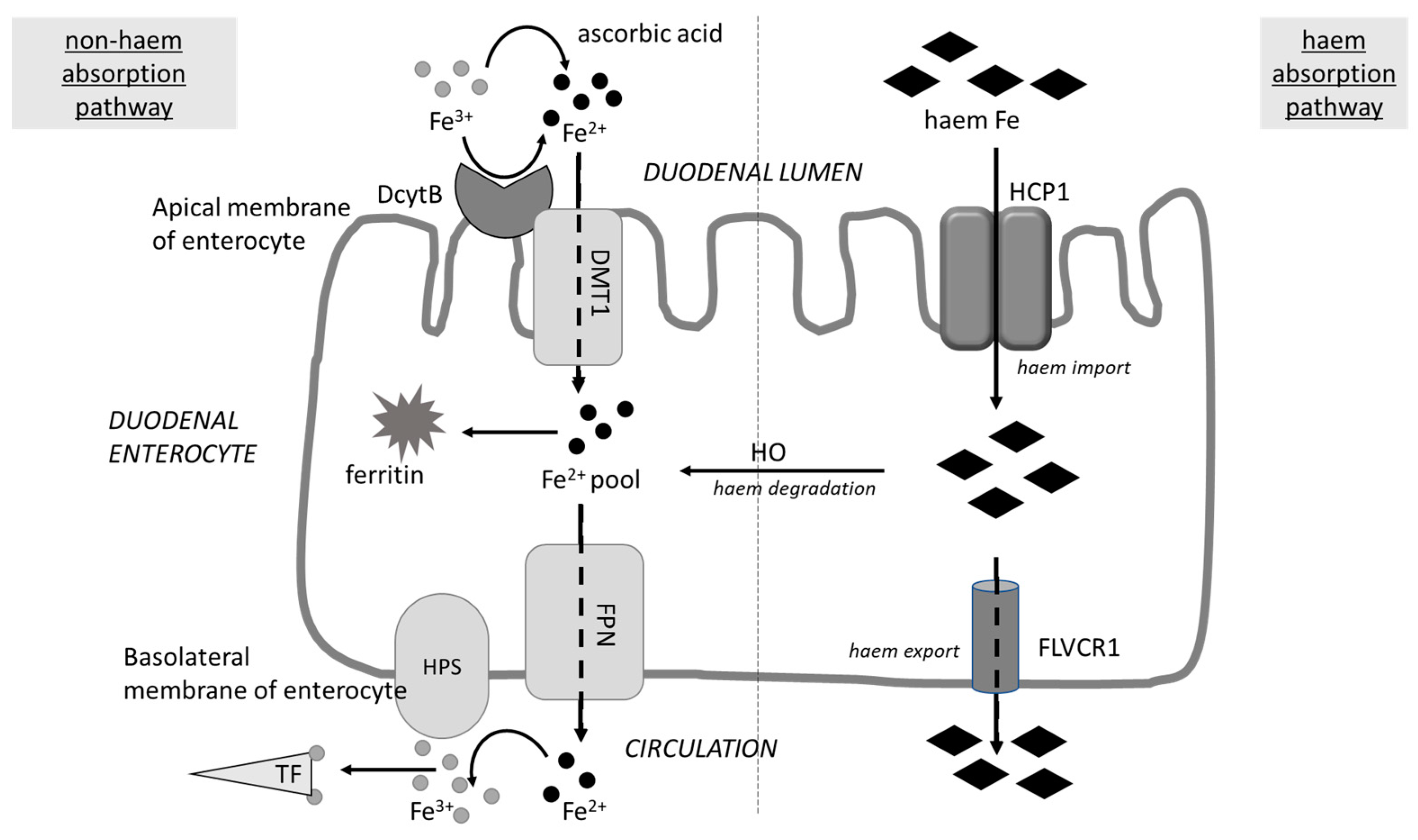
| Enzyme | Polyphenol | Method of Evaluation and Results | References |
|---|---|---|---|
| α-amylase | hesperetin (HES), luteolin (LUT), quercetin (QUE), catechin (CAT) and rutin (RUT) | UV–Vis spectroscopy, fluorescence and molecular docking/α-amylase presented a higher affinity for LUT and LUT was better inhibitor than positive control (acarbose), no inhibition was observed with CAT and RUT; docking analysis showed that flavonoids bound near to enzyme active site | [209] |
| young apple polyphenols (YAP) and nine types of phenolic compounds | fluorescence quenching/tannic acid, chlorogenic acid, and caffeic acid in YAP showed high inhibition against amylase with the IC50 values of 0.30, 1.96, and 3.69 mg/mL, respectively; the order of the apparent static quenching constants was: tannic acid > chlorogenic acid > caffeic acid > epicatechin | [210] | |
| various kinds of tea, catechins and theaflavins | green, oolong and black tea extracts, epigallocatechin gallate, theaflavin-3, 3′-digallate, and tannic acid were competitive inhibitors of PPA, whereas epicatechin gallate, theaflavin-3′-gallate and theaflavin were mixed-type inhibitors with both competitive and uncompetitive inhibitory characteristics; only catechins with a galloyl substituent at the 3-position showed measurable inhibition; 3 and 3′ Galloyl substitution increase the inhibitory activity of theaflavins, and increased the association of catechins and theaflavins with amylase | [211] | |
| sorghum procyanidins (SPC) tetramer | fluorescence, UV-vis absorption, and circular dichroism/SPC-tetramer was bound with human salivary α-amylase at the ratio of 1:1, the conformation of enzyme was altered | [212] | |
| tea polyphenols | depletion assays, fluorescence spectroscopy, and initial rate kinetics/tea polyphenols inhibited the activity of enzyme and increased the binding rate of porcine pancreatic α-amylase to starch | [213] | |
| 4 caffeic and tartaric acid derivates | inhibition assay, kinetics, fluorescence quenching, and molecular docking/caffeic acid had a low inhibitory activity; however, caffeoyl substitution at 2,3-OH of tartaric acid gradually increased its competitive inhibition character/caftaric acid (one caffeoyl-substituted) and chicoric acid (two caffeoyl-substituted) were suggested as mixed-type and competitive inhibitors, tartaric acid was a typical uncompetitive inhibitor of α-amylase; Fluorescence quenching was only observed for compounds with caffeoyl(s), and the effect increased with the moiety number increasing → caffeoyl moiety entered into α-amylase active pocket. | [214] | |
| ellagitannins | ellagitannins inhibit α-amylase activity | [215] | |
| chlorogenic acid (CHA) | kinetic analysis, circular dichroism, fluorescence quenching, and molecular docking/CHA showed a mixed-type inhibitory action on amylase, with the IC50 value of 0.498 ± 0.013 mg/mL; CHA altered the secondary structure of PPA, by interacting with the amino acid residues around or distant from the catalytic site of PPA, mainly through hydrogen bonds, and this interaction was associated with the reduced enzyme’s activity | [216] | |
| apigenin, scutellarein, hispidulin and nepetin | multispectral methods, fluorescence quenching analysis, and molecular docking/nepetin, a competitive inhibitor, exhibited the best inhibitory effect than other tested flavonoids, suggesting that adjacent dihydroxyl group on the B-ring played an important role in inhibiting the activity of α-amylase. | [217] | |
| α-glucosidase and α-amylase | gallocatechin gallate/GCG/ | docking analysis/GCG inhibited α-amylase and α-glucosidase by mixed and non-competitive type. GCG interacted with some amino acid residues located in active site pocket of α-amylase, while it binds to a site close to the active pocket of α-glucosidase GCG form the complexes with enzymes which induced conformational changes | [218] |
| epicatechin gallate (ECG) | molecular simulation/ECG inhibited α-amylase/α-glucosidase in a mixed–type manner, it interacted with some residues in the active pocket of enzymes and induced its conformational changes | [219] | |
| quercetin (1), kaempferol (2), guaijaverin (3), avicularin (4), myricetin (5), hyperin (6) and apigenin (7) isolated from guava leaves | compounds 1, 2, and 5 showed high inhibitory activities, with IC50 values of 3.5 mM, 5.2 mM and 3.0 mM against sucrase, with IC50 values of 4.8 mM, 5.6 mM and 4.1 mM against maltase and with IC50 values of 4.8 mM, 5.3 mM and 4.3 mM against α-amylase, respectively; the hydroxyl group at the 3-position on the A-ring and a number of hydroxyl groups attached to the C-ring played important roles in the inhibition activity | [220] | |
| extracts of raw and heat-processed (roasted or treated in hot water) African pear (Dacryodes edulis) | the extracts inhibited α-amylase activity in a dose-dependent manner; the roasted extract (EC50 = 178.80 μg/mL) had a significantly higher (p < 0.05) inhibitory effect on α-amylase activity than the boiled sample (EC50 = 230.45 μg/mL) and the raw sample extract (EC50 = 266.10 μg/mL). The roasted sample (EC50 = 170.94 μg/mL) also had the highest inhibitory effect on the α-glucosidase activity, while the extracts from the raw pear had the least (EC50 = 178.80 μg/mL) | [221] | |
| herbal extracts containing rosmarinic acid (RA) and purified RA | amylase inhibition correlated with increased concentration of RA; RA-containing oregano extracts yielded higher than expected amylase inhibition than similar amount of purified RA, suggesting that other phenolic compounds or phenolic synergies may contribute to additional amylase inhibitory activity. | [222] | |
| tea polyphenols (TP) and different types of teas (green, black and oolong tea) processed from the same fresh leaves | all three types of teas significantly enhanced α-amylase activity for a wide range of concentrations (0.34–27.14 mg/mL), and green tea showed the highest activation effect, while high TP concentration slightly inhibited it by non-competitive fashion | [223] | |
| extracted and enriched flavonoids from Rubus corchorifolius (12 isolated flavonoids, 6 of the obtained for the first time) | molecular modelling; flavonoid/compound 4 was the strongest inhibitor of α-glucosidase and α-amylase, to improve postprandial hyperglycaemia | [224] | |
| ferulic acid (FA) | enzyme kinetic analysis, circular dichroism (CD), Fourier-transform infrared (FT-IR) spectroscopy, fluorescence quenching, and molecular docking; FA inhibited α-amylase/α-glucosidase by mixed/non-competitive mechanisms; secondary structure of enzymes was changed by binding FA and non-covalent bonding was the main force | [225] | |
| α-glucosidases: maltase and sucrase | 5-caffeoylquinic acid, EGCG, polyphenol-rich green tea extract/GTE/ | GTE efficiently inhibited both human and rat sucrase and maltase activity; 5-caffeoylquinic acid did not significantly inhibit maltase and was only a very weak inhibitor of sucrase. | [226] |
| epicatechin-(4β,8)-epicatechingallate (B2-3′-O-gallate), epicatechin gallate (ECG), epicatechin (EC) | inhibition kinetic/IC50 values were as follows: B2-3′-O-gallate (1.73 ± 1.37 µM and 6.91 ± 3.41 µM), ECG (3.64 ± 2.99 µM and 18.27 ± 3.99 µM), and EC (6.25 ± 1.84 µM and 18.91 ± 3.66 µM,) for maltase and sucrase, respectively. | [227] | |
| α-amylase, lactase, maltase, sucrase | flavonols, theaflavins, gallate esters, 5-caffeoylqunic acid and proanthocyanidins | flavonols, theaflavins, gallate esters, 5-caffeoylqunic acid, and proanthocyanidins inhibit α-amylase activity; anthocyanidins and catechin oxidation products, such as theaflavins and theasinsensins, inhibit maltase; sucrase is less strongly inhibited but anthocyanidins seem somewhat effective; lactase is inhibited by green tea catechins. | [228] |
| lactase (lactase phlorizin hydrolase) | epigallocatechin-3-gallate (EGCG) | EGCG inhibited in vitro hydrolysis of lactose by intestinal lactase and salivary proline-rich proteins (PRPs) shown the protective role against EGCG inhibition of digestive enzymes; inhibition by EGCG of digestive enzymes (α-amylase > chymotrypsin > trypsin > lactase ≫ pepsin) was alleviated ∼2−6-fold by PRPs | [229] |
| pepsin | caffeic acid (CA) | multi-spectroscopy and MD simulations methods; CA affected both the conformation and the activity of pepsin | [230] |
| 10 flavonoids | spectroscopic and molecular docking methods/all flavonoids could bind with pepsin to form flavonoid-pepsin complexes and the interaction was spontaneous mainly through electrostatic forces and hydrophobic interactions with one binding site, the interaction resulted in the reduced enzyme activity | [231] | |
| trypsin | quercetin (Q), luteolin (LUT), kaempferol (KMP) and apigenin (APG) | at a concentration of 2.7 mM, inhibition of trypsin (1.6 U/mL) by Q, LUT, KMP and APG was 46.4%, 32.6%, 26.8% and 17.7%, respectively. The interaction of polyphenol-trypsin caused the fluorescence quenching of trypsin and inhibition of radical scavenging activity of flavonoids; the strength of binding depended on the number and position of hydroxyl group of flavonoids and was in decreasing order Q > LUT > KMP > APG | [232] |
| various polyphenols | Computer Assisted Drug Design studies/5,7-dihydroxy flavonoid have been found to be a perspective trypsin/trypsin-like-enzyme inhibitor; flavanones and isoflavones are less effective trypsin inhibitors due to a loss of the optimal geometry leading to hydrogen bond interactions; quercetin, myricetin and morin have shown to be the best trypsin inhibitors tested. | [233] | |
| hesperetin (HES), luteolin (LUT), quercetin (Q), catechin (CAT), and rutin (RUT) | UV-Vis, intrinsic and extrinsic fluorescence spectroscopies, circular dichroism, and molecular docking/flavonoids-trypsin complexes showed static quenching, and QUE and LUT exhibited higher affinity; the hydrophobic interactions between trypsin and flavonoids were predominant; LUT was the best trypsin inhibitor (IC50 = 45.20 ± 1.00 μM) | [234] | |
| pancreatic lipase | methanolic extract of the leaves of Eremochloa ophiuroides (centipede grass) containing flavonoids | five the C-glycosidic flavones isolated from the extract showed potent inhibitory effects on pancreatic lipase, with IC50 values ranging from 18.5 ± 2.6 to 50.5 ± 3.9 μM | [235] |
| α-amylase, α-glucosidase and lipase | total phenolics, total flavonoids and condensed tannin content in crude, semi-purified extracts from 8 types of foods (black tea, green tea, blueberry, blackberry, red cabbage, broccoli, black turtle bean and black soybean) and five fractions from legumes | semi-purified extracts from legumes, tea and berries showed more potency (lower IC50 values) against α-amylase, α-glucosidase than commercial inhibitors; Myricetin showed the highest potency against α-amylase, α-glucosidase and lipase (IC50: 0.38 mg/mL, 0.87 μg/mL and 15 μg/mL, respectively) | [236] |
| pancreatic lipase (PL), phospholipase A2 (PLA2), and trypsin | tea polyphenols: theaflavin-3,3′-digallate (TFdiG), theaflavin-3′-gallate (TF3′G), theaflavin-3-gallate (TF3G), and theaflavin (TF), catechins, (−)-epigallocatechin-3-gallate (EGCG) | Modelling studies/TfdiG, TF3′G, TF3G, and TF inhibited PL (IC50 = 1.9, 4.2, 3.0, and 32.9 µM, respectively), indicating that the location of the galloyl ester is essential for inhibitory potency; catechins inhibited PL and PLA2; EGCG inhibited trypsin (IC50 = 193 µM) in a non-competitive manner | [237] |
| Drug (Medical Application) | Polyphenol or Food; Type of Study (Protocol If Known) | Impact on Drug Activity → Conclusions or Recommendations | References |
|---|---|---|---|
| Warfarin (preventing blood clots) | green tea/GT/; in vivo human study (a 44-year-old white man was receiving warfarin for thromboembolic prophylaxis secondary to a St. Jude mechanical valve replacement in the aortic position) | the patient had INR * of 3.20 approximately one month prior to entering the clinic, and an INR of 3.79 on entering the clinic, 22 days later his INR was 1.37, 1 month later the INR was 1.14. It was subsequently discovered that the patient began drinking one-half to one gallon of GT/day about one week prior to the INR of 1.37; discontinuation of the green tea enables the patient’s INR increase to 2.55 → concomitant intake of green tea and warfarin should be under medical supervision | [296] |
| resveratrol/RES/; in vivo studies in animal model (rats were orally given (±)warfarin (0.2 mg/kg) without and with RES (100 mg/kg) in a parallel design) | RES significantly increased the AUC0−t of S-warfarin and international normalized ratio. Mechanism is based on the inhibition of BCRP (breast cancer resistance protein)-mediated efflux of R- and S-warfarin. Moreover, RES metabolites activated CYP1A2/3A4, but inhibited CYP2C9 → concomitant intake of RVT increased the systemic exposure of warfarin and enhanced the anticoagulation effect mainly via inhibitions on BCRP and CYP2C9 | [297] | |
| goji berries (Lycium barbarum L.) extract; in vivo studies in animal model (4 experimental groups of Wistar rats: distilled water (negative control); fed daily with the extract (0.18 g/kg); treated daily with water and warfarin (0.5 mg/kg—positive control) and those treated concomitantly with the extract and warfarin, for 7 days) | there were no significant differences between the biochemical and haematological profiles, nor even signs of toxicity of the extract when administered alone → concomitant use intake of goji berries extract with warfarin showed a significant increase in prothrombin time, with the potential for bleeding. | [298] | |
| cranberry; in vivo studies in animal model (rats were orally administered warfarin (0.2 mg/kg) without and with cranberry (5.0 g/kg) at 0.5 h prior to the warfarin, and at 10 h after the warfarin) | cranberry ingested at 0.5 h before warfarin significantly decreased the systemic exposures of S-warfarin and R-warfarin. Conversely, when cranberry was ingested at 10 h after warfarin, the elimination of S-warfarin was significantly inhibited, and the anticoagulation effect of warfarin was significantly enhanced. Probably cranberry activated the breast cancer resistance protein/BCRP/, which mediated the efflux transports of S-warfarin and R-warfarin. The metabolites of cranberry inhibited cytochrome CYP2C9 → the concomitant use of cranberry with warfarin should be avoided. | [299] | |
| cranberry; in vivo human studies (open-label, three-treatment, randomized crossover clinical trial was undertaken and involved 12 healthy male subjects of known CYP2C9 and VKORC1 genotype) | cranberry significantly increased the area under the INR–time curve by 30% when administered with warfarin compared with treatment with warfarin alone. Cranberry did not alter S- and R-warfarin pharmacokinetics or plasma protein binding. Coadministration of garlic did not significantly alter warfarin pharmacokinetics or pharmacodynamics. Both herbal medicines showed some evidence of VKORC1 (not CYP2C9) genotype-dependent interactions with warfarin, which is worthy of further investigation → Co-administration of warfarin and cranberry requires careful monitoring. | [300] | |
| cranberry juice; case study | a man in his 70s had a poor appetite for two weeks and ate almost nothing, taking only cranberry juice and his regular drugs (digoxin, phenytoin, and warfarin). Six weeks after starting cranberry juice he had been admitted to hospital with an INR > 50, although before, his control INR was stable. He died due to a gastrointestinal and pericardial haemorrhage → Uncontrolled, concomitant administration of warfarin and cranberry can cause death due to haemorrhage. | [301] | |
| cranberry juice; case study | a 78-year-old, 86 kg man receiving warfarin at a total weekly dose of 45 mg for atrial fibrillation had INR of 6.45, having reported drinking a half gallon of cranberry/apple juice in the week prior to the elevated INR. After discontinuation of the cranberry juice, maintaining the warfarin dose for 5 days, and resuming the warfarin at a total weekly dose of 40 mg, the INR returned to the therapeutic range of 2 to 3 → combination of warfarin administration and cranberry juice ingestion appeared to be associated with an elevated INR without bleeding in this elderly patient. | [302] | |
| gouqizi (Goji berry) wine; case study | 65-year-old Chinese man taking a prolonged maintenance dose of warfarin who experienced an elevated INR with associated bleeding after drinking Gouqizi wine at large doses → Doctors should advise patients regarding possible interactions between herbs and warfarin when prescribing and should increase the frequency of INR monitoring for those patients concurrently receiving warfarin and medicinal herbs. | [303] | |
| goji juice; case study | 71-year-old Ecuadorean-American woman who was taking warfarin and was hospitalized for a markedly elevated, indeterminate INR (prothrombin time > 120 sec) after consumption of goji juice. She had undergone knee surgery approximately 3 months earlier at which time warfarin therapy was started. She reported no changes in dietary habits or lifestyle other than drinking goji juice for 4 days before hospitalization. On presentation to the emergency department, she described symptoms of epistaxis, bruising, and rectal bleeding → Patients should be educated about avoiding popular herbal drinks or juices, such as goji juice, while they are taking warfarin, while the clinicians should question patients about their use of herbal therapies and document such use in their medical records before prescribing drugs such as warfarin. | [304] | |
| concentrated Chinese herbal tea made from Lycium barbarum L. (goji berry) fruits; case study | a 61-year-old Chinese woman had an elevated INR of 4.1, although before it was stabilized on anticoagulation therapy at level 2–3. There were no changes in her other medications or lifestyle, a review of her dietary habits revealed 4 days of drinking a goji tea (3–4 glasses daily) prior to her clinic visit. After leaving the tea, while maintaining consistency with medications and dietary habits, a follow-up INR seven days later was 2.4, and seven subsequent INR values were in the 2.0–2.5 range → combination of L. barbarum L. and warfarin should be avoided. | [305] | |
| Rivaroxaban (prevent blood clots) | naringenin; in vitro (liver microsomes); in vivo animal model (male Sprague–Dawley rats were randomly divided into the experimental (Ex) group and the control (C) group with six rats in each group; Ex rats were pre-treated with naringenin (10 mg/kg/day) for 2 weeks before the administration of rivaroxaban (10 mg/kg) by oral gavage, while the C rats were given rivaroxaban (10 mg/kg) only once) | (i) in vitro data indicated that naringenin could decrease the metabolic clearance rate of rivaroxaban with the IC50 value of 38.89 μM, and exhibited a mixed inhibition to rivaroxaban; (ii) compared to C group the AUC0–t value was increased in Ex rats from 2406.28 ± 519.69 μg/h/L (in controls) to 4005.04 ± 1172.76 μg/h/L, the Cmax value was increased from 310.23 ± 85.76 μg/L to 508.71 ± 152.48 μg/L, and the Vz/F and CLz/F were decreased from 23.03 ± 4.81 L/kg to 16.2 ± 8.42 L/kg, 4.26 ± 0.91 L/h/kg to 2.57 ± 0.73 L/h/kg, respectively → naringenin had an inhibitory effect on the pharmacokinetics of rivaroxaban in rats | [306] |
| Saquinavir (protease inhibitor used for HIV infection treatment) | garlic supplement; in vivo human study (10 healthy volunteers received 10 doses of saquinavir (Fortovase) at a dosage of 1200 mg, 3 times daily with meals for 4 days on study days 1–4, 22–25, and 36–39, and they received a total of 41 doses of garlic caplets taken 2 times daily on study days 5–25.) | in the presence of garlic, the mean saquinavir area under the curve (AUC) during the 8-h dosing interval decreased by 51%, trough levels at 8 h after dosing decreased by 49%, and the mean maximum concentrations (Cmax) decreased by 54%. After the 10-day washout period, the AUC, trough, and Cmax values returned to 60–70% of their values at baseline → Patients should use caution when combining garlic supplements with saquinavir when it is used as a sole protease inhibitor | [307] |
| Metformin (antihyperglycemic agent used for the treatment of type 2 diabetes, particularly in people who are overweight) | green tea (GT) and EGCG; in vitro studies | (i) metformin uptake was inhibited in a concentration-dependent manner in the presence of GT with IC50 values of 1.4% (v/v) and 7.0% (v/v) for OCT1 and OCT2, respectively; (ii) the inhibitory potency of GT on metformin uptake was stronger for MATE1 compared to MATE2-K; (iii) IC50 of green tea was 4.9% (v/v) for inhibition of MATE1-mediated metformin transport, while the IC50 value for MATE2-K-mediated metformin transport could not be calculated; (iv) OCT1-mediated metformin net uptake (i.e., uptake into transporter-transfected cells minus uptake into vector control cells) was significantly reduced by EGCG to 40% of metformin net uptake without EGCG; (v) OATP1B1-mediated BSP and atorvastatin net uptake (i.e., uptake into transporter-transfected cells minus uptake into vector control cells) were reduced by EGCG to 64% (not significant) and 69% (p < 0.05), respectively, of net uptake without EGCG; (vi) the GT significantly decreased the basal-to-apical digoxin transport to 2.4%/h for 1% (v/v) green tea → green tea and its main catechin ECGC inhibit in vitro transport of prototypical substrates of all seven drug transporters investigated (OCT1, OCT2, MATE1, MATE2-K, OATP1B1, OATP1B3, P-gp). | [293] |
| silibinin, epigallocatechin (ECGC), quercetin and rutin; in vivo animal study (30 male rats were divided into 5 groups and treated as follow: control group treated with olive oil (0.2 mL/day); the other 4 groups were treated with either silibinin (100 mg/kg), ECGC (25 mg/kg), quercetin (50 mg/kg) or rutin (500 mg/kg), administered orally as oily solutions for 30 days. At day 30, a 300 mg/kg metformin and 50 mg/kg atenolol were administered orally) | all polyphenols produced significant increase (p < 0.05) in serum levels of metformin compared with control group, while atenolol levels revealed no significant differences compared with controls, except for silibinin for which significant increase was reported. Silibinin and EGCG long-term use produced significant increase in metformin contents in bran and kidney, and for EGXG also in the liver → Long-term administration of silibinin, EGCG, quercetin or rutin increase oral absorption and tissue distribution of metformin, while atenolol was not affected. | [308] | |
| Digoxin (the oldest medication used to treat various heart conditions, most frequently for atrial fibrillation, atrial flutter, and heart failure) | green tea; in vivo human study (0.5 mg of digoxin was administered orally to 16 healthy volunteers at Day 1, after a 14-day washout period, 630 mg of green tea catechins/GTC/was administered via oral route, followed by 0.5 mg of digoxin 1 h later; from Day 16 through Day 28, 630 mg of GTC was administered alone; At Day 29, 630 mg of GTC and 0.5 mg of digoxin were administered in the same way as Day 15) | compared to digoxin alone, the concomitant administration of digoxin and GTC significantly reduced the systemic exposure of digoxin: geometric mean ratios/GMR/of area under the concentration–time curve from time 0 to the last measurable time/AUClast/and Cmax were 0.69 and 0.72, respectively. The concomitant administration of digoxin and GTC following pretreatment of GTC (Day 29) similarly reduced the AUClast (GMR = 0.67) and Cmax (GMR = 0.74) → the coadministration of GTC reduces the systemic exposure of digoxin regardless of pretreatment of GTC. | [309] |
| Midazolam (a benzodiazepine medication used for anaesthesia and procedural sedation, to treat severe agitation and insomnia) | green tea extract/GT/ and grape seed extract/GSE/; in vitro and in vivo studies in animal model (3 groups of rats with single administration of herbal extract, GTE 400 mg/10 mL b.w.; GSE 80 mg/kg b.w, water-control 10 mL/kg b.d. administered orally after overnight fasting; 3 groups of rats with subchronic treatments: GTE, GSE and water as above but daily administered for 6 successive days) | strong inhibition of these CYP2C9, CYP2D6, and CYP3A4 activities in human liver microsomes by GTE and GSE in vitro; in rats, single treatments with these extracts had negligible effects, 1 week of GTE/GSE treatment resulted in significantly increased elimination rate constant (ke) of intravenously administered midazolam/MDZ/, indicating the induction of CYP3A in the liver. In contrast, 1 week of treatment with GTE, but not GSE, caused a significant increase in the Cmax and AUC∞ of orally administered MDZ without change in the elimination half-life, suggesting a reduction in CYP3A activity in the small intestines → subchronic ingestion of GTE or GSE may alter the pharmacokinetics of midazolam, the effects of GTE on CYP3A activity appear opposite between liver and small intestine. | [310] |
| grapefruit juice; in vivo human study (8 healthy male subjects have administered midazolam/MDZ/intravenously (5 mg) or orally (15 mg) after pretreatment with water or grapefruit juice) | after intravenous administration no changes in the pharmacokinetics or pharmacodynamics of MDZ. After oral administration of MDZ: pretreatment with grapefruit juice led to a 56% increase in peak plasma concentration (Cmax), a 79% increase in time to reach Cmax (tmax), and a 52% increase in the area under the plasma concentration-time curve (AUC) of MDZ, which was associated with an increase in the bioavailability from 24% ± 3% (water) to 35% ± 3% (grapefruit juice, p < 0.01); was also associated with a 105% increase in tmax and with a 30% increase in the AUC of alpha-hydroxyMDZ → pretreatment with grapefruit juice is associated with increased bioavailability and changes in the pharmacodynamics of midazolam that may be clinically important, particularly in patients with other causes for increased midazolam bioavailability such as advanced age, cirrhosis of the liver, and administration of other inhibitors of cytochrome P450. | [311] | |
| Sildenafil (a medication used to treat erectile dysfunction and pulmonary arterial hypertension) + midazolam | green tea (GT); in vivo human study (each of 10 healthy volunteers received one tablet of sildenafil 50 mg and one tablet of midazolam 7.5 mg concurrently either after drinking 250 mL of water or 250 mL of fresh extract of 2 g of green tea; after 1 week washout period, each volunteer received the other intervention) | (i) coadministration of GT with sildenafil increased the extent but not the rate of sildenafil absorption, which resulted in higher plasma concentrations (AUC∞ increased from 484.2 ± 67.27 μg hr/L to 731.5 ± 111.01 μg hr/L and the Cmax from 318.9 ± 46.8 μg/L to 414.9 μg/L ± 67.0 μg/L; (ii) the elimination rate constant of sildenafil was significantly decreased and the elimination half-life was prolonged by about 36%; (iii) The AUC∞ of midazolam increased by 16% and Cmax by 14%; suggesting a small reduction CYP 3A4 activity. → Patients who are taking green tea may need smaller doses of sildenafil, and those at higher risk of developing sildenafil adverse effects | [312] |
| Fluvastatin (belongs to statins, HMG-CoA reductase inhibitors, a class of lipid-lowering medications that reduce illness and mortality in people of high risk of cardiovascular disease; the most common cholesterol-lowering drugs) | green tea/GT/, (−)-epigallocatechin gallate/EGCG/; in vitro study (Bactosomes prepared from Escherichia coli cells coexpressing recombinant human NADPH-P450 reductase and human CYP2C9) in vivo human studies (11 healthy volunteers ingested a single 20-mg dose of fluvastatin with: (1) 300 mL of brewed green tea; (2) 150 mg of EGCG in 300 mL of water/GTE/; or (3) 300 mL of water as control, after overnight fasting) | in vitro EGCG inhibited fluvastatin degradation with IC50 of 48.04 μM. Brewed green tea used in the clinical study also dose-dependently inhibited the metabolism of fluvastatin in vitro. No significant effects of GTE and brewed green tea were observed in plasma concentrations of fluvastatin. The geometric mean ratios with 90% CI for area under the plasma concentration-time curve (AUC0−∞) of fluvastatin were 0.993 (brewed green tea) and 0.977 (GTE) → although in vitro studies indicated that EGCG and brewed green tea produce significant inhibitory effects on CYP2C9 activity, the concomitant administration of green tea and fluvastatin in healthy volunteers did not influence the pharmacokinetics of fluvastatin | [313] |
| Atorvastatin (statin) | green tea extract; in vivo human study (12 healthy volunteers received a single dose of atorvastatin 40 mg alone (control group), atorvastatin 40 mg plus a capsule containing 300 mg of dry green tea extract/GTE300/, or atorvastatin 40 mg plus a capsule containing 600 mg of dry green tea extract/GTE600/) | compared to control, the GTE300 and GTE600 decreased the peak plasma concentration (Cmax) of atorvastatin by 25% and 24%, respectively (p < 0.05), and the area under the plasma concentration-time curve (AUC0−∞) of atorvastatin by 24% and 22%, respectively (p < 0.05); it also increased the apparent oral clearance of atorvastatin by 31% and 29%, respectively. The Tmax and the elimination half-life of atorvastatin did not differ among the three phases. The effects of GTE600 on the pharmacokinetic parameters of atorvastatin were not significantly different from GTE300 → Green tea extract decreases the absorption but not the elimination of atorvastatin, possibly by inhibiting OATP, albeit not in a dose-dependent manner. Coadministration of GTE with atorvastatin may necessitate the monitoring of the drug level in blood. | [314] |
| Simvastatin (statin) | green tea/GT/and soy isoflavones/SIF/; in vivo human study (18 healthy Chinese male volunteers obtained a single dose of 20 mg simvastatin three times: 1. simvastatin only; 2. with green tea extract; 3. with soy isoflavones extract. There was a washout period of at least 4 weeks between phases. The green tea and soy isoflavone extracts were given at a dose containing EGCG 800 mg once daily or soy isoflavones about 80 mg once daily for 14 days before simvastatin dosing) | SIF intake was associated with reduced systemic exposure to simvastatin acid (AUC0–24 h from 16.1 h∙mg/L to 12.1 h∙mg/L, p < p0.05), but not the lactone. The interaction between simvastatin and SIF only resulted in a significant reduction of AUC in subjects with the SLCO1B1 521TT genotype and not in those with the 521C variant allele. There was no effect of GTE on simvastatin pharmacokinetics, only the group with the SLCO1B1 521TT genotype showed reduced AUC values for simvastatin acid → repeated administration of soy isoflavones reduced the systemic bioavailability of simvastatin in healthy volunteers, which was dependent on the SLCO1B1 genotype suggesting that SIF-simvastatin interaction is impacted by genotype-related function of this liver uptake transporter. | [315] |
| Rosuvastatin (statin) | green tea/GT/; in vivo human studies (healthy volunteers aged 20–55 years received a 20-mg rosuvastatin tablet with 150 mL of water by oral route on Day 1. After a 3-day washout period, they received 300 mg of EGCG followed by 20 mg of rosuvastatin 1 h later. From Day 5 through 14, subjects only received 300 mg of EGCG. On Day 15, just like Day 4, they received 300 mg of EGCG followed by 20 mg of rosuvastatin 1 h later) | compared with the administration of rosuvastatin alone, the concomitant use at Day 4 significantly reduced the area under the concentration–time curve from time 0 to the last measurable time (AUClast) by 19% (geometric mean ratio 0.81, 90% confidence interval [CI] 0.67–0.97) and the peak plasma concentration (Cmax) by 15% (geometric mean ratio 0.85, 90% CI 0.70–1.04). AUClast or Cmax of rosuvastatin on Day 15 was not significantly different from that on Day 1 → Coadministration of EGCG reduces the systemic exposure of rosuvastatin by 19%, and pretreatment of EGCG can eliminate that effect of co-administration of EGCG. | [316] |
| green tea extract/GTE/and (–)-epigallocatechin-3- gallate/EGCG/; in vitro (Caco-2 cells and OATP1B1-HEK293T cells) in vivo animal studies | in the Caco-2 cell model, the uptake and transport of rosuvastatin in the GTE groups were 1.94-fold (p < 0.001) and 2.11-fold (p < 0.050) higher, respectively, than those of the control group. However, in the EGCG group, the uptake and transport of rosuvastatin were decreased by 22.62% and 44.19%, respectively (p < 0.050). In the OATP1B1-HEK293T cell model, the OATP1B1-mediated rosuvastatin uptake was decreased by GTE to 35.02% of that in the control (p < 0.050) and was decreased by EGCG to 45.61% of that in the control (p < 0.050). GTE and EGCG increased the AUC0−∞ of rosuvastatin (p < 0.050) → GTE increased the systemic rosuvastatin exposure in rats, and the mechanism may include an increase in rosuvastatin absorption and a decrease in liver distribution by inhibiting OATP1B1 | [317] | |
| honey flavonoids (galangin, myricetin, pinocembrin, pinobanksin, chrysin and fisetin); in vitro study (cell lines overexpressing the hOATP2B1 or hOATP1A2 transporter) | chrysin, galangin, and pinocembrin inhibited both hOATP2B1 and hOATP1A2 in lower or comparable concentrations as the known flavonoid OATP inhibitor quercetin. Galangin, chrysin and pinocembrin effectively inhibited rosuvastatin uptake by hOATP2B1 with IC50 ∼1–10 μM. The inhibition of the hOATP1A2-mediated transport of rosuvastatin by these flavonoids was weaker. → several natural flavonoids present in honey can affect drug cellular uptake by hOATP2B1 and/or hOATP1A2 at relative low concentrations suggesting the possibility of food-drug interactions. | [318] | |
| green tea extract/GTE/ and soy isoflavonoids/SIF/; in vivo human study (20 healthy Chinese males were given a single dose of rosuvastatin 10 mg three times: 1. rosuvastatin alone; 2. with GTE; 3. with SIF. The GTE and SIF were given at a dose containing EGCG 800 mg once daily or soy isoflavones 80 mg once daily for 14 days before statin dosing and at the same time as the statin dosing with at least 4-weeks washout period between phases). | GTE intake significantly reduced the systemic exposure to rosuvastatin by about 20% reducing AUC0–24 h from 108.7 h·μg/L to 74.1 h·μg/L and Cmax from 13.1 μg/L to 7.9 μg/L (p < 0.001 for both), without affecting the elimination half-life. SIF had no significant effect on rosuvastatin pharmacokinetics. → repeated administration of GTE significantly reduced the systemic exposure of rosuvastatin in healthy volunteers. | [319] | |
| Nadolol (β-blocker) | green tea extract/GTE/and (–)-epigallocatechin-3-gallate/EGCG/; in vivo animal model (Male Sprague-Dawley rats received GTE (400 mg/kg), EGCG (150 mg/kg) or saline (control) by oral gavage, 30 min before a single intragastric administration of 10 mg/kg nadolol) | pretreatment with GTE resulted in marked reductions in the Cmax and AUC of nadolol by 85% and 74%, respectively, as compared with control. In addition, EGCG alone significantly reduced Cmax and AUC of nadolol. Amounts of nadolol excreted into the urine were decreased by pretreatments with GTE and EGCG, while the terminal half-life of nadolol was not different among groups. → the coadministration with GT catechins, particularly EGCG, causes a significant alteration in the pharmacokinetics of nadolol, possibly through the inhibition of its intestinal absorption mediated by uptake transporters. | [320] |
| green tea/GT/; in vitro study (human embryonic kidney 293) and in vivo human studies (10 healthy volunteers received a single oral dose of 30 mg nadolol with GT or water after repeated consumption of 700 mL GT/day or water for 14 days) | GT markedly decreased Cmax and AUC0–48 of nadolol by 85.3% and 85.0%, respectively (p < 0.01), without altering renal clearance of nadolol. The effects of nadolol on systolic blood pressure were significantly reduced by green tea. [3H]-Nadolol uptake assays in human embryonic kidney 293 cells stably expressing the organic anion–transporting polypeptides OATP1A2 and OATP2B1 revealed that nadolol is a substrate of OATP1A2, but not of OATP2B1 and that GT significantly inhibited OATP1A2-mediated nadolol uptake → These results suggest that green tea reduces plasma concentrations of nadolol possibly in part by inhibition of OATP1A2-mediated uptake of nadolol in the intestine. | [321] | |
| (−)-epigallocatechin-3-gallate/EGCG/; in vivo animal study (male rats aged 12–13 weeks were divided into 4 groups: control, EGCG (pretreated 14 days with EGCG), nadolol (received single dose of nadolol), and EGCG-nadolol (pretreated 14 day with EGCG and received a single dose of nadolol). EGCG (10 mg/kg body weight/day) was given orally for consecutively 13 days at the same time of the day. The rats were fasted for a night and on Day 14, a single dose of nadolol (10 mg/kg body weight) was given orally 30 min after the last dose of EGCG administration) | systolic blood pressure (SBP) of rats EGCG-nadolol was significantly higher than in those which received nadolol alone. Pre-treatment of EGCG resulted in a marked reduction of Cmax and AUC by 53% and 51%, respectively, compared to control. → exposure to EGCG lead to reduced nadolol bioavailability and therefore, uncontrolled raised blood pressure and higher risks of cardiovascular events. | [322] | |
| (−)-epigallocatechin-3-gallate/EGCG/; in vivo human studies (3 healthy volunteers received single doses of 30 mg nadolol orally with water (control), or an aqueous solution of EGCG-concentrated green tea extract/GTE/at low or high dose) | a single coadministration of low- and high-dose GTE significantly reduced the plasma concentrations of nadolol, AUC0–∞ of nadolol (0.72 for the low and 0.60 for the high GTE dose). There were no significant differences in Tmax, elimination half-life, and renal clearance between GTE and water phases. No significant changes were observed for blood pressure and pulse rate between phases. EGCG competitively inhibited OATP1A2-mediated uptake of sulphobromophthalein and nadolol with Ki values of 21.6 and 19.4 μM, respectively → due to EGCG even a single coadministration of green tea may significantly affect nadolol pharmacokinetics. | [323] | |
| green tea/GT/; in vivo human studies (1 healthy volunteers received an oral administration of nadolol with, or 1 h after pre-ingestion of brewed GT, or with water in a volume of 150 mL) | in control group AUC0–48 of nadolol was 830.5 h∙ng/mL, concomitant GT ingestion and GT ingestion 1 h before nadolol administration resulted in a significant reduction of AUC0–48 to 359.0 and 453.6 h∙ng/mL, respectively. There were no differences in time to maximal plasma concentration and renal clearance of nadolol among groups → single concomitant ingestion of GT substantially decreases plasma concentrations of nadolol. Moreover, the reduction in nadolol bioavailability could persist for at least 1 h after drinking a cup of GT | [324] | |
| Lisinopril (highly hydrophilic long-acting angiotensin-converting enzyme inhibitor, is frequently prescribed for the treatment of hypertension and congestive heart failure) | aqueous solution of EGCG; in vivo human studies (10 healthy subjects ingested 200 mL of an aqueous solution of GTE containing ~300 mg of EGCG, or water (control) when receiving 10 mg of lisinopril after overnight fasting) | lisinopril Cmax, AUC0–24, and AUC0–∞ in the GTE phase were significantly decreased by 71% (p < 0.001), 69% (p < 0.001), and 67% (p < 0.001), respectively, compared with values in the control phase. The geometric mean ratio (GTE/control) for Cmax and AUC0–∞ of lisinopril were 0.289 and 0.337, respectively. No significant differences were observed in Tmax and renal clearance of lisinopril → the extent of intestinal absorption of lisinopril was significantly impaired in the presence of GTE, whereas it had no major effect on the absorption rate and renal excretion of lisinopril | [325] |
| Diltiazem (calcium channel blockers) | morin; in vivo animal study (rats were orally administrated with diltiazem (15 mg/kg) in the presence and absence of morin at various concentrations (1.5, 7.5 and 15 mg/kg) | compared to the control given diltiazem alone, the Cmax and AUC of diltiazem increased by 30–120% in the rats co-administered with a 1.5 or 7.5 mg/kg of morin, while there was no significant change in Tmax and terminal plasma half-life of diltiazem in the presence of morin. Therefore, absolute and relative bioavailability values of diltiazem in the rats co-administered with morin were significantly higher (p < 0.05) than those from the control group → morin significantly enhanced the oral exposure of diltiazem, suggesting that concurrent use of morin or morin-containing dietary supplement with diltiazem should require close monitoring for potential drug interactions. | [326] |
| resveratrol/RES/; in vivo animal study (rats were divided into groups with oral administration of 15 mg/kg of diltiazem dissolved in water (3.0 mL/kg) without (control) or with 0.5, 2.5, and 10 mg/kg of resveratrol (mixed in distilled water; total oral volume of 3.0 mL/kg); an intravenous group had injected 5 mg/kg of diltiazem, total injection volume of 1.5 mL/kg). | RES presence significantly (p < 0.05) increased AUC of diltiazem, except for resveratrol 0.5 mg/kg, compared to the control group, therefore the absolute bioavailability of diltiazem in the presence of 2.5 and 10 mg/kg RES was significantly higher (10.2–11.1%) than that of the control (6.9%). The relative bioavailability of diltiazem in the presence 2.5 and 10 mg/kg RES was increased by 1.48- to 1.60-fold, respectively. RES did not change absorption rate constant and Tmax of diltiazem. → resveratrol significantly increased the bioavailability of diltiazem due to the inhibition of both the cytochrome P450 (CYP) 3A4-mediated metabolism and the efflux pump P-glycoprotein (P-gp) in the intestine and/or liver. | [327] | |
| Amlodipine (calcium channel blockers) | (−)-epigallocatechin-3-gallate/EGCG/; in vivo animal study (rats had orally administered amlodipine (1 mg/kg) with or without EGCG pretreatment at the dose of 30 mg/kg/day for 10 days) | rats pretreated with EGCG had the Cmax of amlodipine increased from 16.32 ± 2.57 to 21.44 ± 3.56 ng/mL (p < 0.05), the Tmax decreased from 5.98 ± 1.25 to 4.01 ± 1.02 h (p < 0.05), the AUC0−t increased from 258.12 ± 76.25 to 383.34 ± 86.95 μg∙h/L (p < 0.05), and the metabolic half-life was prolonged from 31.3 ± 5.6 to 52.6 ± 7.9 min (p < 0.05), suggesting that EGCG affected the pharmacokinetic behaviour of amlodipine → the drug-drug interaction between EGCG and amlodipine might occur, due to the metabolism inhibition of amlodipine by EGCG when they were co-administered. | [328] |
| Verapamil (calcium channel blockers) | (−)-epigallocatechin-3-gallate/EGCG/; in vivo animal study (9 mg/kg verapamil was administered orally to Sprague-Dawley rats 30 min after the oral administration of 2 and 10 mg/kg of oral EGCG) | compared with the controls, the AUC value of verapamil were greater in the presence of EGCG (74.3% and 111% increase for 2 and 10 mg/kg EGCG, respectively) → probably the inhibition of P-glycoprotein was the mechanism. | [329] |
| Felodipine (calcium channel blockers) | grapefruit juice; in vivo human study (10 healthy men were given 8 oz of grapefruit juice 3× a day for 6 days. Before and after receiving grapefruit juice, small bowel and colon mucosal biopsies were obtained endoscopically, oral felodipine kinetics were determined, and liver CYP3A4 activity was measured) | grapefruit juice did not affect liver CYP3A4 activity, colon levels of CYP3A5, or small bowel concentrations of P-glycoprotein, villin, CYP1A1, and CYP2D6. In contrast, the concentration of CYP3A4 in small bowel epithelia (enterocytes) fell 62% (p < 0.001) with no corresponding change in CYP3A4 mRNA levels. Enterocyte concentrations of CYP3A4 measured before grapefruit juice consumption correlated with the increase in Cmax when felodipine was taken with grapefruit juice → mechanism of the impact of grapefruit juice on oral felodipine kinetics is its selective downregulation of CYP3A4 in the small intestine. | [330] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. https://doi.org/10.3390/molecules28062536
Duda-Chodak A, Tarko T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules. 2023; 28(6):2536. https://doi.org/10.3390/molecules28062536
Chicago/Turabian StyleDuda-Chodak, Aleksandra, and Tomasz Tarko. 2023. "Possible Side Effects of Polyphenols and Their Interactions with Medicines" Molecules 28, no. 6: 2536. https://doi.org/10.3390/molecules28062536
APA StyleDuda-Chodak, A., & Tarko, T. (2023). Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules, 28(6), 2536. https://doi.org/10.3390/molecules28062536