The Analgesic Properties of Corydalis yanhusuo
Abstract
:1. Introduction
2. Plant Extracts and Pain Management
2.1. Corydalis yanhusuo W. T. Wang
2.1.1. Botany and Traditional Uses
2.1.2. Most Recent Studies in Analgesia Induced by Corydalis yanhusuo
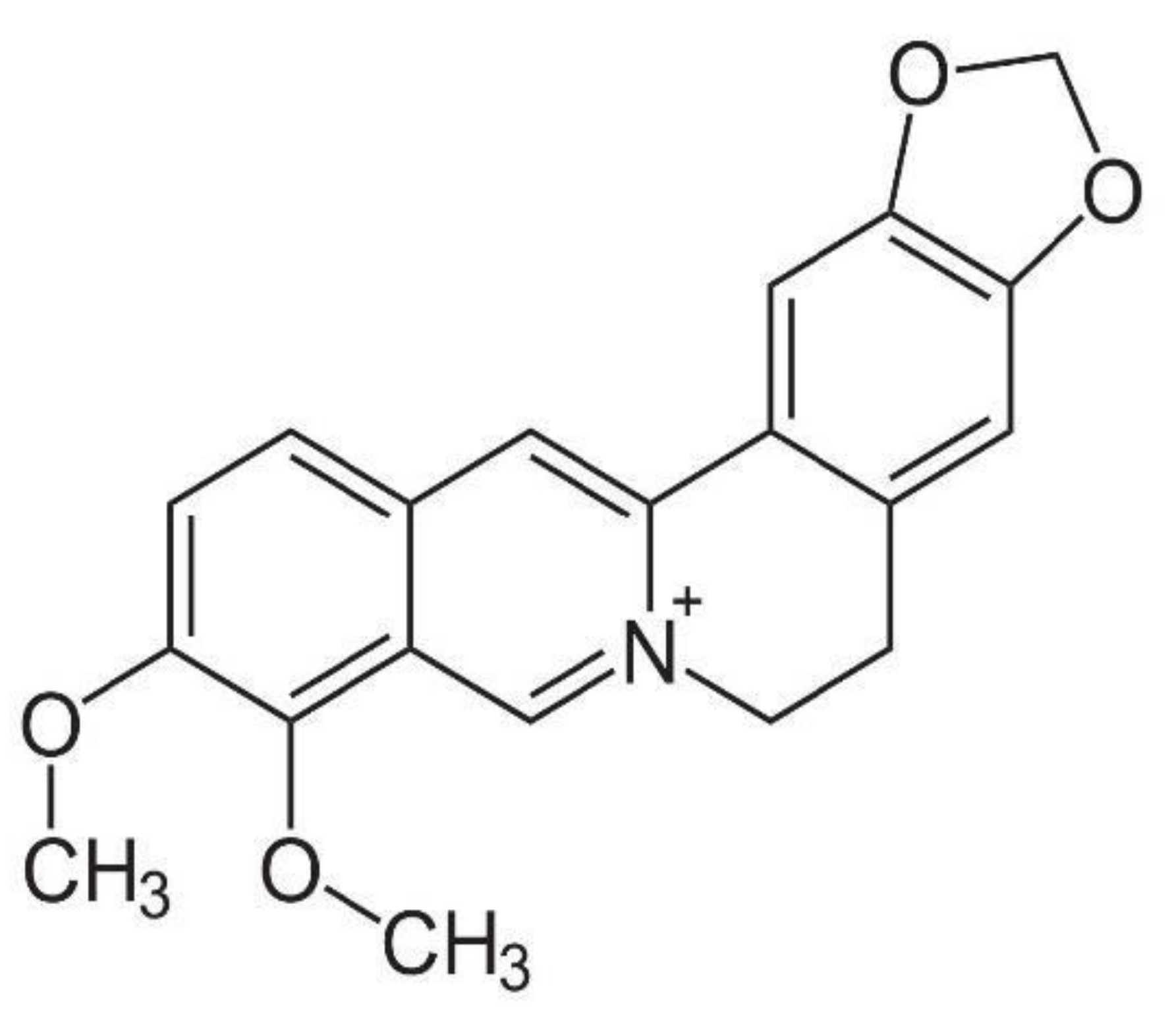
3. YHS Chemical Components
3.1. Alkaloids
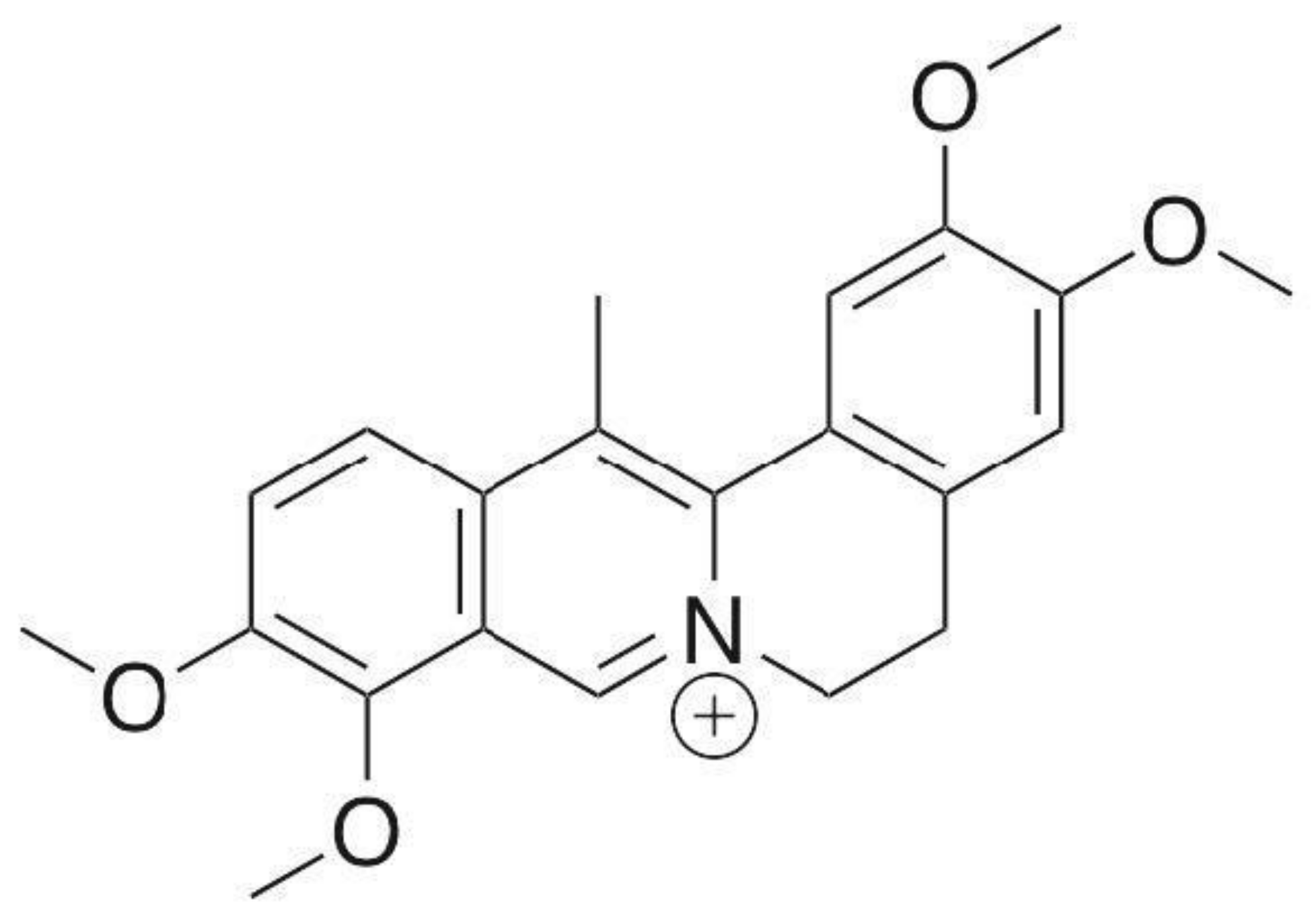
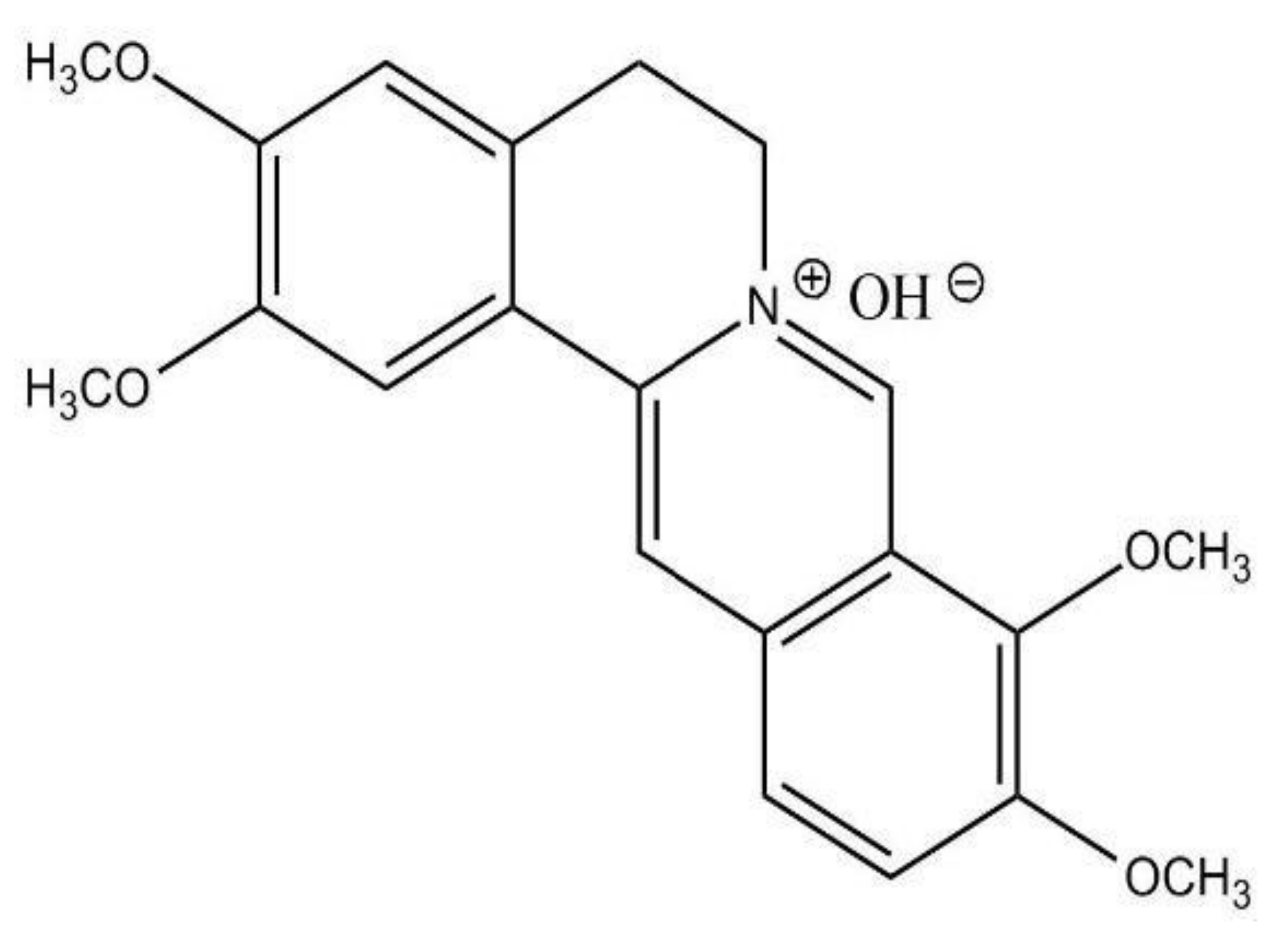
3.1.1. Dehydrocorydaline (DHC)
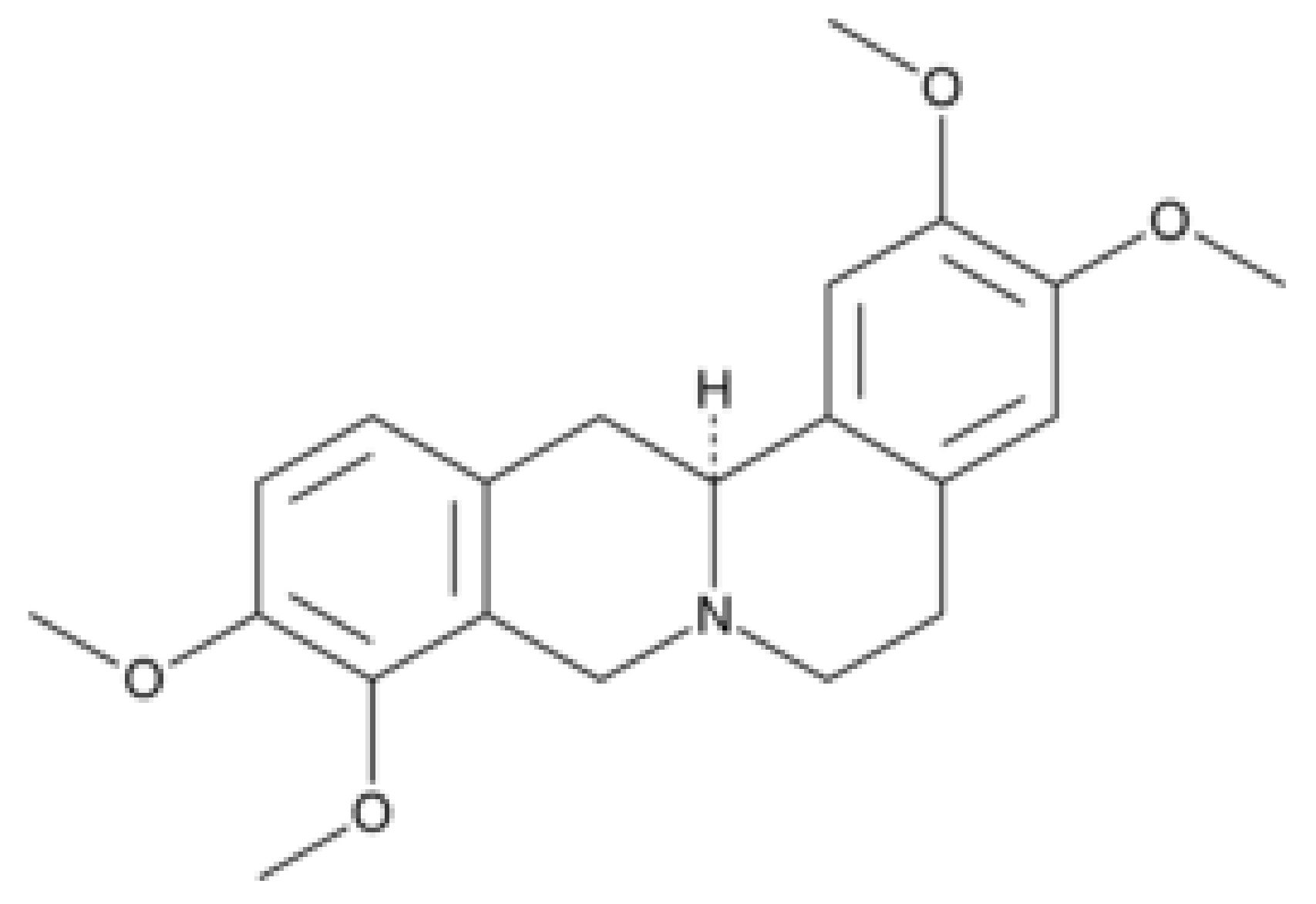
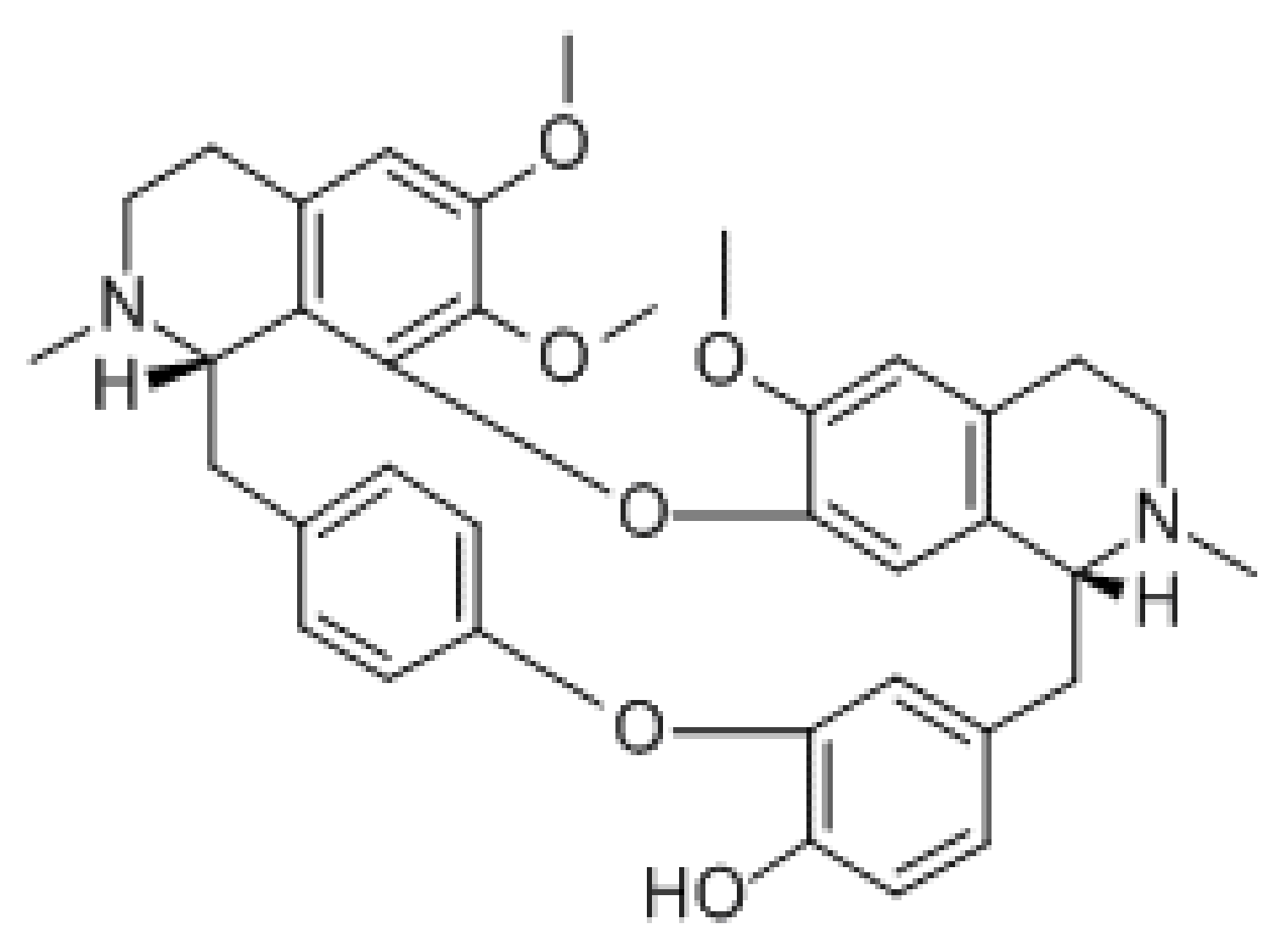
3.1.2. Levo-Tetrahydropalmatine (l-THP)
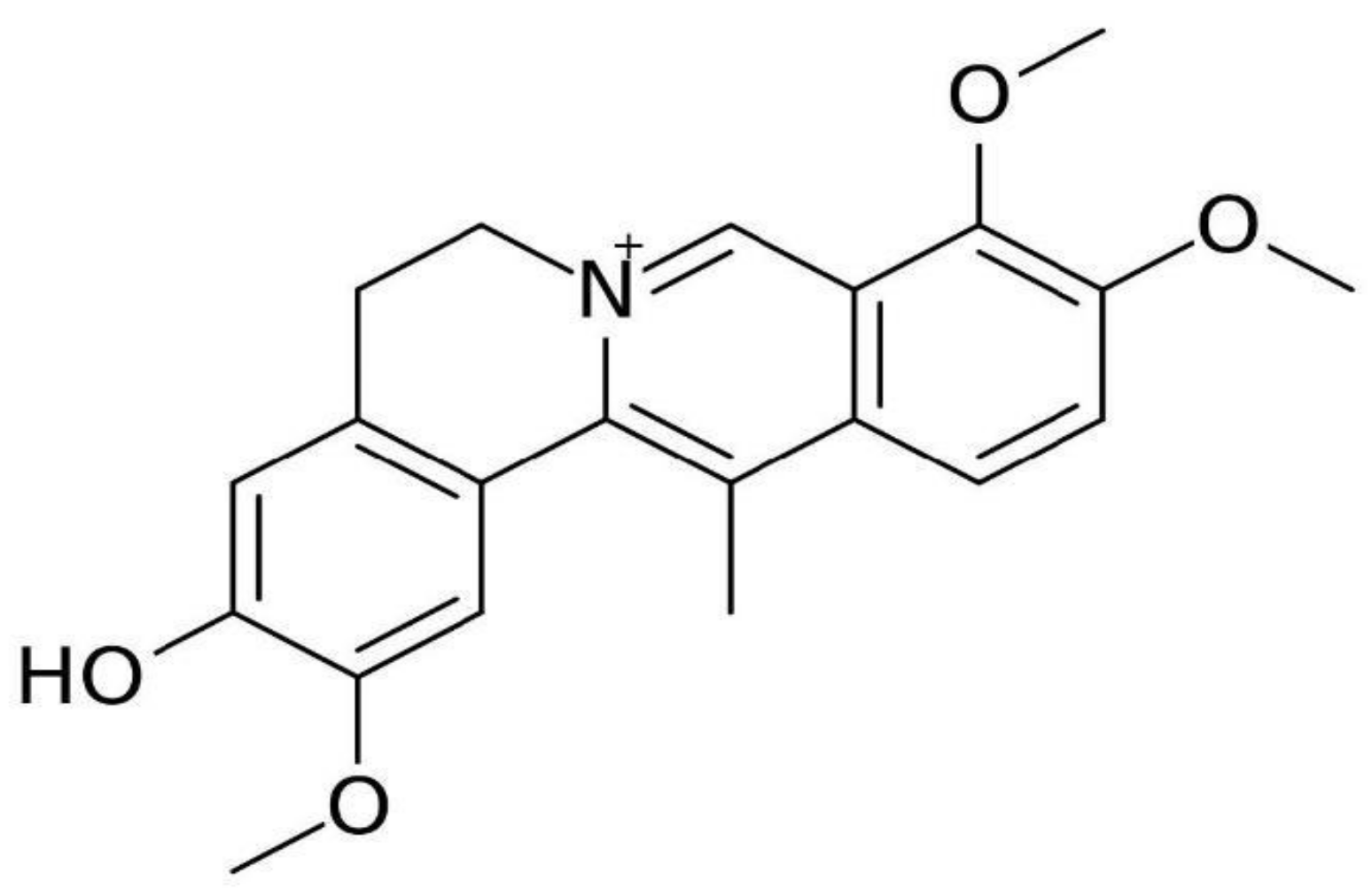
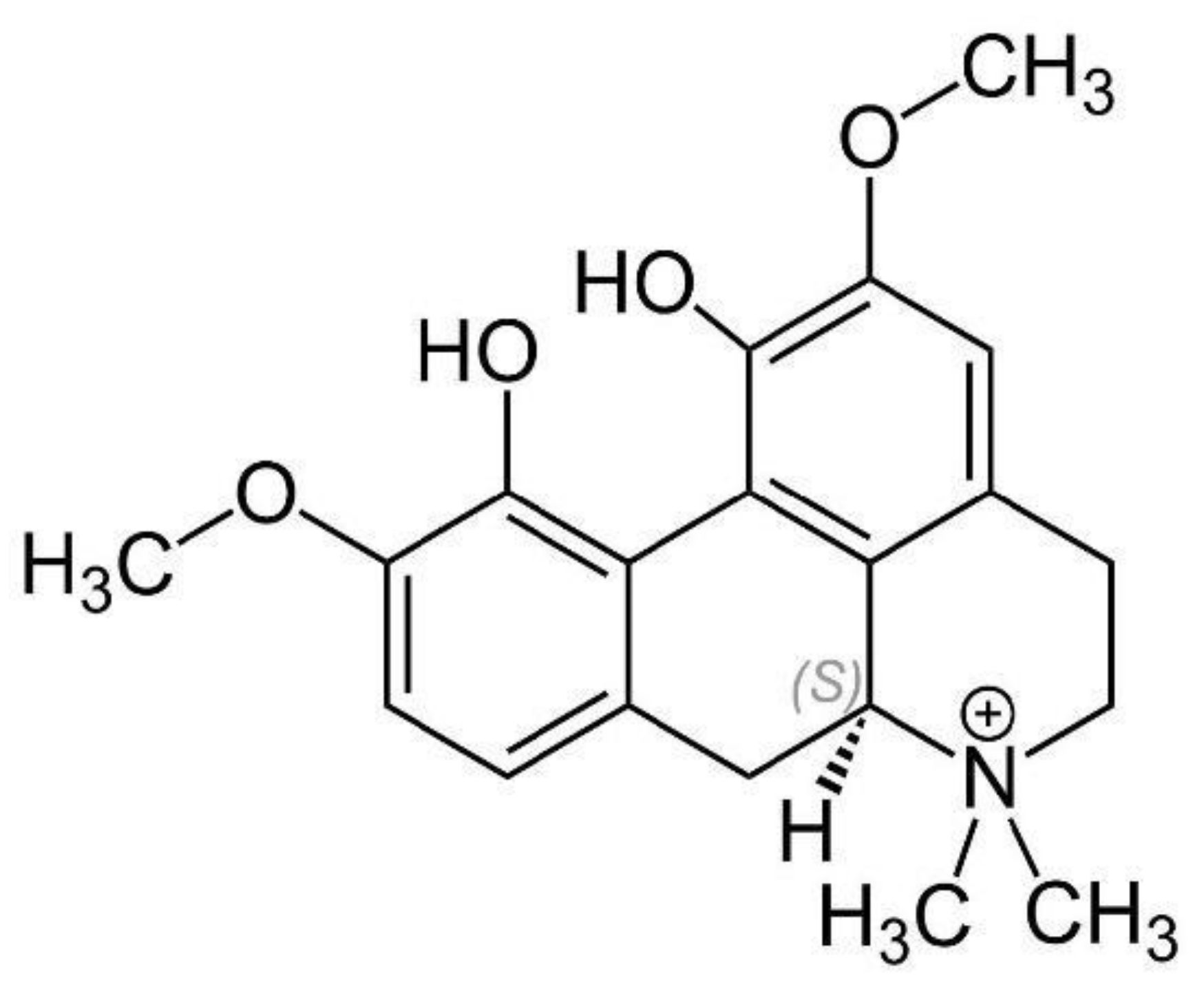
3.1.3. Dehydrocorybulbine (DHCB)
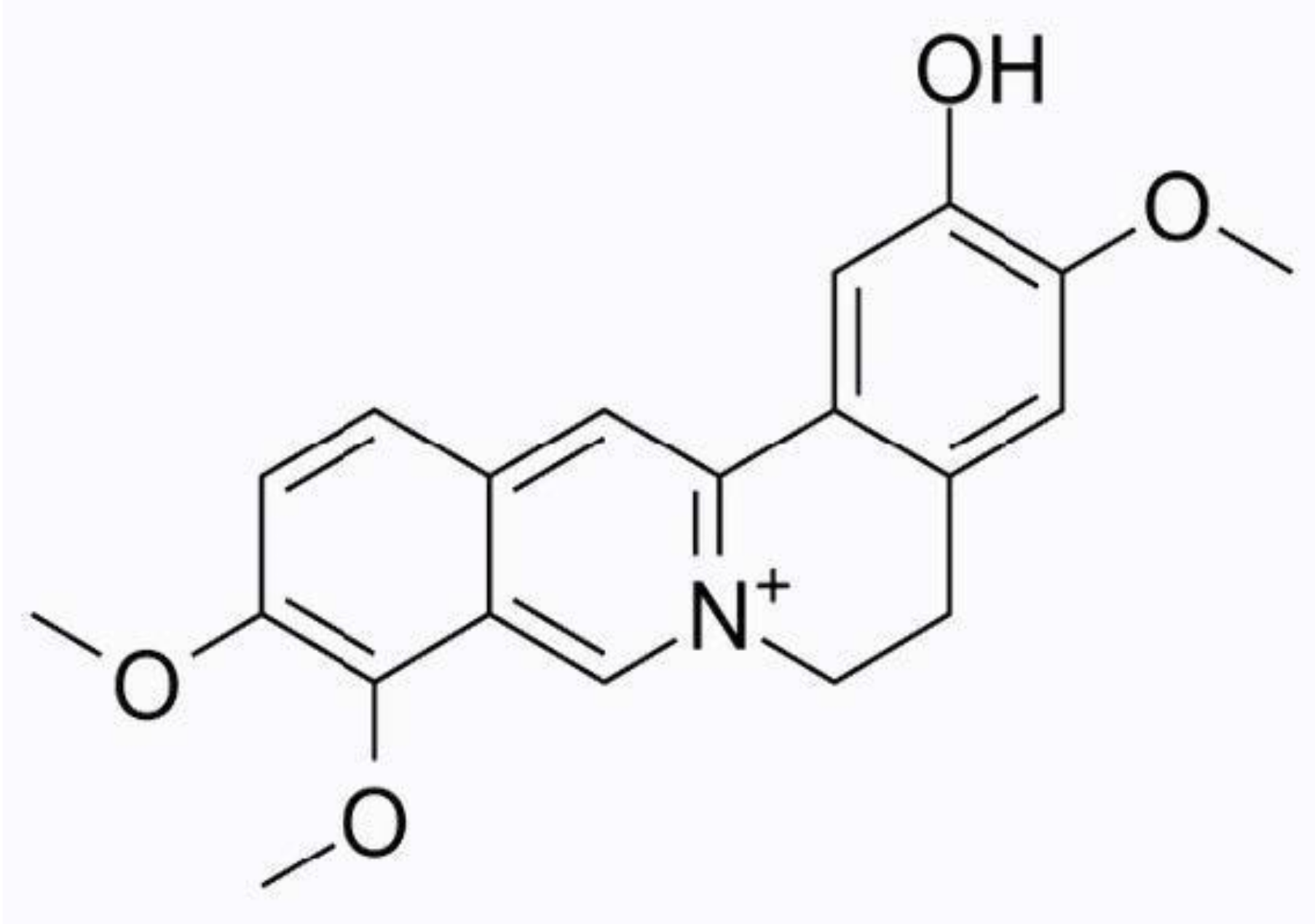
3.1.4. Berberine
3.1.5. Palmatine
3.1.6. Oxyacanthine
3.1.7. Magnoflorine
3.1.8. Columbamine
3.1.9. Corydine and Corydaline
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garland, E.L. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Primary Care 2012, 39, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.E.; Watson, C.P. The pharmacotherapy of chronic pain: A review. Pain Res. Manag. 2006, 11, 11–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [Green Version]
- Khansari, M.; Sohrabi, M.; Zamani, F. The Usage of Opioids and their Adverse Effects in Gastrointestinal Practice: A Review. Middle East J. Dig. Dis. 2013, 5, 5–16. [Google Scholar]
- Kawai, K.; Kawai, A.; Wollan, P.; Yawn, B.P. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Family Practice 2017, 34, 656–661. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Norn, S.; Kruse, P.R.; Kruse, E. History of opium poppy and morphine. Dan Medicinhist Arbog 2005, 33, 171–184. [Google Scholar] [PubMed]
- Rauf, A.; Jehan, N.; Ahmad, Z.; Mubarak, M.S. Analgesic Potential of Extracts and Derived Natural Products from Medicinal Plants. IntechOpen 2017. [Google Scholar] [CrossRef] [Green Version]
- Turnaturi, R.; Aricò, G.; Ronsisvalle, G.; Pasquinucci, L.; Parenti, C. Multitarget Opioid/Non-opioid Ligands: A Potential Approach in Pain Management. Curr. Med. Chem. 2016, 23, 4506–4528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, M.; Zhao, F.; Chu, S.; Zha, L.; Xu, T.; Peng, H.; Zhang, W. Molecular Identification and Taxonomic Implication of Herbal Species in Genus Corydalis (Papaveraceae). Molecules 2018, 23, 1393. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Tian, M.; Huang, S.-M. Advances in phytochemical and modern pharmacological research of Rhizoma Corydalis. Pharm. Biol. 2020, 58, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-J.; Kim, D.-H.; Park, S.-J.; Kim, J.-M.; Ryu, J.-H. Ginseng in Traditional Herbal Prescriptions. J. Ginseng Res. 2012, 36, 225–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, S.; Wang, J.; Wang, C.; Wu, J.; Wang, W.; Li, F.; Li, S.; Zhao, C.; Li, F. A Review of the Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology of Corydalis yanhusuo. SAGE J. 2020, 15. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wang, Z.; Gong, N.; Kweon, T.D.; Vo, B.; Wang, C.; Zhang, X.; Chung, J.Y.; Alachkar, A.; et al. The Antinociceptive Properties of the Corydalis yanhusuo Extract. PLoS ONE 2016, 11, e0162875. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, L.; Parks, G.S.; Zhang, X.; Guo, Z.; Ke, Y.; Li, K.-W.; Kim, M.K.; Vo, B.; et al. A Novel Analgesic Isolated from a Traditional Chinese Medicine. Curr. Biol. 2014, 24, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, Z.; Li, K.; Wang, P.; Cao, L. Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats. Molecules 2012, 17, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-Q.; Chu, H.-P.; Park, C. The alkaloids of Chinese Corydalis ambigua, Cham. Et Sch. (Yen- Hu- So). Chin. J. Physiol. 1928, 25, 544–547. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv. Nat. Prod. Anal. 2020, 505–567. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Z.; Xia, Y.; Liu, E.Y.L.; Ren, H.; Wang, C.; Hu, W.W.H.; Dong, T.T.X.; Pi, R.; Tsim, K.W.K. Dehydrocorydaline Accounts the Majority of Anti-Inflammatory Property of Corydalis Rhizoma in Cultured Macrophage. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Yin, Z.-Y.; Li, L.; Chu, S.-S.; Sun, Q.; Ma, Z.-L.; Gu, X.-P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 2016, 6, 27129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, W.; Zhang, Y.; Liu, Y.; Lei, Y.; Sun, R.; Zhang, W.; Huang, Y.; Mao, Y.; Wang, C.; Ma, Z.; et al. Dehydrocorydaline attenuates bone cancer pain by shifting microglial M1/M2 polarization toward the M2 phenotype. Mol. Pain 2018, 14. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Wu, D.-L.; Gao, L.-Y.; Fang, Y.; Ge, W.-H. L-Tetrahydropalmatine alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. NeuroReport 2016, 27, 476–480. [Google Scholar] [CrossRef]
- Liu, J.; Dai, R.; Damiescu, R.; Efferth, T.; Lee, D.Y. Role of Levo-tetrahydropalmatine and its metabolites for management of chronic pain and opioid use disorders. Phytomedicine 2021, 90, 153594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Mantsch, J.R. l-tetrahydropalamatine: A potential new medication for the treatment of cocaine addiction. Future Med. Chem. 2012, 4, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.Z.; Xu, W.; Jensen, N.H.; Roth, B.L.; Liu-Chen, L.Y.; Lee, D.Y.W. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affinities at the dopamine D1 receptor. Molecules 2008, 13, 2303–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagelberg, N.; Martikainen, I.K.; Mansikka, H.; Hinkka, S.; Någren, K.; Hietala, J.; Scheinin, H.; Pertovaara, A. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 2002, 99, 273–279. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, W.; Wang, Q.; Guo, R.; Liu, P.; Wang, Y.; Zhang, Z.; Wang, L. Role of Dehydrocorybulbine in Neuropathic Pain After Spinal Cord Injury Mediated by P2X4 Receptor. Mol. Cells 2019, 42, 143–150. [Google Scholar] [CrossRef]
- Hahn, F.E.; Ciak, J.B. Mechanism of Action of Antimicrobial and Antitumor Agents; Corcoran, J.W., Hahn, F.E., Snell, J.F., Arora, K.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 577–584. [Google Scholar]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Hashemzaei, M.; Rezaee, R. A review on pain-relieving activity of berberine. Phytotherapy Res. 2020, 35, 2846–2853. [Google Scholar] [CrossRef]
- Chen, C.; Lu, M.; Pan, Q.; Fichna, J.; Zheng, L.; Wang, K.; Yu, Z.; Li, Y.; Li, K.; Song, A.; et al. Berberine Improves Intestinal Motility and Visceral Pain in the Mouse Models Mimicking Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) Symptoms in an Opioid-Receptor Dependent Manner. PLoS ONE 2015, 10, e0145556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.-Y.; Li, B.; Zhu, W.-L.; Shi, J.-Y.; Jia, Q.; Li, Y.-M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2016, 38, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yu, Z.; Sun, W.; Jiang, C.; Ba, X.; Zhou, Q.; Xiong, D.; Xiao, L.; Deng, Q.; Hao, Y. The antiviral alkaloid berberine ameliorates neuropathic pain in rats with peripheral nerve injury. Acta Neurol. Belg. 2018, 120, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.D.; Mallak, K.A.; James, R.L.; Tuttle, R.; Eisenach, J.C. Enhancement of analgesia from systemic opioid in humans by spinal cholinesterase inhibition. J. Pharmacol. Exp. Ther. 1997, 282, 86–92. [Google Scholar] [PubMed]
- Eldufani, J.; Blaise, G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: A review of recent clinical applications. Alzheimers Dement. 2019, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Jing, J.; Mackie, B.; Zheng, X.; Zhang, X.; Wang, J.; Li, X. The anti-sepsis activity of the components of Huanglian Jiedu Decoction with high lipid A-binding affinity. Int. Immunopharmacol. 2017, 46, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Wang, D.; Dong, S.; Cheng, Z.; Na, L.; Sang, M.; Yang, H.; Yang, Z.; Zhang, S.; Yan, Z. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cell. Int. Immunopharmacol. 2017, 45, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Guan, S.; Ge, H.; Xiong, W.; He, L.; Liu, L.; Yin, C.; Liu, H.; Li, G.; Xu, C.; et al. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res. Bull. 2018, 139, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, N.; Philipov, S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996, 18, 553–561. [Google Scholar] [CrossRef]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A review of its pharmacology, pharmacokinetics and toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.-H. Potential Biological Activities of Magnoflorine: A Compound from Aristolochia debilis Sieb. et Zucc. Korean J. Plant Res. 2014, 27, 223–228. [Google Scholar] [CrossRef]
- Sun, D.; Han, Y.; Wang, W.; Wang, Z.; Ma, X.; Hou, Y.; Bai, G. Screening and identification of Caulis Sinomenii bioactive ingredients with dual-target NF-κB inhibition and β2-AR agonizing activities. Biomed. Chromatogr. 2016, 30, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, K.; Wu, H.; Yang, C.; Yang, Y.; Yang, J.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide induced acute lung injury via suppressing NF-κB and MAPK activation. Front. Pharmacol. 2018, 30, 982. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Protective effect of magnoflorine on LPS-induced acute lung injury. World Latest Medicine Information. 2019. [Google Scholar]
- Zhou, J.; Sun, J.B.; Zheng, P.; Liu, J.; Cheng, Z.H.; Wang, F.Q. Orthogonal array design for optimization of hollow-fiber-based liquid-phase microextraction combined with high-performance liquid chromatography for study of the pharmacokinetics of magnoflorine in rat plasma. Anal. Bioanal. Chem. 2012, 403, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Z.; Lin, Y.; Chen, M.; Pan, G.; Huang, C. Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS. Anal. Bioanal. Chem. 2013, 406, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhao, Y.; Miao, Q.; Miao, P.; Yang, X.; Sun, G.; Su, J.; Ye, J.; Wei, B.; Zhang, Y.; et al. In vitro and in vivo identification of metabolites of magnoflorine by LC LTQ-Orbitrap MS and its potential pharmacokinetic interaction in Coptidis Rhizoma decoction in rat. Biomed. Chromatogr. 2015, 29, 1235–1248. [Google Scholar] [CrossRef]
- Tao, C.; Hu, S.-Q.; Chen, J.; Chen, Y.-J.; Sun, K.-H.; Cui, G.-Z.; Ma, M.; Wu, Z.-Z. Highly efficient synthesis and monoamine oxidase B inhibitory profile of demethyleneberberine, columbamine and palmatine. Neurochem. Int. 2020, 139, 104807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Guo, M.-Q.; Li, X.-M.; Yang, X.-W. Simultaneous Qualitative and Quantitative Evaluation of the Coptidis Rhizoma and Euodiae Fructus Herbal Pair by Using UHPLC-ESI-QTOF-MS and UHPLC-DAD. Molecules 2020, 25, 4782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Z.; Shi, Q.; Zeng, H.; Shen, Y.; Jin, H.; Zhang, W. Anti-inflammatory and anti-nociceptive activities of compounds from Tinospora sagittata (Oliv.) Gagnep. Arch. Pharmacal Res. 2010, 33, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Kaserer, T.; Steinacher, T.; Kainhofer, R.; Erli, F.; Sturm, S.; Waltenberger, B.; Schuster, D.; Spetea, M. Identification and characterization of plant-derived alkaloids, corydine and corydaline, as novel mu opioid receptor agonists. Sci. Rep. 2020, 10, 13804. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Qiu, X.; Wang, C.; Liu, Y.; Wang, Z.; Yu, Y.; Ye, R.D.; Zhang, Y. Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay. Molecules 2018, 23, 2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.Y.; Lee, H.; Kim, J.-H.; Kim, K.H.; Lee, K.R.; Shim, H.J.; Son, M.; Lee, H.S. In vitro metabolism of corydaline in human liver microsomes and hepatocytes using liquid chromatography-ion trap mass spectrometry. J. Sep. Sci. 2012, 35, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhassen, L.; Dabbous, T.; Ha, A.; Dang, L.H.L.; Civelli, O. The Analgesic Properties of Corydalis yanhusuo. Molecules 2021, 26, 7498. https://doi.org/10.3390/molecules26247498
Alhassen L, Dabbous T, Ha A, Dang LHL, Civelli O. The Analgesic Properties of Corydalis yanhusuo. Molecules. 2021; 26(24):7498. https://doi.org/10.3390/molecules26247498
Chicago/Turabian StyleAlhassen, Lamees, Travis Dabbous, Allyssa Ha, Leon Hoang Lam Dang, and Olivier Civelli. 2021. "The Analgesic Properties of Corydalis yanhusuo" Molecules 26, no. 24: 7498. https://doi.org/10.3390/molecules26247498
APA StyleAlhassen, L., Dabbous, T., Ha, A., Dang, L. H. L., & Civelli, O. (2021). The Analgesic Properties of Corydalis yanhusuo. Molecules, 26(24), 7498. https://doi.org/10.3390/molecules26247498




