Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions
Abstract
1. Introduction
2. Results and Discussion
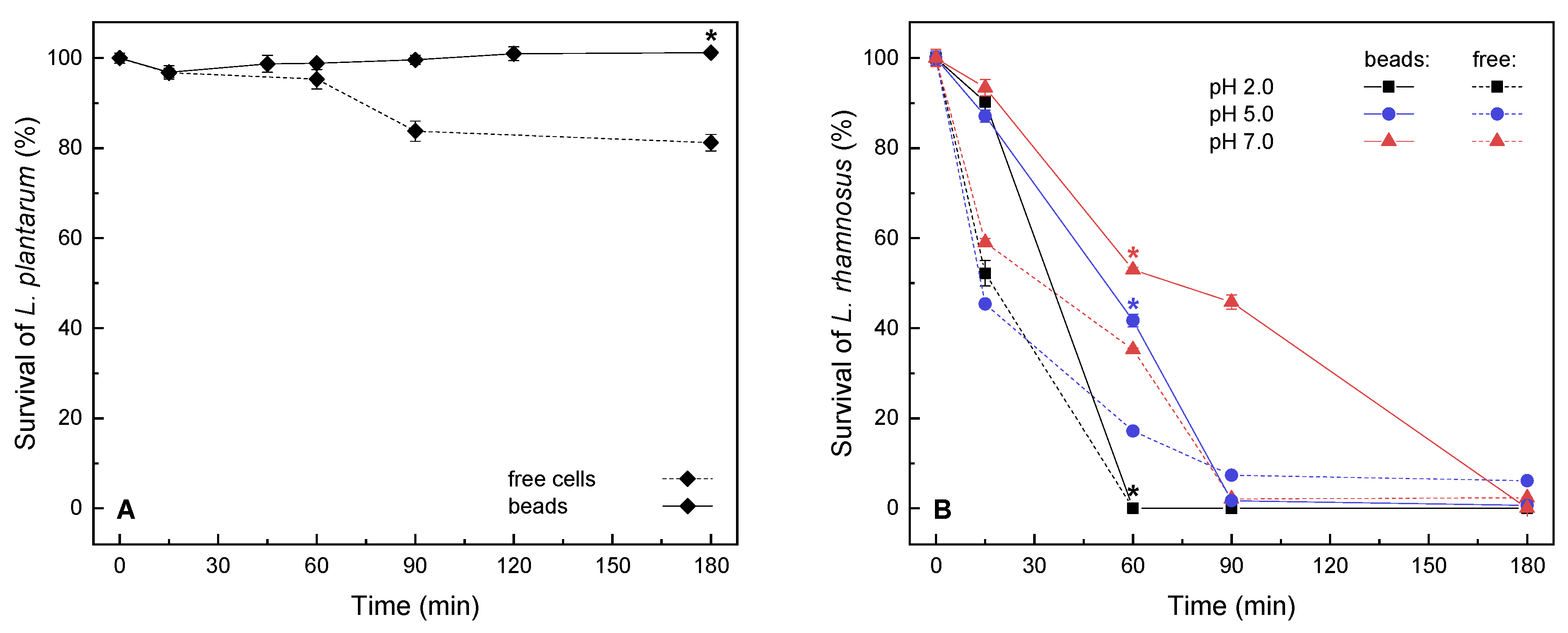
2.1. Short-Term Survival of Encapsulated Lactic Acid Bacteria
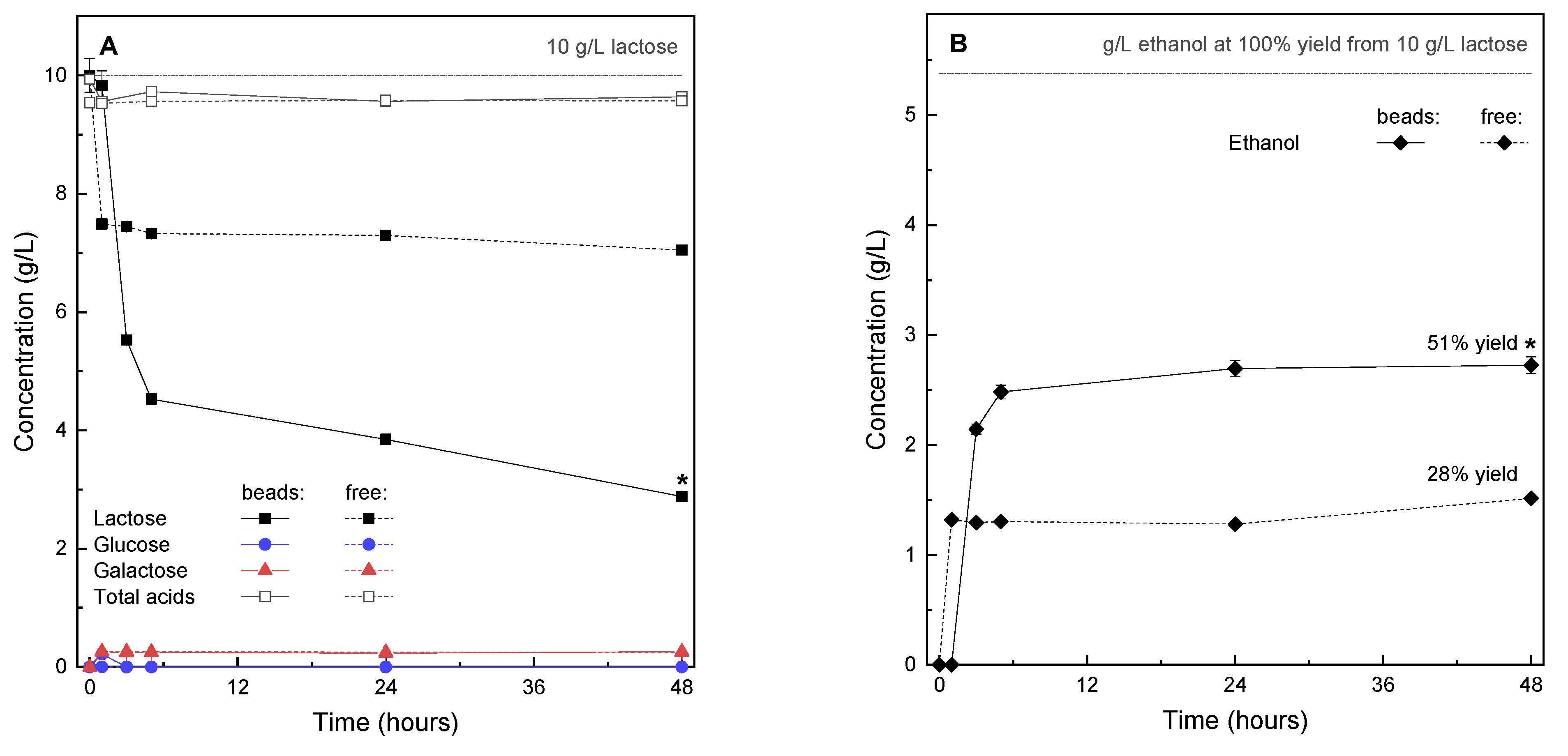
2.2. Fermentation Activity in Encapsulated Yeasts
3. Materials and Methods
3.1. Materials
3.2. Microorganisms and Initial Culture Media
3.3. Encapsulation
3.4. Survival of Free and Encapsulated Strains at Low pH
3.5. Total Viable Cell Counts
3.6. Microscopy
3.7. HPLC
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Mechanism for Development of Nanobased Drug Delivery System. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–67. [Google Scholar]
- Ojha, K.S.; Tiwari, B.K. Novel Food Fermentation Technologies. In Novel Food Fermentation Technologies, Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–5. [Google Scholar]
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast Immobilization Systems for Alcoholic Wine Fermentations: Actual Trends and Future Perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Sathyabama, S.; Ranjith kumar, M.; Bruntha devi, P.; Vijayabharathi, R.; Brindha priyadharisini, V. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT-Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Gaudana, S.B.; Dhanani, A.S.; Bagchi, T. Probiotic attributes of lactobacillus strains isolated from food and of human origin. Br. J. Nutr. 2010, 103, 1620–1628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Ashwar, B.A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Sun, C.; Gu, L.; Liu, Z.; Yu, W.; Chen, L.; Jiang, Z.; Hou, J. Effects of environmental stresses on the physiological characteristics, adhesion ability and pathogen adhesion inhibition of Lactobacillus plantarum KLDS 1.0328. Process Biochem. 2020, 92, 426–436. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of various encapsulating materials on the stability of probiotic bacteria. J. Food Sci. 2009, 74, M100–M107. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L.S. Preserving viability of Lactobacillus rhamnosus GG in vitro and in vivo by a new encapsulation system. J. Control. Release 2016, 230, 79–87. [Google Scholar] [CrossRef]
- Sandoval-Mosqueda, I.; Llorente-Bousquets, A.; Montiel-Sosa, J.F.; Corona, L.; Guadarrama-Álvarez, Z. Encapsulation of Lactobacillus plantarum ATCC 8014 and Pediococcus acidilactici ATCC 8042 in a freeze-dried alginate-gum arabic system and its in vitro testing under gastrointestinal conditions. J. Microencapsul. 2019, 36, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bostrom, T.; Bhandari, B. Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method. Int. J. Food Microbiol. 2011, 145, 162–168. [Google Scholar] [CrossRef]
- De Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Stamford Arnaud, T.M.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Campos-Montiel, R.G.; Lobato-Calleros, C.; Pedroza-Islas, R.; Vernon-Carter, E.J. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res. Int. 2009, 42, 292–297. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Jodat, V.; Sahebi, J.; Ataei, A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bhandari, B. The Viability of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM Following Double Encapsulation in Alginate and Maltodextrin. Food Bioprocess Technol. 2013, 6, 2763–2769. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 100679. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of lactobacillus casei, lactobacillus plantarum and lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Tee, W.F.; Nazaruddin, R.; Tan, Y.N.; Ayob, M.K. Effects of encapsulation on the viability of potential probiotic Lactobacillus plantarum exposed to high acidity condition and presence of bile salts. Food Sci. Technol. Int. 2014, 20, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequencing-Based Analysis of the Bacterial and Fungal Composition of Kefir Grains and Milks from Multiple Sources. PLoS ONE 2013, 8, e69371. [Google Scholar] [CrossRef]
- Baruzzi, F.; Quintieri, L.; Caputo, L.; Cocconcelli, P.S.; Borcakli, M.; Owczarek, L.; Jasińska, U.T.; Skąpska, S.; Morea, M. Improvement of Ayran quality by the selection of autochthonous microbial cultures. Food Microbiol. 2016, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Kok-Tas, T.; Greene, A.K.; Seydim, A.C. Review: Functional properties of kefir. Crit. Rev. Food Sci. Nutr. 2011, 51, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Vardjan, T.; Mohar Lorbeg, P.; Rogelj, I.; Čanžek Majhenič, A. Characterization and stability of lactobacilli and yeast microbiota in kefir grains. J. Dairy Sci. 2013, 96, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Grishina, A.; Kulikova, I.; Alieva, L.; Dodson, A.; Rowland, I.; Jin, J. Antigenotoxic effect of kefir and ayran supernatants on fecal water-induced DNA damage in human colon cells. Nutr. Cancer 2011, 63, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Steels, H.; Novodvorska, M.; Archer, D.B.; Avery, S.V. Extreme osmotolerance and halotolerance in food-relevant yeasts and the role of glycerol-dependent cell individuality. Front. Microbiol. 2019, 9, 3238. [Google Scholar] [CrossRef]
- Beshkova, D.M.; Simova, E.D.; Simov, Z.I.; Frengova, G.I.; Spasov, Z.N. Pure cultures for making kefir. Food Microbiol. 2002, 19, 537–544. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Florez-Rojas, J.S.; Pradilla, D.; Valderrama-Rincón, J.D.; Cruz, J.C.; Reyes, L.H. Formulation and characterization of gelatin-based hydrogels for the encapsulation of kluyveromyces lactis-Applications in packed-bed reactors and probiotics delivery in humans. Polymers 2020, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Patarroyo, J.L.; Fonseca, E.; Cifuentes, J.; Salcedo, F.; Cruz, J.C.; Reyes, L.H. Gelatin-graphene oxide nanocomposite hydrogels for kluyveromyces lactis encapsulation: Potential applications in probiotics and bioreactor packings. Biomolecules 2021, 11, 922. [Google Scholar] [CrossRef]
- Gabardo, S.; Pereira, G.F.; Rech, R.; Ayub, M.A.Z. The modeling of ethanol production by Kluyveromyces marxianus using whey as substrate in continuous A-Stat bioreactors. J. Ind. Microbiol. Biotechnol. 2015, 42, 1243–1253. [Google Scholar] [CrossRef]
- Methner, Y.; Hutzler, M.; Matoulková, D.; Jacob, F.; Michel, M. Screening for the brewing ability of different non-Saccharomyces yeasts. Fermentation 2019, 5, 101. [Google Scholar] [CrossRef]
- Barranco-Florido, E.; García-Garibay, M.; Gómez-Ruiz, L.; Azaola, A. Immobilization system of Kluyveromyces marxianus cells in barium alginate for inulin hydrolysis. Process Biochem. 2001, 37, 513–519. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Díaz Vergara, L.I.; Rossi, Y.E.; Aminahuel, C.A.; Mauri, A.N.; Cavaglieri, L.R.; Montenegro, M.A. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces marxianus VM004 during storage and in simulated gastrointestinal conditions. LWT 2020, 118, 108844. [Google Scholar] [CrossRef]
- Güneşer, O.; Karagül-Yüceer, Y.; Wilkowska, A.; Kregiel, D. Volatile metabolites produced from agro-industrial wastes by Na-alginate entrapped Kluyveromyces marxianus. Braz. J. Microbiol. 2016, 47, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vergara, L.; Pereyra, C.M.; Montenegro, M.; Pena, G.A.; Aminahuel, C.A.; Cavaglieri, L.R. Encapsulated whey–native yeast Kluyveromyces marxianus as a feed additive for animal production. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, H.; Servetas, Y.; Bosnea, L.A.; Kanellaki, M.; Koutinas, A.A. Novel technology development through thermal drying of encapsulated kluyveromyces marxianus in micro- and nano-tubular cellulose in lactose fermentation and its evaluation for food production. Appl. Biochem. Biotechnol. 2012, 168, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Kregiel, D.; Guneser, O.; Karagul Yuceer, Y. Growth and by-product profiles of Kluyveromyces marxianus cells immobilized in foamed alginate. Yeast 2015, 32, 217–225. [Google Scholar] [CrossRef]
- Becerra, M.; Baroli, B.; Fadda, A.M.; Blanco Méndez, J.; González Siso, M.I. Lactose bioconversion by calcium-alginate immobilization of Kluyveromyces lactis cells. Enzyme Microb. Technol. 2001, 29, 506–512. [Google Scholar] [CrossRef]
- Tomaska, M.; Gemeiner, P.; Materlin, I.; Sturdik, E.; Handrikova, G. Calcium pectate gel beads for cell entrapment: A study on the stability of Kluyveromyces marxianus whole-cell lactase entrapped in hardened calcium pectate and calcium alginate gels. Biotechnol. Appl. Biochem. 1995, 21, 347–356. [Google Scholar]
- Marieb, E.N.; Hoehn, K.N. Human Anatomy & Physiology, 11th ed.; Pearson: Harlow, UK, 2019; ISBN 9780134580999. [Google Scholar]
- Osojnik Črnivec, I.G.; Istenič, K.; Skrt, M.; Poklar Ulrih, N. Thermal protection and pH-gated release of folic acid in microparticles and nanoparticles for food fortification. Food Funct. 2020, 11, 1467–1477. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, X.; Huang, R.; Tao, X.; Shah, N.P.; Wei, H.; Wan, C. A physiological comparative study of acid tolerance of Lactobacillus plantarum ZDY 2013 and L. plantarum ATCC 8014 at membrane and cytoplasm levels. Ann. Microbiol. 2017, 67, 669–677. [Google Scholar] [CrossRef]
- Mirlohi, M.; Soleimanian-Zad, S.; Dokhani, S.; Sheikh-Zeinodin, M.; Abghary, A. Investigation of acid and bile tolerance of native lactobacilli isolated from fecal samples and commercial probiotics by growth and survival studies. Iran. J. Biotechnol. 2009, 7, 233–240. [Google Scholar]
- Rogelj, I.; Bogovič Matijašić, B.; Hacin, B.; Čitar, M.; Štempelj, M.; Švigelj, K. Method for the Isolation and Selection of Bacterial Strains, the Bacterial Strains, and the Method of Their Use. Patent SI24543 (A), 29 May 2015. [Google Scholar]
- Fuochi, V.; Petronio, G.P.; Lissandrello, E.; Furneri, P.M. Evaluation of resistance to low pH and bile salts of human Lactobacillus spp. isolates. Int. J. Immunopathol. Pharmacol. 2015, 28, 426–433. [Google Scholar] [CrossRef]
- Hang, Y.D.; Woodams, E.E.; Hang, L.E. Utilization of corn silage juice by Klyuveromyces marxianus. Bioresour. Technol. 2003, 86, 305–307. [Google Scholar] [CrossRef]
- Hang, N.J. The rapid degradation of sauerkraut brine by free and immobilized yeast cells. Electron. J. Pol. Agric. Univ. Food Sci. Technol. 2003, 6. Available online: http://www.ejpau.media.pl/volume6/issue2/food/art-13.html (accessed on 10 November 2021).
- Wongson, D.D. Optimisation of Industrial Whey Ethanol Fermentation Process; Massey University: Palmerston North, New Zealand, 1993; 236p. [Google Scholar]
- Lo, S.C.; Yang, C.Y.; Mathew, D.C.; Huang, C.C. Growth and autolysis of the kefir yeast Kluyveromyces marxianus in lactate culture. Sci. Rep. 2021, 11, 14552. [Google Scholar] [CrossRef] [PubMed]
- Smetanková, J.; Hladíková, Z.; Valach, F.; Zimanová, M.; Kohajdová, Z.; Greif, G.; Greifová, M. Influence of aerobic and anaerobic conditions on the growth and metabolism of selected strains of Lactobacillus plantarum. Acta Chim. Slovaca 2018, 5, 204–210. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Köksoy, A.; Kiliç, M. Effects of water and salt level on rheological properties of ayran, a Turkish yoghurt drink. Int. Dairy J. 2003, 13, 835–839. [Google Scholar] [CrossRef]
- Odet, G. Fermented milks. Bull. IDF 1995, 300, 98–100. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
- Shah, V.P.; Lesko, L.J.; Fan, J.; Fleischer, N.; Handerson, J. Dissolution Testing of Immediate Release Solid Oral Dosage Forms. Dissolut. Technol. 1997, 4, 15–22. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osojnik Črnivec, I.G.; Neresyan, T.; Gatina, Y.; Kolmanič Bučar, V.; Skrt, M.; Dogša, I.; Bogovič Matijašić, B.; Kulikova, I.; Lodygin, A.; Poklar Ulrih, N. Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions. Molecules 2021, 26, 7484. https://doi.org/10.3390/molecules26247484
Osojnik Črnivec IG, Neresyan T, Gatina Y, Kolmanič Bučar V, Skrt M, Dogša I, Bogovič Matijašić B, Kulikova I, Lodygin A, Poklar Ulrih N. Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions. Molecules. 2021; 26(24):7484. https://doi.org/10.3390/molecules26247484
Chicago/Turabian StyleOsojnik Črnivec, Ilja Gasan, Tigran Neresyan, Yuliana Gatina, Vid Kolmanič Bučar, Mihaela Skrt, Iztok Dogša, Bojana Bogovič Matijašić, Irina Kulikova, Aleksei Lodygin, and Nataša Poklar Ulrih. 2021. "Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions" Molecules 26, no. 24: 7484. https://doi.org/10.3390/molecules26247484
APA StyleOsojnik Črnivec, I. G., Neresyan, T., Gatina, Y., Kolmanič Bučar, V., Skrt, M., Dogša, I., Bogovič Matijašić, B., Kulikova, I., Lodygin, A., & Poklar Ulrih, N. (2021). Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions. Molecules, 26(24), 7484. https://doi.org/10.3390/molecules26247484











