Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica
Abstract
:1. Introduction
2. The Results and Discussion
2.1. Phenolic Yield and Total Phenolic Contents
2.2. Profile by UPLC-ESI-MS/MS Analysis
2.2.1. Hydroxycinnamic Acids and Derivatives
2.2.2. Flavonoids
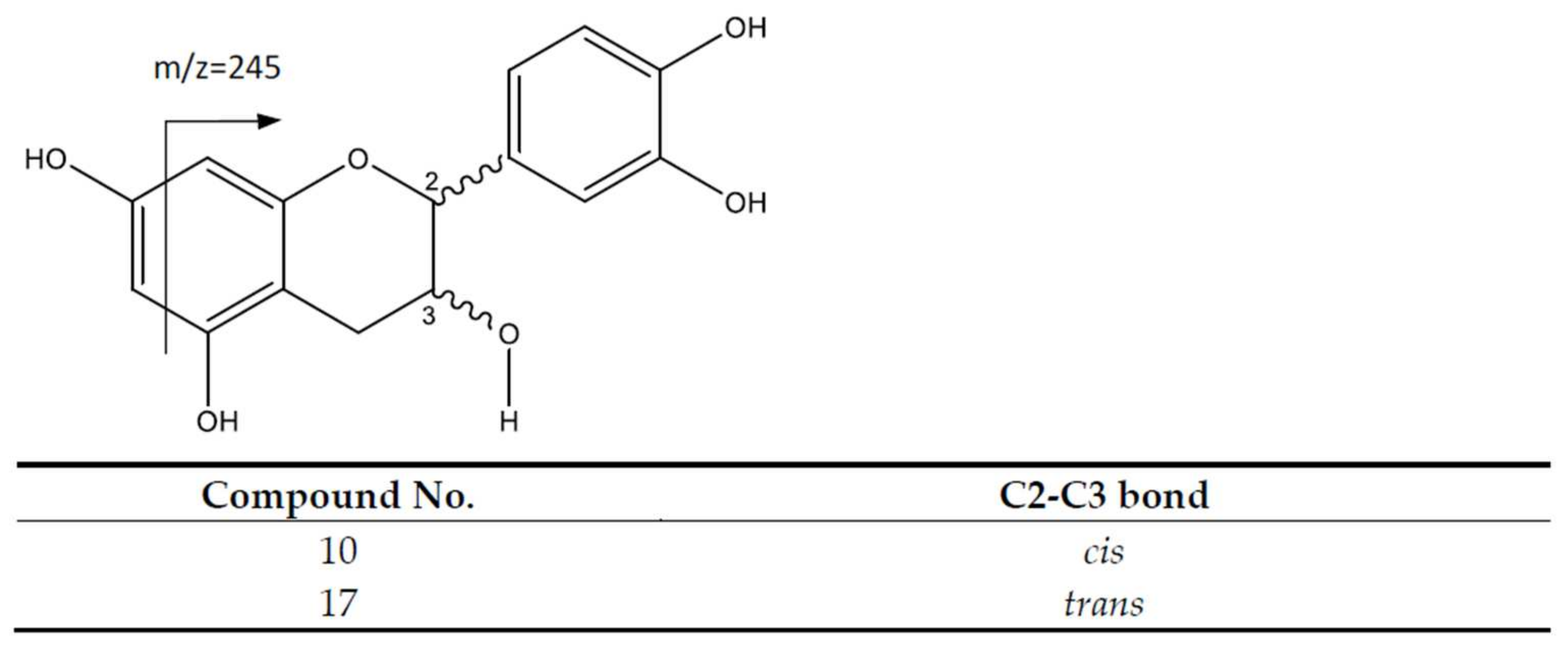
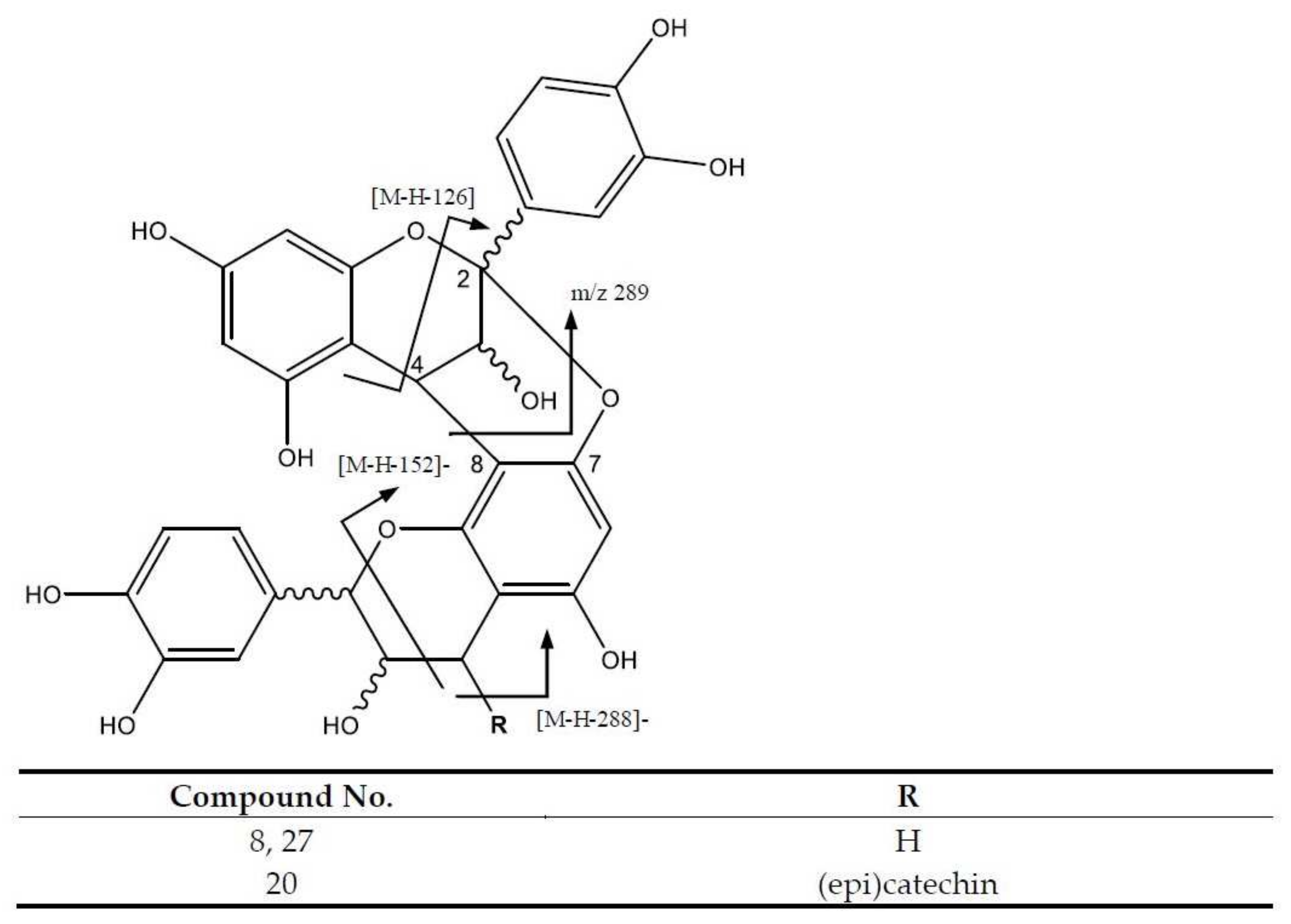
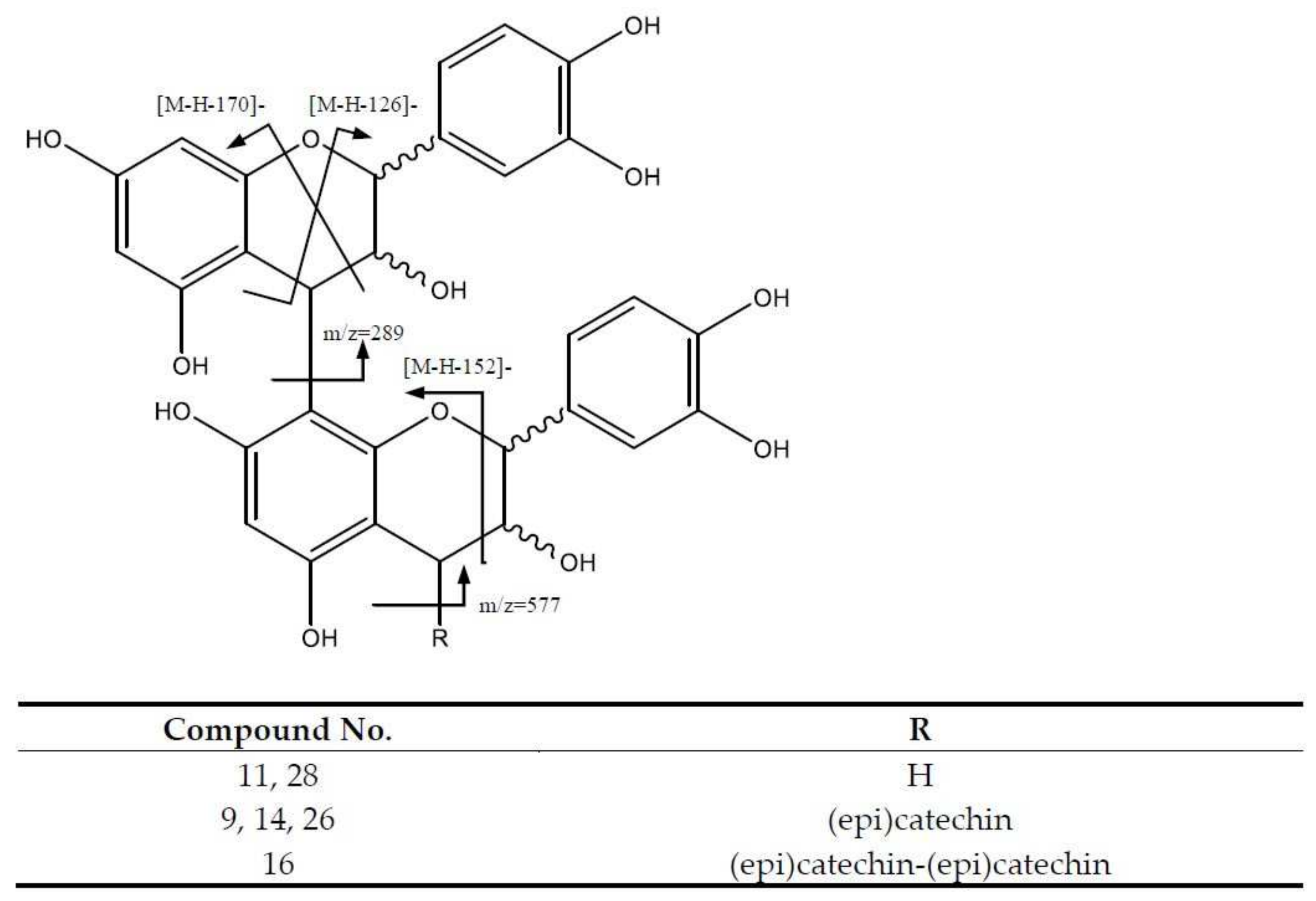
2.2.3. Flavan-3-ols Derivatives
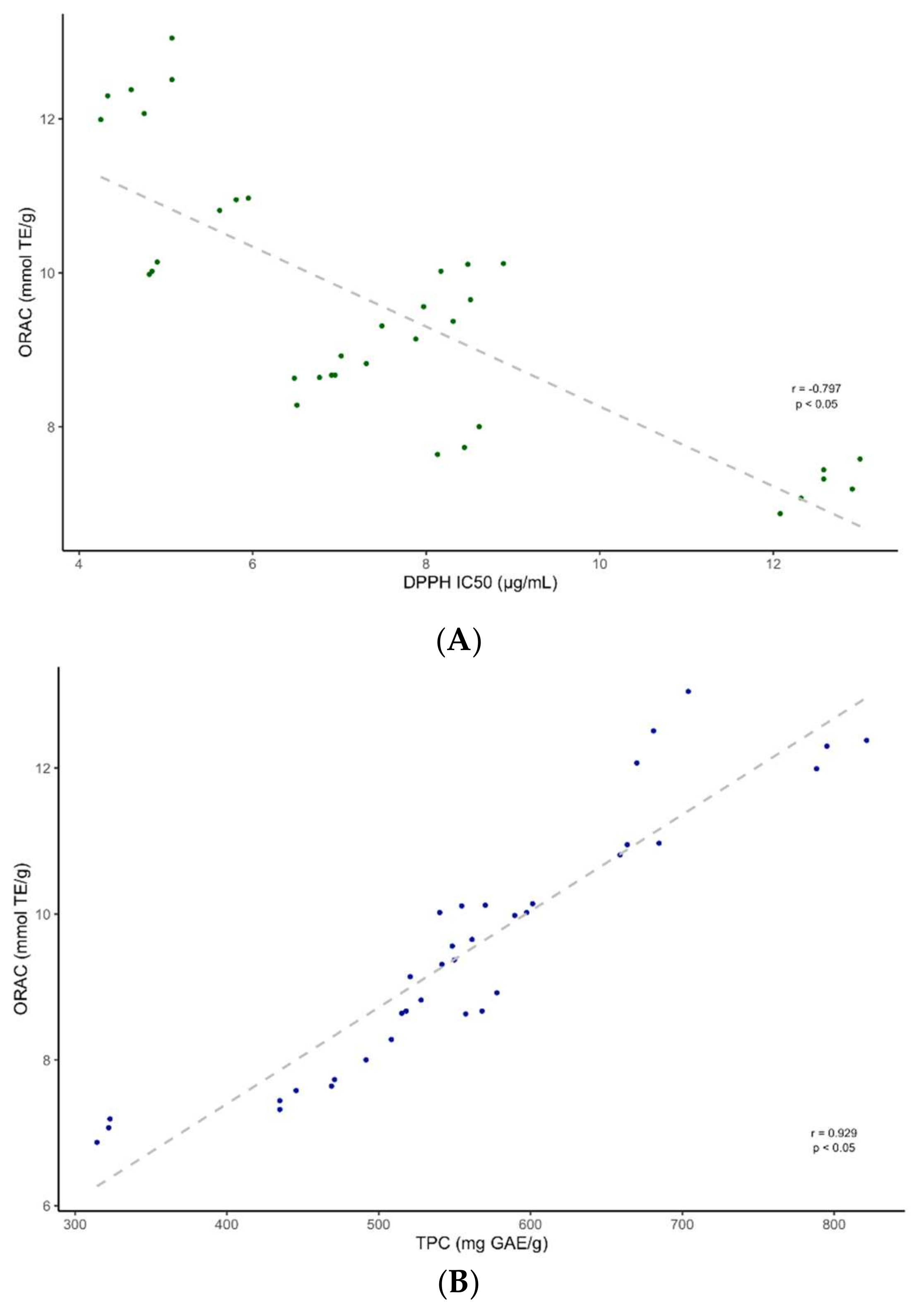
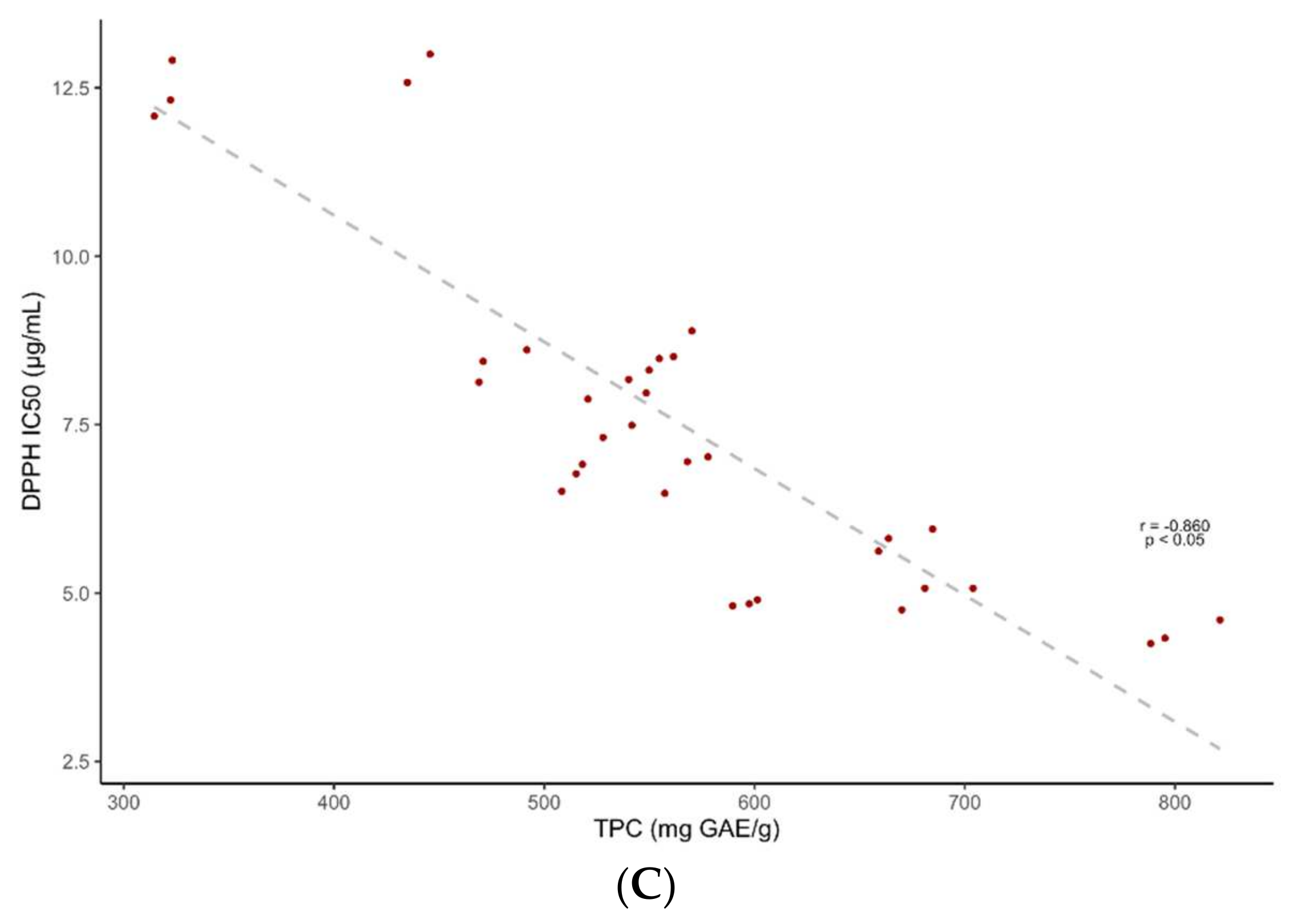
2.3. Antioxidant Activity
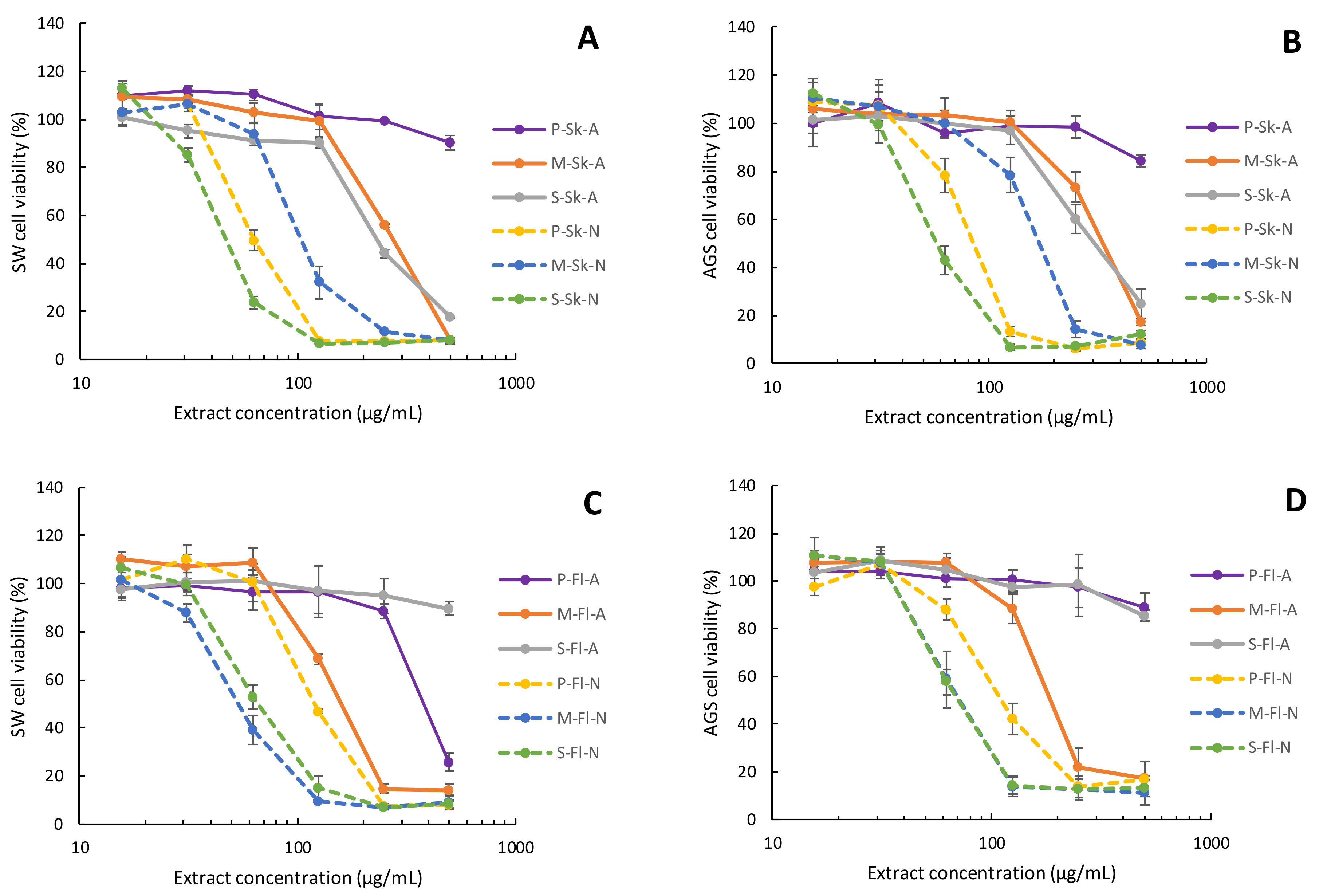
2.4. Cytotoxicity
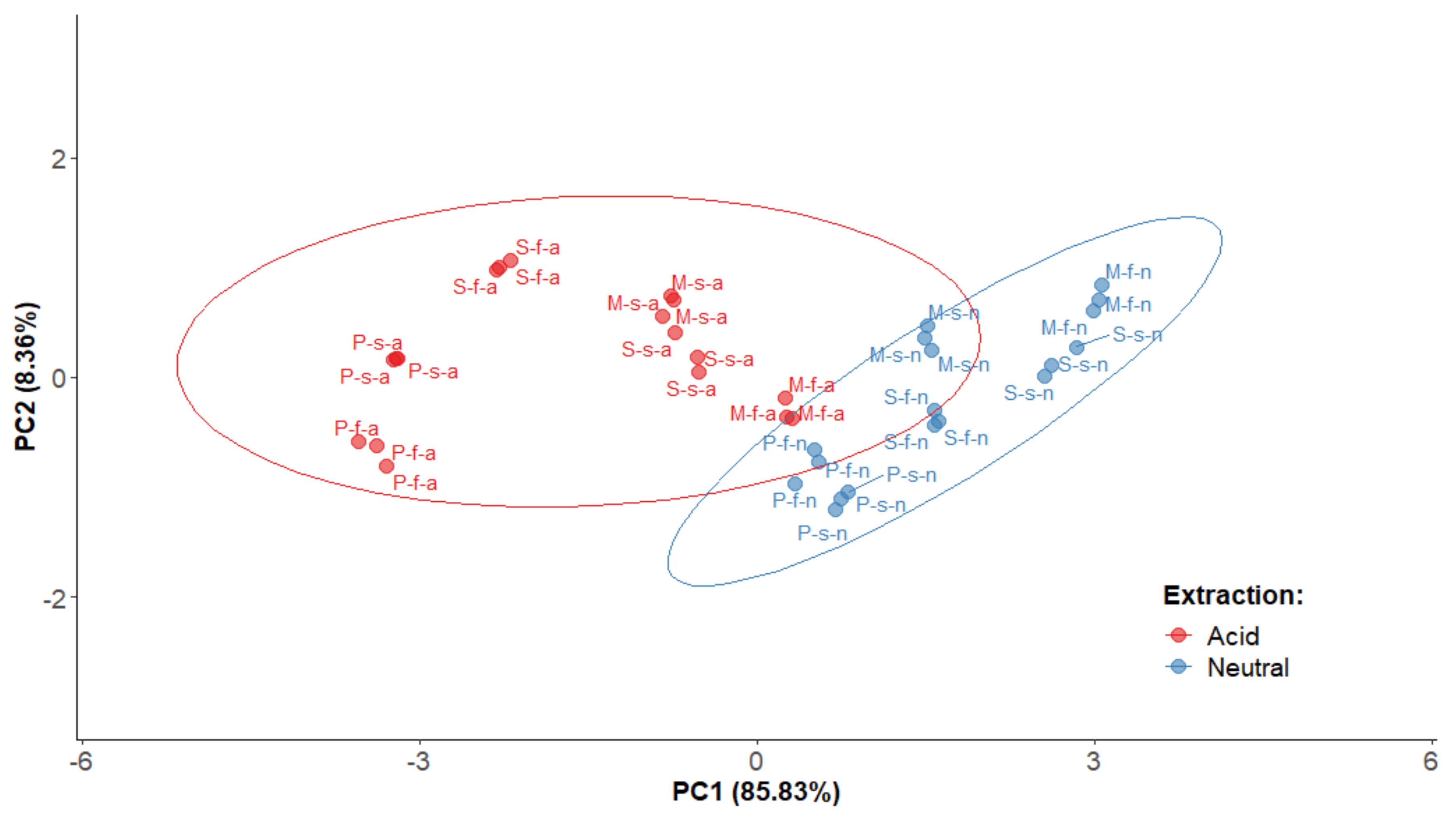
2.5. Principal Component Analysis for Polyphenolic Extracts of P. domestica
3. Materials and Methods
3.1. Materials, Reagents and Solvents
3.2. Phenolic Extracts from Prunus domestica Fruits
3.3. Total Phenolic Content
3.4. UPLC-ESI-MS Analysis
3.5. DPPH Radical-Scavenging Activity
3.6. ORAC Antioxidant Activity
3.7. Evaluation of the Cytotoxicity of the Extracts
3.7.1. Cell Culture
3.7.2. Assessment of the Cytotoxicity by the MTT Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kim, H.; Barnes, R.C.; Talcott, S.T.; Mertens-Talcott, S.U. Obesity-Associated Diseases Biomarkers Are Differently Modulated in Lean and Obese Individuals and Inversely Correlated to Plasma Polyphenolic Metabolites After 6 Weeks of Mango (Mangifera Indica L.) Consumption. Mol. Nutr. Food Res. 2018, 62, e1800129. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Karakose, H.; Ruhmann, S.; Goldner, K.; Neumuller, M.; Treutter, D.; Kuhnert, N. Identification of phenolic compounds in plum fruits (Prunus salicina L. and Prunus domestica L.) by HPLC/MS and characterization of varieties by quantitative phenolic fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M. Dried Plums and Their Products: Composition and Health Effects–An Updated Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.; Rühmann, S.; Neumüller, M. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. J Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P. Influence of Acidity and Extraction Time on the Recovery of Flavonoids from Grape Skin Pomace Optimized by Response Surface Methodology. Chem. Biochem. Eng. Q. J. 2017, 30, 455–464. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic characterization and antioxidant activity of malus domestica and prunus domestica cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, M.; Zaiter, A.; Modarressi, A.; Baudelaire, E.; Dicko, A. The positive impact of a new parting process on antioxidant activity, malic acid and phenolic content of Prunus avium L., Prunus persica L. and Prunus domestica subsp. Insititia L. powders. Microchem. J. 2019, 149, 103962. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Moon, H.Y.; Kang, H.G.; Lee, C.Y. Contribution of Individual Polyphenolics to Total Antioxidant Capacity of Plums. J. Agric. Food Chem. 2003, 51, 7240–7245. [Google Scholar] [CrossRef]
- DiNardo, A.; Subramanian, J.; Singh, A. Investigation of Antioxidant Content and Capacity in Yellow European Plums. Int. J. Fruit Sci. 2018, 18, 99–116. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Total Phenolic, Flavonoid Distribution and Antioxidant Capacity in Skin, Pulp and Fruit Extracts of Plum Cultivars. J. Food Biochem. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Melgarejo, P. Chemical, Functional and Quality Properties of Japanese Plum (Prunus salicina Lindl.) as Affected by Mulching. Sci. Hortic. 2012, 134, 114–120. [Google Scholar] [CrossRef]
- Vlaic, R.A.; Mureșan, V.; Mureșan, A.E.; Mureșan, C.C.; Păucean, A.; Mitre, V.; Chiș, S.M.; Muste, S. The Changes of Polyphenols, Flavonoids, Anthocyanins and Chlorophyll Content in Plum Peels during Growth Phases: From Fructification to Ripening. Not. Bot. Horti. Agrobo. 2017, 46, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Mocan, A.; Diuzheva, A.; Carradori, S.; Andruch, V.; Massafra, C.; Moldovan, C.; Sisea, C.; Petzer, J.P.; Petzer, A.; Zara, S.; et al. Development of novel techniques to extract phenolic compounds from Romanian cultivars of Prunus domestica L. and their biological properties. Food Chem. Toxicol. 2018, 119, 189–198. [Google Scholar] [CrossRef]
- Willems, J.L.; Khamis, M.M.; Mohammed Saeid, W.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef]
- de la Luz Cadiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Fujimoto, K.; Nakamura, S.; Matsumoto, T.; Ohta, T.; Yoshikawa, M.; Ogawa, K.; Kashiwazaki, E.; Matsuda, H. Structures of acylated sucroses from the flower buds of Prunus mume. J. Nat. Med. 2014, 68, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Ciric, A.; Prosen, H.; Jelikić-Stankov, M.; Đurđević, P. Evaluation of matrix effect in determination of some bioflavonoids in food samples by LC–MS/MS method. Talanta 2012, 99, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Regueiro, J.; Sánchez-Gonzalez, C.; Vallverdu-Queralt, A.; Simal-Gandara, J.; Lamuela-Raventos, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Cluzet, S.; Madani, K.; Pakina, E.; Gadzhikurbanov, A.; Mesnil, M.; Monvoisin, A.; Richard, T. HPLC-DAD-MS/MS profiling of phenolics from different varieties ofpeach leaves and evaluation of their antioxidant activity: A comparative study. Int. J. Mass Spectrom. 2019, 445, 116192. [Google Scholar] [CrossRef]
- Hammed, A.I.; Al-Ayed, A.S.; Moldoch, J.; Piacente, S.; Oleszek, W.; Stochmal, A. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun-an ancient Egyptian palm) by LC-ESI-MS/MS. J. Mass Spectrom. 2014, 49, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Alsolmei, F.A.; Li, H.; Pereira, S.L.; Krishnan, P.; Johns, P.W.; Siddiqui, R.A. Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells. Nutrients 2019, 11, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Beltagi, H.S.; El-Ansary, A.E.; Mostafa, M.A.; Kamel, T.A.; Safwat, G. Evaluation of the Phytochemical, Antioxidant, Antibacterial and Anticancer Activity of Prunus domestica Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Tomić, J.; Štampar, F.; Glišić, I.; Jakopič, J. Phytochemical assessment of plum (Prunus domestica L.) cultivars selected in Serbia. Food Chem. 2019, 299, 125113. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Zabaras, D.; Sze, D.M.-Y. Potential Antioxidant, Antiinflammatory, and Proapoptotic Anticancer Activities of Kakadu Plum and Illawarra Plum Polyphenolic Fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef]
- Arion, C.; Tabart, J.; Kevers, C.; Niculaua, M.; Filimon, R.; Beceanu, D.; Dommes, J. Antioxidant Potential of Different Plum Cultivars during Storage. Food Chem. 2014, 146, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Lizcano, L.J.; Bakkali, F.; Ruiz-Larrea, B.; Ruiz-Sanz, J.I. Antioxidant activity and polyphenol content of aqueous extracts from Colombia Amazonian plants with medicinal use. Food Chem. 2010, 119, 1566–1570. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; Koh, J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Muzzaffar, S. Plum Cultivars Grown in Himalayan Temperate Conditions: Physicochemical, Antioxidant and Antiproliferative Activity against Three Cancer Cell Lines. J. Food Meas. Charact. 2018, 12, 2247–2255. [Google Scholar] [CrossRef]
- Avelar, M.M. Procyanidin B2 Cytotoxicity to MCF-7 Human Breast Adenocarcinoma Cells. Indian J. Pharm. Sci. 2012, 74, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S.; Kingsley, K. Cranberry and Grape Seed Extracts Inhibit the Proliferative Phenotype of Oral Squamous Cell Carcinomas. Evid. Based Complement. Alternat. Med. 2011, 2011, 467691. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Agarwal, R.; Agarwal, C. Grape Seed Extract Induces Anoikis and Caspase-Mediated Apoptosis in Human Prostate Carcinoma LNCaP Cells: Possible Role of Ataxia Telangiectasia Mutated–P53 Activation. Mol. Cancer 2006, 5, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.-Y.; de Mejia, E.G.; Lila, M.A. Cytotoxicity of Bioactive Polymeric Fractions from Grape Cell Culture on Human Hepatocellular Carcinoma, Murine Leukemia and Non-Cancerous PK15 Kidney Cells. Food Chem. Toxicol. 2006, 44, 1758–1767. [Google Scholar] [CrossRef]
- Quesada, M.; Azofeifa, G.; Campone, L.; Pagano, I.; Perez, A.M.; Cortés, C.; Rastrelli, L.; Quesada, S. Bactris guineensis (Arecaceae) extract: Polyphenol characterization, antioxidant capacity and cytotoxicity against cancer cell lines. J. Berry Res. 2020, 10, 329–344. [Google Scholar] [CrossRef]
- Dinicola, S. Antiproliferative and Apoptotic Effects Triggered by Grape Seed Extract (GSE) versus Epigallocatechin and Procyanidins on Colon Cancer Cell Lines. Int. J. Mol. Sci. 2012, 14, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, S.; Wagner, W.; Podsedek, A.; Szewczyk, K.; Koziołkiewicz, M.; Dastych, J. Procyanidins From Japanese Quince (Chaenomeles Japonica) Fruit Induce Apoptosis in Human Colon Cancer Caco-2 Cells in a Degree of Polymerization-Dependent Manner. Nutr. Cancer 2011, 63, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-P.; Lin, Y.-H.; Chou, C.-C.; Zhou, S.-P.; Hsu, Y.-C.; Liu, C.-L.; Ku, F.-M.; Chung, Y.-C. Mechanisms of Grape Seed Procyanidin-Induced Apoptosis in Colorectal Carcinoma Cells. Anticancer Res. 2009, 9, 283–290. [Google Scholar]
- Kaur, M.; Singh, R.P.; Gu, M.; Agarwal, R.; Agarwal, C. Grape Seed Extract Inhibits In Vitro and In Vivo Growth of Human Colorectal Carcinoma Cells. Clin. Cancer Res. 2006, 12, 6194–6202. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Mandair, R.; Agarwal, R.; Agarwal, C. Grape Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Colon Carcinoma Cells. Nutr. Cancer 2008, 60, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnesecchi, S.; Schneider, Y.; Lazarus, S.A.; Coehlo, D.; Gosse, F.; Raul, F. Flavanols and Procyanidins of Cocoa and Chocolate Inhibit Growth and Polyamine Biosynthesis of Human Colonic Cancer Cells. Cancer Lett. 2002, 175, 147–155. [Google Scholar] [CrossRef]
- Navarro, M.; Zamora, W.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M. Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship. Antioxidants 2017, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Mahavorasirikul, W. Cytotoxic Activity of Thai Medicinal Plants against Human Cholangiocarcinoma, Laryngeal and Hepatocarcinoma Cells in Vitro. BMC Complement. Altern. Med. 2010, 28, 55. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L. Blueberry Phytochemicals Inhibit Growth and Metastatic Potential of MDA-MB-231 Breast Cancer Cells Through Modulation of the Phosphatidylinositol 3-Kinase Pathway. Cancer Res. 2010, 70, 3594–3605. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S.; Alia, M.; Bravo, L.; Goya, L. Comparative Effects of Food-Derived Polyphenols on the Viability and Apoptosis of a Human Hepatoma Cell Line (HepG2). J. Agric. Food Chem. 2005, 53, 1271–1280. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.Y.; Kong, Z.H.; Ding, D.G. Induction of Cell Cycle Arrest and Apoptosis by Grape Seed Procyanidin Extract in Human Bladder Cancer BIU87 Cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3282–3291. [Google Scholar]
- Navarro, M.; Sanchez, F.; Murillo, R.; Martín, P.; Zamora, W.; Monagas, M.; Bartolomé, B. Phenolic assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, stem, bark and wood extracts. Molecules 2015, 20, 22703–22717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Arnaez, E.; Moreira, I.; Hurtado, A.; Monge, D.; Monagas, M. Polyphenolic Composition and Antioxidant Activity of Uncaria tomentosa Commercial Bark Products. Antioxidants 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Hernandez, B.; Garrido, I.; Martin-Álvarez, P.J.; Gomez, C.; Bartolomé, B. Quality assessment of commercial dietary antioxidant products from Vitis vinifera L. grape seeds. Nutr. Cancer 2005, 53, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
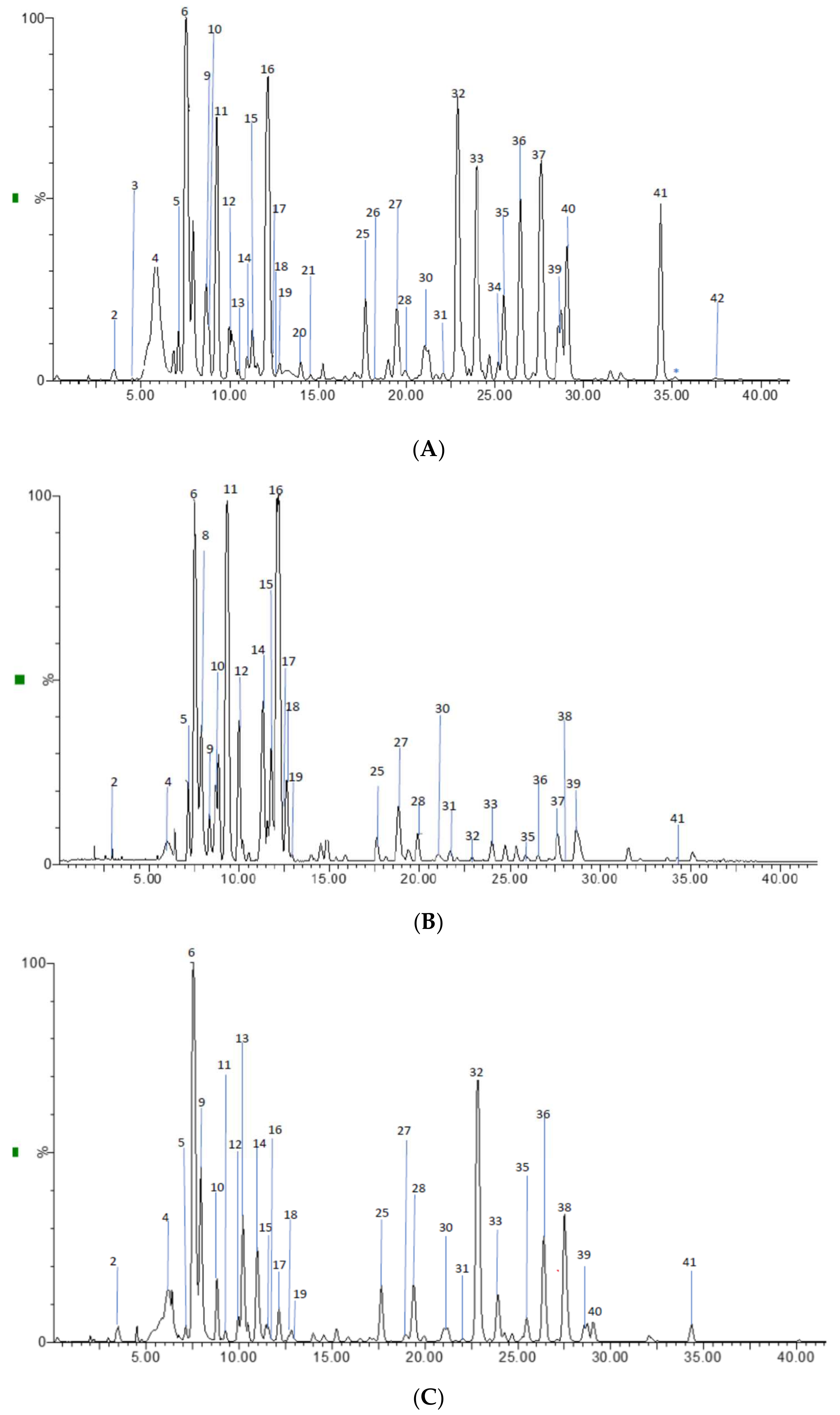
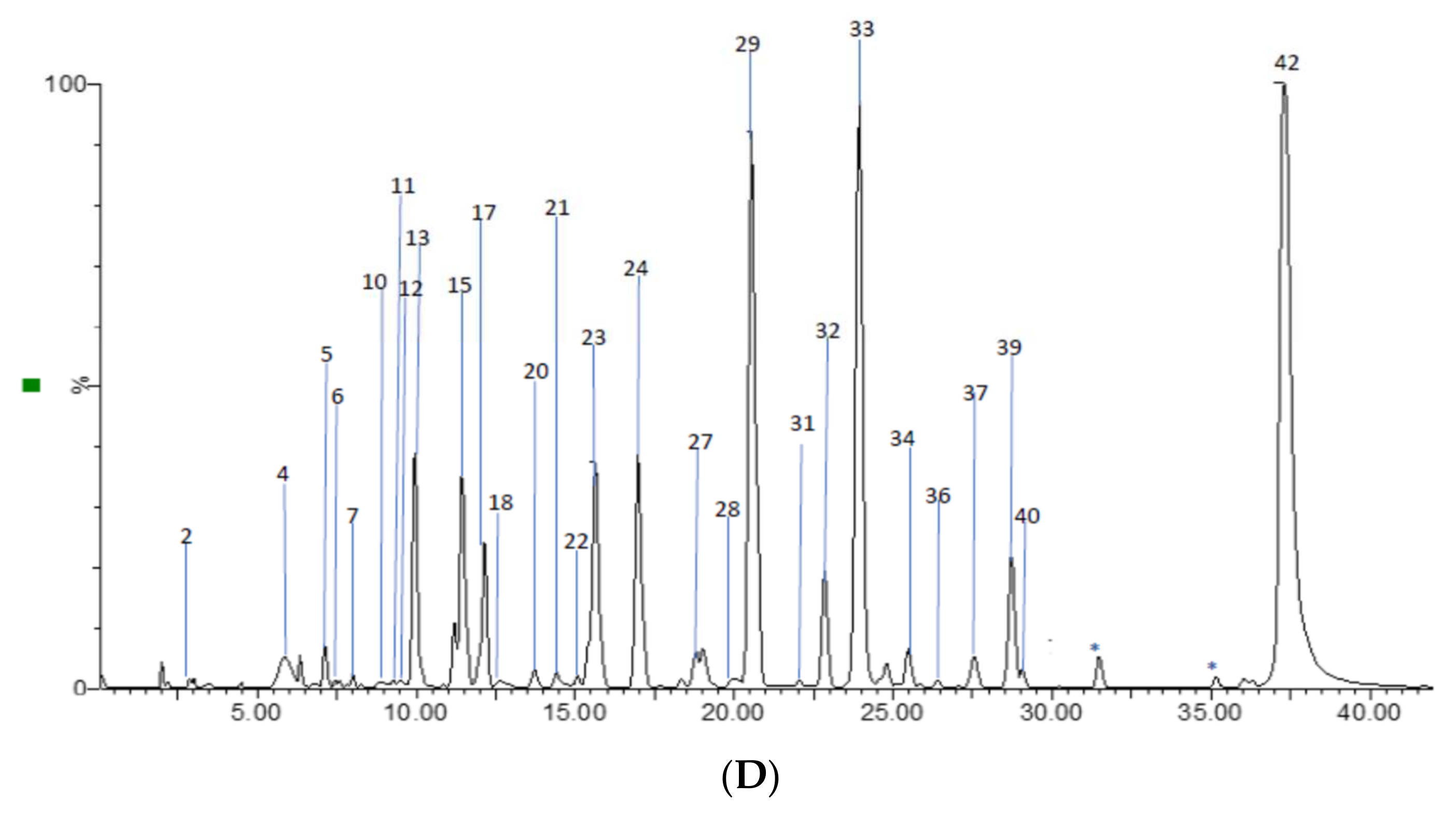
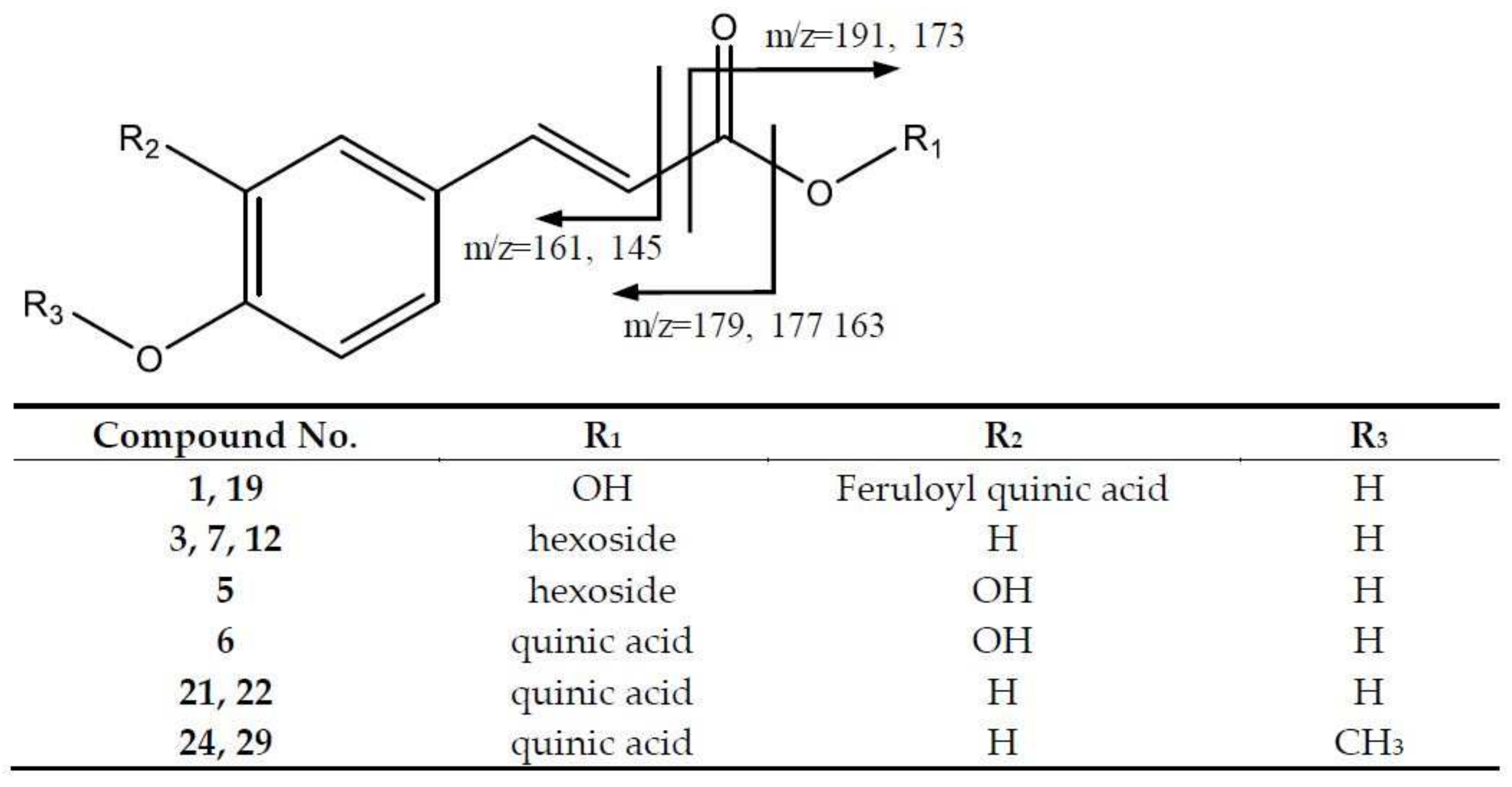
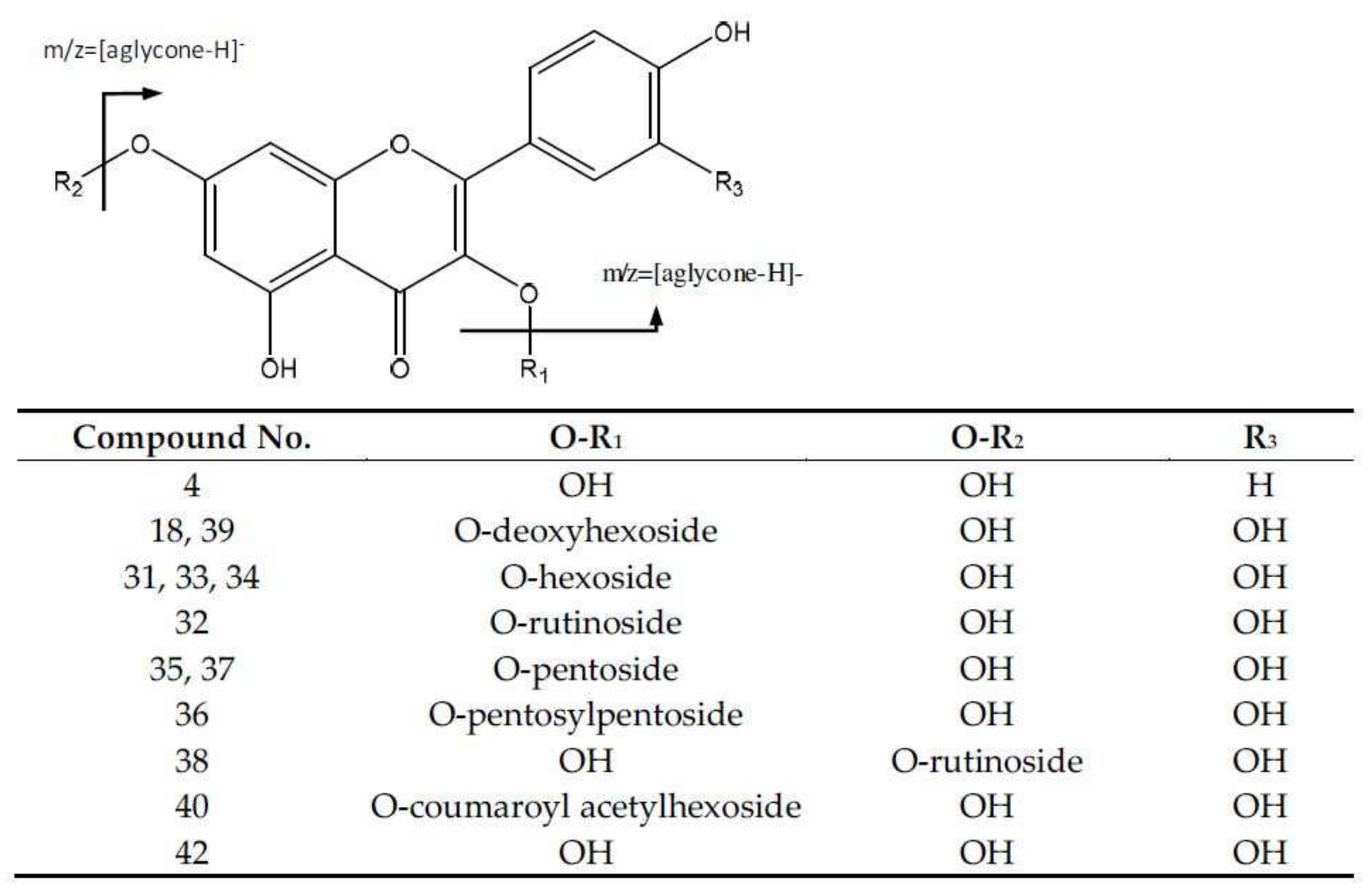
| Sample | Total Phenolic Content (TPC) (mg/g) 1,2,3 | |
|---|---|---|
| Acid Extraction | Neutral Extraction | |
| Methley | ||
| Skin | 539.3 a,* ± 8.55 | 669.1 a,# ± 11.2 |
| Flesh | 567.7 b,* ± 8.40 | 801.6 b,# ± 14.2 |
| Pisardii | ||
| Skin | 438.5 c,* ± 5.08 | 560.5 cd,# ± 8.34 |
| Flesh | 319.9 d,* ± 3.86 | 538.5 d,# ± 13.9 |
| Satsuma | ||
| Skin | 513.9 e,* ± 4.10 | 685.0 a,# ± 14.1 |
| Flesh | 477.2 f,* ± 10.3 | 596.1 c,# ± 4.89 |
| # | Tentative Identification | [M-H]− | tR (min) | MS2 Fragments | Formula |
|---|---|---|---|---|---|
| Hydroxycinnamic acids and derivatives | |||||
| 1 | Caffeoylferuloylquinic acid (I of II) | 529.1365 | 2.89 | [529]: 353, 367, 191, 179 | C26H25O12 |
| 3 | p-Coumaroyl hexoside (I of III) | 325.0917 | 4.42 | [325]: 145, 163, 187 | C15H17O8 |
| 5 | Caffeoyl hexoside | 341.0884 | 7.13 | [341]: 161, 179 | C15H17O9 |
| 6 | Caffeoylquinic acid isomer | 353.0869 | 7.56 | [353]: 191, 145 | C16H17O9 |
| 7 | p-Coumaroyl hexoside (II of III) | 325.0917 | 7.96 | [325]: 145, 163, 187 | C15H17O8 |
| 12 | p-Coumaroyl hexoside (III of III) | 325.0917 | 9.98 | [325]: 145 | C15H17O8 |
| 13 | Feruloylquinic acid isomer (I of III) | 367.1021 | 10.23 | [367]: 161, 134 | C17H19O9 |
| 15 | Feruloylquinic acid isomer (II of III) | 367.1021 | 11.45 | [367]: 193, 134 | C17H19O9 |
| 19 | Caffeoylferuloylquinic acid (II of II) | 529.1365 | 12.8 | [529]: 353, 367, 191, 179 | C26H25O12 |
| 21 | p-coumaroylquinic acid isomer (I of II) | 337.0919 | 14.78 | [337]:173 | C16H17O8 |
| 22 | p-coumaroylquinic acid isomer (II of II) | 337.0919 | 15.29 | [337]:173 | C16H17O8 |
| 23 | Feruloylquinic acid isomer (III of III) | 367.1021 | 15.65 | [367]: 193, 134 | C17H19O9 |
| 24 | Methyl-p-Coumaroylquinic acid (I of II) | 351.1082 | 16.99 | [351]: 177, 293, 235, 191, 133 | C17H19O8 |
| 25 | Di-O-acetyl-O-p-coumaroylsucrose (I of II) | 571.1675 | 17.44 | [571]: 553, 529, 511, 487, 307 | C25H31O15 |
| 29 | Methyl-p-Coumaroylquinic acid (II of II) | 351.1082 | 20.55 | [351]:177, 293, 235, 191, 133 | C17H19O8 |
| 30 | Di-O-acetyl-O-p-coumaroylsucrose (II of II) | 571.1675 | 21.03 | [571]: 529, 511, 307, 175 | C25H31O15 |
| Flavonoids | |||||
| 4 | Kaempferol-deoxyhexoside | 447.0913 | 5.89 | [447]: 300, 285 | C21H19O11 |
| 18 | Quercetin-deoxyhexoside (I of II) | 447.0913 | 12.57 | [447]: 300, 301 | C21H19O11 |
| 31 | Quercetin-hexoside (I of III) | 463.0901 | 21.95 | [463]: 300, 301 | C21H19O12 |
| 32 | Quercetin-rutinoside (I of II) | 609.1488 | 22.86 | [609]:300, 301 | C27H29O16 |
| 33 | Quercetin-hexoside (II of III) | 463.0875 | 23.89 | [463]: 300, 301 | C21H19O12 |
| 34 | Quercetin-hexoside (III of III) | 463.0875 | 25.46 | [463]: 300, 301 | C21H19O12 |
| 35 | Quercetin-pentoside (I of II) | 433.0764 | 25.95 | [433]: 300, 301 | C20H17O11 |
| 36 | Quercetin-pentosylpentoside | 565.1208 | 26.24 | [565]: 300, 301 | C25H25O15 |
| 37 | Quercetin-pentoside (II of II) | 433.0769 | 27.5 | [433]: 300, 301 | C20H17O11 |
| 38 | Quercetin-rutinoside isomer (II of II) | 609.1488 | 27.86 | [609]:300, 301 | C27H29O16 |
| 39 | Quercetin-deoxyhexoside (II of II) | 447.0913 | 28.68 | [447]: 300, 301 | C21H19O11 |
| 40 | Quercetin-acetylhexoside | 505.0974 | 29.09 | [505]: 300, 301 | C23H21O13 |
| 41 | Quercetin 3-O-p-coumaroyl acetylhexoside | 651.1553 | 34.27 | [651]: 633, 505, 487, 301, 300 | C32H27O15 |
| 42 | Quercetin | 301.0353 | 37.25 | [301]: 283, 273, 257, 255, 179, 151 | C15H9O7 |
| Flavan-3-ols | |||||
| 8 | Procyanidin dimer A (I of II) | 575.1183 | 8.05 | [575]: 285, 289, 423, 449 | C30H23O12 |
| 9 | Procyanidin B-type trimer (I of III) | 865.2014 | 8.28 | [865]: 287, 289, 575, 577, 695, 713, 739 | C45H37O18 |
| 10 | Catechin | 289.0704 | 8.81 | [289]: 245, 271 | C15H13O6 |
| 11 | Procyanidin B-type dimer (I of II) | 577.1332 | 9.27 | [577]: 287, 289, 407, 425, 451, 559 | C30H25O12 |
| 14 | Procyanidin B-type trimer (II of III) | 865.2014 | 11.25 | [865]: 287, 289, 575, 577, 695, 713, 739 | C45H37O18 |
| 16 | Procyanidin B-type tetramer | 1153.2579 | 11.86 | [1153]: 287, 289, 575, 577, 863, 865, 983, 1001, 1027, 1135 | C60H49O24 |
| 17 | Epicatechin | 289.0704 | 12.16 | [289]: 245, 271 | C15H13O6 |
| 20 | Procyanidin A-type trimer | 863.1790 | 13.71 | [863]: 287, 575, 711 | C45H35O18 |
| 26 | Procyanidin B-type trimer (III of III) | 865.1993 | 18.28 | [865]: 287, 289, 575, 577, 695, 713, 739 | C45H37O18 |
| 27 | Procyanidin A-type dimer | 575.1183 | 18.74 | [575]: 285, 289, 423, 449 | C30H23O12 |
| 28 | Procyanidin B-type dimer (II of II) | 577.1332 | 19.89 | [577]: 287, 289, 407, 425, 451, 559 | C30H25O12 |
| Other acids | |||||
| 2 | Quinic acid | 191.0554 | 3.08 | [191]: 173, 127 | C7H11O6 |
| Sample | DPPH IC50 (μg/mL) 1,2 | ORAC (mmol TE/g Extract)1,3 | ||
|---|---|---|---|---|
| Acid Extraction | Neutral Extraction | Acid Extraction | Neutral Extraction | |
| Methley | ||||
| Skin | 7.59 ab,* ± 0.28 | 5.79 a,# ± 0.13 | 9.23 a,^ ± 0.31 | 10.91 b,& ± 0.07 |
| Flesh | 6.81 bc,* ± 0.24 | 4.39 b,# ± 0.15 | 8.74 ab,^ ± 0.13 | 12.22 a,& ± 0.17 |
| Pisardii | ||||
| Skin | 12.72 d,* ± 0.20 | 8.57 c,# ± 0.24 | 7.45 cd,^ ± 0.11 | 9.71 c,& ± 0.31 |
| Flesh | 12.44 d,* ± 0.35 | 8.17 c,# ± 0.25 | 7.04 d,^ ± 0.13 | 9.76 c,& ± 0.44 |
| Satsuma | ||||
| Skin | 6.73 c,* ± 0.17 | 4.85 b,# ± 0.04 | 8.53 b,^ ± 0.18 | 12.55 a,& ± 0.40 |
| Flesh | 8.40 a,* ± 0.20 | 4.96 b,# ± 0.15 | 7.79 c,^ ± 0.15 | 10.05 bc,& ± 0.07 |
| Sample | AGS | SW620 | Vero |
| IC50 (μg/mL) 1,2 (SI) 3 | |||
| Acid Extraction | |||
| Methley | |||
| Skin | 403 a,* ± 13 (1.2) | 318 a,# ± 5 (1.6) | >500 a,& |
| Flesh | 196 b,* ± 9 (2.6) | 161 b,# ± 5 (3.1) | >500 a,& |
| Pisardii | |||
| Skin | >500c | >500 c | >500 a |
| Flesh | >500 c,* | 387 d,# ± 24 (1.3) | >500 a,* |
| Satsuma | |||
| Skin | 340 d,* ± 29 (1.5) | 255 e,# ± 16 (2.0) | >500 a,& |
| Flesh | >500 c | >500 c | >500 a |
| AGS | SW620 | Vero | |
| IC50 (μg/mL) 1,2 (SI) 3 | |||
| Neutral Extraction | |||
| Methley | |||
| Skin | 186 a,* ± 7 (2.7) | 122 a,# ± 7 (4.1) | >500 a,& |
| Flesh | 76.3 b,* ± 6.8 (6.6) | 60.9 bc,* ± 4.1 (8.2) | >500 a,# |
| Pisardii | |||
| Skin | 83.1 b,* ± 5.2 (6.0) | 67.9 b,# ± 2.4 (7.4) | >500 a,& |
| Flesh | 125 c,* ± 10 (4.0) | 132 a,* ± 3 (3.8) | >500 a,# |
| Satsuma | |||
| Skin | 60.7 b,* ± 4.6 (6.2) | 46.7 c,* ± 1.5 (8.1) | 378 b,# ± 33 |
| Flesh | 75.0 b,* ± 5.1 (6.6) | 73.0 b,* ± 4.1 (6.8) | >500 a,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules 2021, 26, 6493. https://doi.org/10.3390/molecules26216493
Navarro-Hoyos M, Arnáez-Serrano E, Quesada-Mora S, Azofeifa-Cordero G, Wilhelm-Romero K, Quirós-Fallas MI, Alvarado-Corella D, Vargas-Huertas F, Sánchez-Kopper A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules. 2021; 26(21):6493. https://doi.org/10.3390/molecules26216493
Chicago/Turabian StyleNavarro-Hoyos, Mirtha, Elizabeth Arnáez-Serrano, Silvia Quesada-Mora, Gabriela Azofeifa-Cordero, Krissia Wilhelm-Romero, María Isabel Quirós-Fallas, Diego Alvarado-Corella, Felipe Vargas-Huertas, and Andrés Sánchez-Kopper. 2021. "Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica" Molecules 26, no. 21: 6493. https://doi.org/10.3390/molecules26216493
APA StyleNavarro-Hoyos, M., Arnáez-Serrano, E., Quesada-Mora, S., Azofeifa-Cordero, G., Wilhelm-Romero, K., Quirós-Fallas, M. I., Alvarado-Corella, D., Vargas-Huertas, F., & Sánchez-Kopper, A. (2021). Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules, 26(21), 6493. https://doi.org/10.3390/molecules26216493







