Abstract
Chalcones are secondary metabolites belonging to the flavonoid (C6-C3-C6 system) family that are ubiquitous in edible and medicinal plants, and they are bioprecursors of plant flavonoids. Chalcones and their natural derivatives are important intermediates of the flavonoid biosynthetic pathway. Plants containing chalcones have been used in traditional medicines since antiquity. Chalcones are basically α,β-unsaturated ketones that exert great diversity in pharmacological activities such as antioxidant, anticancer, antimicrobial, antiviral, antitubercular, antiplasmodial, antileishmanial, immunosuppressive, anti-inflammatory, and so on. This review provides an insight into the chemistry, biosynthesis, and occurrence of chalcones from natural sources, particularly dietary and medicinal plants. Furthermore, the pharmacological, pharmacokinetics, and toxicological aspects of naturally occurring chalcone derivatives are also discussed herein. In view of having tremendous pharmacological potential, chalcone scaffolds/chalcone derivatives and bioflavonoids after subtle chemical modification could serve as a reliable platform for natural products-based drug discovery toward promising drug lead molecules/drug candidates.
1. Introduction
Chalcones (or 1,3-diaryl-2-propen-1-ones) are one of the major secondary metabolites of plants belonging to the flavonoid family. These metabolites are abundantly present in edible plants. A majority of naturally occurring chalcones is polyhydroxylated aromatic compounds, and they are considered the bioprecursors of open chain flavonoids, flavonoids, and isoflavonoids. Due to the presence of phenolic groups, chalcones have a radical quenching property, which has created interest among researchers to investigate chalcone-rich plant extracts in search for therapeutically useful compounds. The therapeutic applications of chalcones have been associated since time immemorial for the treatment of different diseases []. Chalconarngenin, phloretin, and its glucosidephloridzin (phloretin 2′-O-glucose) are some of the most common chalcones present in food [].
Chalcones and their structural analogues, either natural or synthetic, are known to exhibit diverse therapeutic and pharmacological activities such as antioxidant, anti-inflammatory, antiplasmodial (antimalarial), antileishmanial, antitubercular, antimicrobial, antiviral, anticancer, modulation of P-glycoprotein (P-gp) mediated multi-drug resistance, and immunosupportive potential. Studies have revealed that compounds with a chalcone-based structure and/or chalcone template also show a profound pharmacological influence on the cardiovascular, cerebrovascular, and neurovascular systems. Some chalcones have been associated with anti-peptic ulcer and antihypertensive activities. Some other activities have been also reported as anti-spasmodic, tranquilizing, analgesic, sedative, anti-thrombic, vasodilatory, estrogenic, anesthetic, anti-coagulating, anti-convulsant, and diuretic activities. Moreover, chalcones are considered as important pharmacophores of various bioactive natural products and therefore display a variety of biological potential. Representative examples of naturally occurring bioactive chalcones are cardamonin, a hydroxychalcone isolated from a Zingiberous plant species, which possesses antimutagenic, vasorelaxant, and anti-inflammatory properties, and xanthohumol, the principal prenylated flavonoid of the hop plant, which is characterized as a broad-spectrum cancer chemopreventing agent in vitro [,].
This review aims to discuss detailed aspects of naturally occurring chalcones including their biosynthesis, chemistry, and spectrum of bioactivities. In addition, this review also highlights the bioavailability issues associated with natural chalcones, along with pharmacokinetics and toxicities. All the relevant databases available in electronic search engines such as Web of Science, ScienceDirect, Pubmed, and Scopus were explored to collect relevant information for the terms such as chalcones, natural and dietary chalcones, chalcone derivatives, pharmacological activities, and the bioavailability or pharmacokinetics of naturally occurring chalcones.
2. Chalcone: Structure, Nomenclature, and Chemistry
Chalcone is a vital intermediate substance in the biosynthetic pathway of flavonoids. The term chalcone was coined by Kostanecki and Tomar who first demonstrated chalcone as banzalacetophenone or benzylidene acetophenone []. In recent years, the chemistry and synthesis of chalcone-based bioactive molecules have become an interesting area of research in the field of medicinal chemistry and drug discovery for their potential as a good structural synthon with wide molecular diversity (natural as well as synthetic) and having an array of biodynamic or pharmacological activities.
2.1. Chemical Structure
Chalcones are α,β-unsaturated ketones containing a reactive ketoethylenic group i.e., –CO-CH=CH-. These compounds are also known as benzalacetophenone or benzylidene acetophenone. Chemically, chalcones are 1,3-diaryl-2-propen-1-one, in which two aromatic rings are linked by an aliphatic three-carbon α,β-unsaturated carbonyl system (Figure 1a). Chalcones possess conjugated double bonds and a completely delocalized π-electron system on both benzene rings. They constitute the skeleton of open-chain flavonoids in which the three-carbon aliphatic system is used as an adjunct between two aromatic rings A and B []. Chalcones are small, low molecular weight (in the range of 300–600 g/mol), non-chiral molecules with relatively high lipophilicity (Log P ≈ 5–7). As a result of the presence of the chromophore -CO-CH=CH-, chalcones are colored compounds. Chalcones may exist as either cis (E, 1) or trans (Z, 2) isomeric forms. The trans form is thermodynamically more stable than the cis form [].
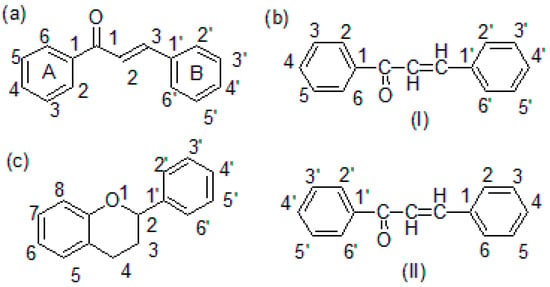
Figure 1.
(a) Chalcone structure, (b) chalcone nomenclature (I and II), and (c) flavonoid skeleton.
2.2. Nomenclature
Chalcone or chalconoid is an enone and an aromatic ketone, which forms the central core for several important biological compounds. Benzylideneacetophenone is the parent member of the chalcone series. The alternative names given to chalcone are benzalacetophenone, phenyl styryl ketone γ-oxo-α,γ-diphenyl-α-propylene, α-phenyl-β-benzoylethylene, and β-phenylacrylophenone. Different methods of nomenclatures for chalcone are available. The following two nomenclatures have been adopted by the “Chemical Abstracts” published by American Chemical Society (I) and the British Chemical Abstract and Journal of Chemical Society (II) (Figure 1b). The IUPAC name of chalcone is 1,3-diphenyl-2-propen-1-one [].
2.3. Occurrence of Chalcones
Chalcones are secondary plant metabolites, belonging to the flavonoid family that are abundantly present in edible plants, particularly fruits and vegetables. Therefore, chalcones belong to an important class of plant flavonoids (C6-C3-C6 system) (Figure 1c). Chalcones and their derivatives are important intermediates of the flavonoid biosynthetic pathway. Flavonoids are an important group of naturally occurring bioactive compounds. The majority of naturally occurring chalcones are polyhydroxylated aromatic compounds abundantly found in fruits, grains, legumes, vegetables, and beverages such as tea, coffee, red wine, beer, etc. The medicinal benefits of polyhydroxylated chalcones are mainly attributed due to their free radical scavenging activity (antioxidant property), which in turn mitigates oxidative stress-induced tissue damage associated with some chronic disorders such as cardiovascular diseases, inflammatory diseases and neurological disorders, and certain infectious diseases [,,].
3. Biosynthesis of Chalcones
Chalcone is one of the precursors in the biosynthesis of flavonoids, isoflavonoids, anthocyanidins, proanthocyanidins, and other polyphenolic compounds []. Chalcone synthase (CHS) is the major enzyme that plays a vital role in the biosynthesis of chalcones [,]. The effectiveness of chalcone synthase (CHS) as an enzyme for chalcone formation is brought about by the presence of two active sites in the enzyme. One of the active sites referred to as the upper domain consists of four amino acids. The second active site referred as the lower domain is also essential for chalcone formation []. Phenylalanine is the major precursor for chalcones biosynthesis (phenylalanine is formed from chorismate as a precursor). p-Coumaroyl CoA and malonyl CoA are other important biomolecules required for the formation of chalcones. However, p-coumaroyl CoA is formed from phenylalanine []. Phenylalanine undergoes deamination at the aliphatic chain to form cinnamic acid. This is catalyzed by phenylalanine ammonia-lyase (PAL), which is followed by hydroxylation at the para position of the phenylalanine aromatic ring mediated by cinnamate-4-hydroxylase to form p-coumaric acid. Succinyl-CoA substitution of the hydroxyl group occurs at the aliphatic carboxyl group of the p-coumaric acid to yield p-coumaroyl CoA by the enzyme 4-coumaroyl-coenzyme A ligase. CHS catalyzes the condensation of three molecules of malonyl CoA and p-coumaroyl CoA (one molecule) successively. The process also involves the decarboxylation, cyclization, and aromatization of malonyl CoA, which is mediated by the four amino acids (Asn 336, His 303, Phe 215, and Cys 164) present in the active site of CHS []. The biosynthesis of chalcones is depicted in Figure 2.
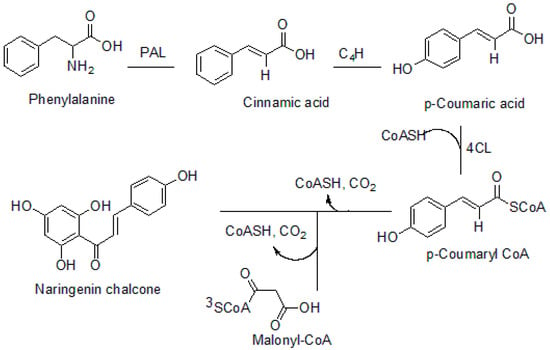
Figure 2.
Biosynthesis of chalcone. PAL: phenylalanine ammonia-lyase, C4H: cinnamate 4-hydroxylase, 4CL: 4-coumarate-CoA ligase.
The chalcone formed is a biosynthetic precursor for various polyphenolic classes of natural products such as flavanones, flavonols, flavanols, dihydroflavonols, isoflavones, flavones, isoflavonoids, aurone, and anthocyanidins []. The biosynthesis of various chalcone bioprecursors is represented in Figure 3.
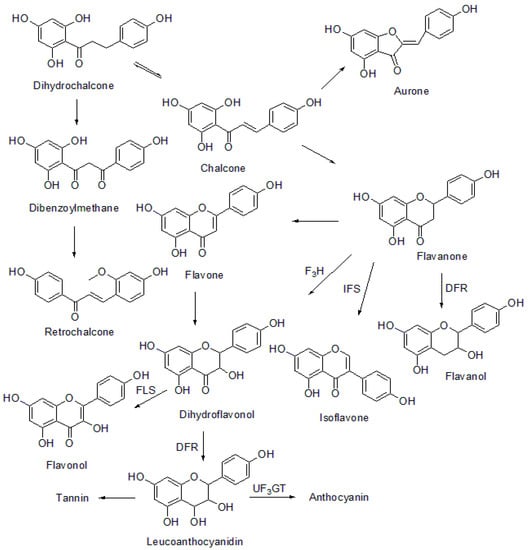
Figure 3.
Biosynthesis of chalcone precursors. DFR: dihydroflavonol-4-reductase, IFS: isoflavonone synthase, F3H: flavanone-3-hydroxylase, FLS: flavonol synthase, UF3GT: UDP-glucose flavonoid-3-O-glucosyltransferase.
The formation of prenylated chalcones has been reported to be mediated by prenyltransferase, which plays a significant role in transferring prenyl units to an acceptor molecule from an isoprenyl source, which is usually dimethylallyl pyrophosphate (DMAPP) (Figure 4a) [].
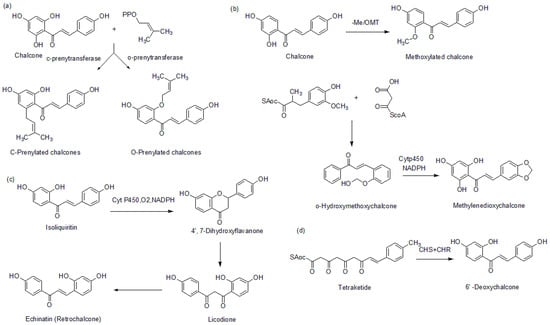
Figure 4.
Biosynthesis of (a) prenylated chalcones, (b) methoxychalcone and methylenedioxychalcone, (c) retro chalcones, and (d) dedoxychalcones.
In the formation of methoxylated chalcones, methylation takes place through a catalytic mediation of S-adenosyl-L-methionine-dependent-O-methyltransferase (OMTs) []. It mediates the transfer of a methyl group from a donor (S-adenosyl-L-methionine) to an acceptor molecule. Methylenedioxy chalcone is generated through the formation of methylenedioxy bridges and catalyzed by cytochrome P450-dependent enzymes alongside NADPH, which acts as a cofactor (Figure 4b) [,]. Retro chalcones have been reported to be formed by the inversion of α, β-unsaturated ketone during the biosynthesis of 6′-deoxychalconeisoliquiritigenin (Figure 4c) []. It has been reported that the presence of CHS and a polyketide reductase (CHR) as the active enzymes in a biosynthetic process generates 6′-deoxychalcones (Figure 4d) [].
During chalcone biosynthesis, the linkage of a sugar molecule catalyzed by the enzyme uridine diphosphate glycosyltransferase yields glycosylated chalcones. In this case, a nucleophilic substitution reaction is used to transfer the sugar molecule from a donor molecule (UDP-glycoside) to an acceptor molecule [,].
4. Naturally Occurring Chalcones
Chalcones occurring in nature have plants as their major source. They are usually found either in medicinal plants or in dietary plants. In nature, chalcones can be found as chalcone derivatives and flavonoids []. Chalcone derivatives of medicinal importance can be chemically synthesized in the laboratory by chemical modification of the parent chalcone scaffolds with a diverse range of structural substitutions [].
4.1. Chalcones from Medicinal and Dietary Plants
Several chalcones with proven therapeutic activities have been isolated from various medicinal and potential medicinal plants. Star et al. (1978) carried out the isolation of Pityrogramma triangularis [] exudate, which yielded a chalcone, 2,6-dihydroxy-4-methoxy-3-methyl chalcone, which was reported as a new compound. Isoliquiritigenin, isoliquiritin, neoisoliquiritin [], licochalcone A, licochalcone B [], echinatin [], licuroside [], and neolicurosid [] have earlier been isolated from liquorice (Glycyrrhiza glabra), which is a medicinal plant having therapeutic uses against many human diseases []. Two dihydrochalcones, 2,6-dihydroxy-4-methoxy-3,5-dimethyl dihydrochalcone and 4,4,6-trimethyl-2-(3-phenyl propionyl)-cyclohexane-1,3,5-trione from Myrica gale have been reported by Uyar et al. (1978) []. Crotalaria prostrata, an Indian medicinal plant, has been reported to yield crotaoprostrin on isolation []. Psoralea corylifolia, a known traditional medicine for Indians and Chinese, has also yielded bavachromanol, a novel natural chalcone []. Dihydrochalcone, dihydroisocordon, and flemistrictin B have been isolated from Lonchocarpus xuul root extract []. In a comprehensive review by Wang et al. (2020), about 42 chalcones isolated from licorice have been reported []. Brackenin is a dimeric dihydrochalcone isolated from Brackenridgea zanguebarica belonging to the Ochnaceae plant family []. Six chalcones have been isolated from Angelica keiskei extracts by column chromatography []. Alongside a flavonoid mixtecacin, oaxacin had been isolated from Tephrosia woodii [] and epoxychalcone has been isolated from Tephrosia carrollii []. Furthermore, 3,4-dimethoxy chalcone and 3,4-dihyroxy-3′,4,4′-trimethoxy chalcone have been isolated from Arrabidaea brachypoda flowers []. Three chalcones, flavokawain B, pinostrobn, and pashanone have been also been reported from seeds of Periscariala pathifolia through chromatographic separations []. Four chalcones, 5,7-dihydroxy-4-phenyl-8-(3-phenyl-trans-acry-loyl)-3,4-dihydro-1-benzopyran-2-one, 2′-hydroxy-4′,6′dimethoxychalcone, 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone, and 2′,4′-dihydroxy-6′-methoxy-3′-methylchalcone (along with three new chalcone derivatives, parasiticin A, parasiticin B, and parasiticin C) have been isolated from the fern Cyclosorus parasiticus []. Chalcones has been identified as a complex mixture of multicomponents in Helichrysum rugulosum []. Glycyrrhizae radix has been identified as a source of licuraside and isoliquiritin, which are nothing but chalcone derivatives []. Mallotophilippens C, D, and E are chalcone derivatives that have been isolated from the fruits of Malotus philippinensis []. About five chalcones have been isolated from Artocarpus nobilis 2′,4′,4-trihydroxy-3′-geranylchalcone, 2′,3,4,4′-tetrahydroxy-3′-geranylchalcone, 2′,4′,4-trihydroxy-3′-[′2-hydroxy-7-methyl-3-methylene-6-oetaenyl] chalcone, 2′,4′,4-trihydroxy-3′-[6-hydroxy-3,7-dimethyl-′2(E),7-oetadienyl] chalcone, and 2′3,4,4′-tetrahydroxy-3′-[6-hydroxy-3,7-dimethyl-2(E),7-octadienyl] chalcone []. 2-hydroxy-4′, 6′-dibenzyloxy chalcone, and 4′, 6′, 8′-trihydroxy chalcones have been isolated from Helichrysum gymnocomum []. Other compounds that have been isolated from Bidens tripartitus are 2′-hydroxy-4,4′-dimethoxychalcone []. Ponganones I and II have been identified as chalcone constituents of Pongamia pinnata []. 2′,4′-dihydroxy-3′-methoxychalcone and 2′,4′-dihydroxychalcone have been reported as constituents of Zuccagnia punctata []. 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone has been reported from Dalea versicolor []. Cycloaltilisin 6, a dimeric dihydrochalcone, has been identified as a constituent of the bud cover of Artocarpus altilis []. Stipalen, which is a diprenylated chalcone, has been reported as a constituent of Dalbergia stipulacea root []. 3,3′dihyroxy chalcone has been isolated from primula macrophylla []. Even though flemistrictin A has been previously isolated from Tephrosia spinosa, two chalcones later, spinochalcones A and B, have been identified []. 4′-O-α-D-(2″-p-coumaroyl)glucopyranosyl-4,2′,3′-trihydroxychalcone, 4′-O-α-D-(2″-p-coumaroyl-6″-acetyl)glucopyranosyl-4,2′,3′-trihydroxychalcone, and 3′-(3-methyl-2-butenyl)-4′-O-â-D-glucopyranosyl-4,2′-dihydroxychalcone, and 4′-O-α-D-(2″-acetyl-6″-cinnamoyl)glucopyranosyl-4,2′,3′-trihydroxychalcone, which are chalcone glycosides, have been isolated from Maclura tinctoria []. Calythropsis aurea crude extract yielded calythropsin and dihydrocalythropsin on isolation []. Cedreprenone, 2′-methoxy helikrausic chalcone, cedrediprenone, 5,7-dimethylpinocembrine, flavokawin B, and uvangoletin have been isolated from the fruits and seeds of Cedrelopis grevei []. Anneslea fragrans var. lanceolata yielded eight dihydrochalcones, davidigenin-2′-O-(6″-O-4‴-hydroxybenzoyl)-β-glucoside, davidigenin-2′-O-(2″-O-4‴-hydroxybenzoyL)-β-glucoside, davidigen-2′-O-(3″-O-4‴-hydroxybenzoyl)-β-glucoside, davidigenin-2′-O-(6″-O-syringoyl)-β-glucopyranoside, 1-O-3,4-dimethoxy-5-hydroxyphenyl-6-O-(3,5-di-O-methylgalloyl)-β-gluco-pyranoside, davidioside, 4′-O-methyldavidioside, and davidigenin on isolation by chromatography []. Another two dihydrochalcones, 2′,4,4′,6′-tetrahydroxy-5-(E-3, 7-dimethylocta-2,6-dienyl)-3-(3-methylbut-2-enyl)dihydrochalcone, and 2′,4,4′,6′-tetrahydroxy-3,5-di(3-methylbut-2-enyl)dihydrochalcone have also been isolated from the aerial parts of boronia inconspicua []. Hostmanin A, B, C, and D, 2′,6′-dihydroxy-4′-methoxy, linderatone, aductine E, and (-)-methyl linderatin are all dihydrochalcones isolated from piper hostmannianum var. berbicense []. 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone has been extracted from the dried flower, Cleistocalyx operculatus []. The roots of lonchocarpus sericeus yielded derricin and lonchocarpin on isolation of its hexane extract []. In addition to pedicin, two novel condensed chalcones, fissistin and isofissistin, have been isolated from the ethyl acetate extract of Fissistigma lanuginosum []. Four dihydrochalcones, 10′,6′-diacetoxy-4,4′-dimethoxydihydrochalcone, 4,2′,6′-trihydroxy-4′-methoxy dihydrochalcone, 2′,6′-dihydroxy-4′-methoxydihydrochalcone, and chalcone 2′,4′-diacetoxy chalcone have been reported from the leaves of Carthamus arborescens []. Syzygium samarangense has been identified as a source of stercurensin, cardamonin, and 4′, 6′-dihydroxy-2′-methoxy-3′,5′-dimethyl chalcone []. Litseaone A and B have been isolated from the stem bark of Litsea rubescens and Litsea pedunculata []. Cyclohexanyl chalcone and panduratin have been found to be a constituent of Boesenbergia rotunda []. Crotalaria trifoliastrum yielded munchiwarin, which has a 2,2,6-tri-isoprenyl-cyclohex-5-ene-1,3-dione skeleton []. Glycyrrhiza inflate has been reported to contain kanzonol, licochalcone A, D, and G, licoagrochalcone A, isoliquiritigenin, 5-prenyl butein, and echinantin []. Isoliquiritigenin, syzalterin, L-farrenol, and L-liquiritigenin have been isolated from Pancratium maritium []. Xanthohumol has been reported from Humulus lupulus []. Glabridin, licochalcone A, isoliquiritigenin, glycycoumarin, glycerin, glycerol, and liquiritigenin have been reported from Glycyrrhiza uralensis []. α-Hydroxy dihydrochalcones together with the novel isoflavanone, norisojamicin have been isolated from the Millettiaus aramensis stem bark []. A prenylated chalcone, 2′,4′-dihydroxy-5- prenylchalcone has been isolated from the aerial parts of Lonchocarpus cultratus []. Ulvaria dulcis has yielded 2′,3′-dihydroxy- 4′,6′-dimethoxychalcone []. Pashanone, pinostrobin, and flavokawain chalcones have been identified as constituents of persicaria lapathifolia seeds []. p-Hydroxy benzaldehyde, dorsmanin A, 4,2,4-trihydroxy-3-prenylchalcone, and 4,2,4-trihydroxychalcone have been isolated from Dorstenia zenkeri []. Ethanolic extract of Haematoxylum campechia num. L. has been reported to contain two chalcones, sappanchalcone and 3-deoxy sappanchalcone []. Some newer chalcones such as (-)-hydroxypanduratin A, a cyclohexenyl chalcone derivative, dihyro-5,6-dehydro kawain, pinocembrin, panduratin A, pinostrobin, and sakuranetin have been investigated by Tuchinda et al. (2002) []. Perez- Gutierrez et al. isolated six flavonoids from the bark of Eysenhardtia polystachiya with 2′,4′-dihydroxychalcone-6′-O-β-d-glucopyranoside, α,4,4′-trihydroxydihydrochalcone-2′-O-β-d-glucopyranoside, α,3,2′,4′-tetrahydroxy-4-methoxy-dihydrochalcone, and 3′-C-β-glucopyranosy-6′-O-β-d-glucopyranoside bearing the chalcone moiety []. Artoindonesianin J, a prenylated chalcone has been isolated from the root bark of Artocarpus bracteate []. More recently, Nchiozem-Ngnitedem et al. have isolated eight known chalcones alongside four new steroidal sapogenins and a conjugated chalcone–stilbene [].
Chalcone has been identified as one of the vital constituents of some edible plants []. In general, the phenolic chalcones present in edible plants play an essential role in maintaining good health condition for humans, with their basic function ranging from being good antioxidants to antimicrobial activities, among others []. Chalcones have been identified, isolated, and characterized from edible plants in many research works. Phloretin-3′,5′-di-C-β-glucopyranoside, a dihydrochalcone, and chalconaringenin have been identified from Solanaceae specie of tomatoes []. Iijima et al. (2008) reported the presence of eriodictyol chalcone in tomatoes (Solanum lycopersicum). They also reported narigenin chalcones []. Slimestad and Verheul (2011) reported the presence of chalconarigenin from fresh cherry tomatoes []. Two hydroxylated polymethoxychalcones have been isolated from sweet orange (citrus sinensis) peel []. 2′-hydroxy-3,4,4′,5′,6′-pentaethoxychalcone and 2′-hydroxy-3,4,3′,4′,5′,6′-pentaethoxychalcone, which are C-methylated chalcones, have been isolated from the edible syzygium samarangense methanolic extract []. Apple fruit (Malus domestica) has been reported to possess phloridzin, seboldin, and trilobatin []. Angelica keiski (Ashitaba), which is vital as a food supplement, constitutes some chalcone compounds. Nine chalcones have been isolated from this plant alongside four coumarins in different research; 4-hydroxy derricin and xantholangelol were isolated from the ethanolic extract []. Glycyrrhiza glabra, a licorice species, is a vital constituent of candies, snacks, beverages, and sweets []. Many compounds including isoliquiritin apioside [], lucuraside [], butein-4-O-β-D-glucopyranoside [], neoisoliquiritin [], licochalcone C, licoagrochalcone B, licoagrochalcone C, licoagrochalcone D, kanzonol Y [], echinatin, licochalcone B, morachalcone A, 2,3′,4,4′-tetrahydroxy-3,5′-diprenyl chalcone, 3,3′,4,4′-tetrahydroxy-2′-methoxy-5-prenylchalcone, paratocarpin B, 2,3′,4,4′,α-pentahydroxy-3,5′-diprenyl-dihydrochalcone, 2,3′,4,4′,α-pentahydroxy-3-prenyl-dihydrochalcone [], kanzonol B, 4-hydroxylonchocarpin [], licochalcone G [], 3,4,3′,4′-tetrahydroxy-2-methoxychalcone [], glypallichalcone [], paratocarpin A and B [], glycybridin A, B, and C have been isolated from this plant []. Trankoontivakorn et al. (2001) isolated six chalcones, panduratin A, pinostrobin, cardamonin, pinocembrin, 4-hydroxy panduratin A, and 2′,4′,6′-triydroxychalcone from finger root rhizomes (boesenbergia pandurate) [].
4.2. Bioactivities of Naturally Occurring Chalcones
Generally, chalcones exhibit a wide range of biological activities: antioxidant, antimalarial, anti-inflammatory, antimicrobial, antiosteoporosis, antiplasmodial, anticancer, antifungal, antihyperglycemic, and many others (Figure 5) []. Specifically, chalcones from medicinal plants exhibit these biological activities, and as a consequence, plants containing chalcones are used as therapeutic agents in various diseases. Many plants containing chalcones have shown inhibition against cancer growth. Licochalcone A, xanthohumol, 4-hydroxyderricin, butein, phloretin, garcinol, flavokawain A, B, and C, broussochalcone, dimethyl amino chalcones, cardamonin, and 2′-hydroxy-2,3,4′,6′-tetramethoxy chalcone have been reported to exhibit anticancer activity against various cancer cells [,,,].
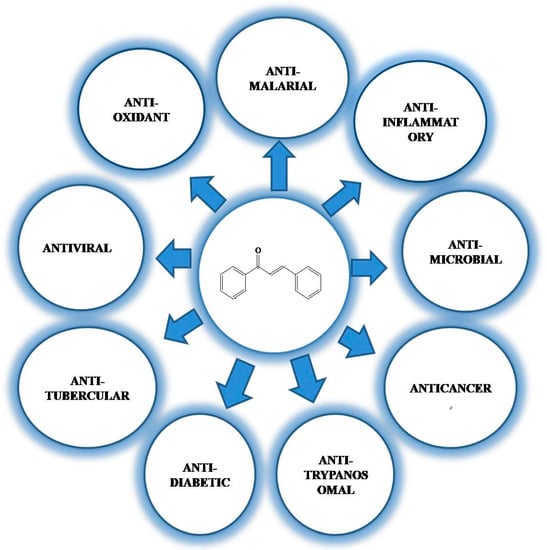
Figure 5.
Diverse biological activities of chalcones.
The antimalarial and antileishmanial activities of some chalcones, for example, Licochalcone A, have also been investigated []. Chalcones from the plants Mallotus hilippinensis and Maclura tinctoria have been shown to possess antifungal activity []. Xanthoangelol and 4-hydroxyderricin, constituents of ashitaba, have been reported to possess a considerable extent of hyperglycemic activity []. Protein tyrosin phosphatase IB (PTBIB) plays a significant role in the regulation of hyperglycemia []. Some chalcone derivatives from medicinal plants are essential PTPIB inhibitors [].
Chalcones from dietary sources also possess many biological activities. This enables edible plants containing chalcones to be used as therapeutic agents []. Tomatoes have been reported to exhibit anticardiovascular, antidiabetic, and anticancer activities [,,]. Anti-inflammatory, antiallergic [], and antiobesity [] activities have been reported with naringenin chalcone. It is one of the major bioconstituents of tomatoes [,,]. Other chalcone constituents such as phloretin-3′,5′-di-C-glucoside present in tomatoes have been reported to possess antioxidant properties []. Panduratin A, boesenbergin A, and pinostrobin chalcone in tomatoes have been reported for their aphrodisiac properties [].
In a separate description, panduratin A has been reported for its antioxidant, antiobesity, anti-inflammatory, and antimicrobial activities [,,,]. Even though boesenbergin has been reported to be highly hepatoxic, it has been demonstrated to exhibit anti-inflammatory, antioxidant, and anticancer activities []. Protease inhibition, anticancer, and antipyretic activities have been attributed to cardamonin []. Antiretroviral activity has been reported for hydroxypanduratin A, pinostrobin, and panduratin chalcone [,]. Licochalcone A, a constituent of licorice, has been reported to have a good inhibition of TNF-α, IL-β, and IL-6 inflammatory markers [,]. This chalcone along with licochalcone B, C, and D has been associated with antiviral [], anti-inflammatory [], antidiabetic [], antitrypanosomal [], anticancer [], and antibacterial [] activities. Apple containing dihydrochalcone constituents has biological activities against many diseases [,]. Phloretin is the most important chalcone present in apple. Phloretin has been demonstrated to possess antioxidant, anticancer, and anti-inflammatory effects [,]. As an anticancer agent, it targets the inhibition of GLUT2. It also inhibits the anti-inflammatory markers such as NF-Kβ, TNF-α, etc. [].
The bioactivities of chalcones obtained from medicinal plants are illustrated in Table 1.

Table 1.
Bioactivities of important naturally occurring chalcones.
The structures of naturally occurring chalcones are presented in Figure 6.
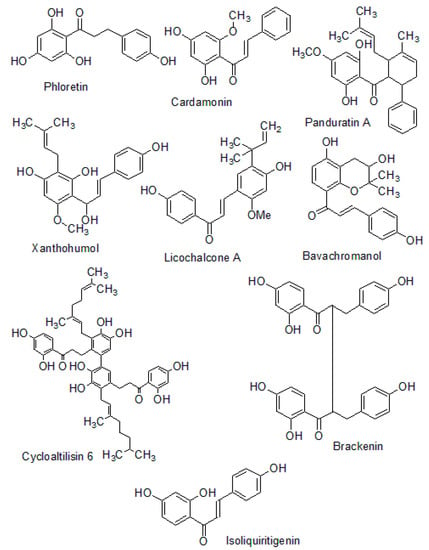
Figure 6.
Structures of naturally occurring chalcones.
5. Pharmacokinetics and Toxicities of Chalcones
Although chalcones have a wide range of pharmacological activities, the unavailability of sufficient bioavailability and bioaccessibility data in humans is a major challenge toward their development as therapeutic agents []. Synthetic chalcones have been widely studied, whereas the bioavailability of chalcones from natural sources is limited. The expected level of in vivo efficacy in preclinical evaluation has not been reached yet due to poor bioavailability profile. However, optimization of the physiochemical properties of chalcone derivatives could be an important step in their further development as lead molecules or drug candidates. The adsorption, distribution, metabolism, excretion, and toxicity (ADMET) of some naturally occurring chalcones have been studied, but the data do not satisfactorily support their ADMET profile [,] (Figure 7).
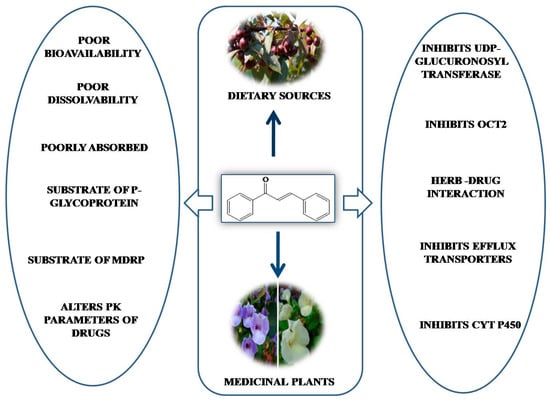
Figure 7.
Challenges associated with pharmacokinetics of naturally occurring chalcones.
Studies have shown that amongst many natural chalcones, prenylated derivatives are bioavailable, but they exhibit low bioaccessibility. One such chalcone is xanthohumol obtained in hop plant (Humulus lupulus), which upon oral administration by force feeding at extremely higher dosage to rodents (1 g/kg body weight) produces good oral bioavailability, but it does not obtain appreciable accessibility at the site of action. Xanthohumal 4′-O-glucoronide has been found to be the major metabolite in plasma, and unmetabolized xanthohumol has also been detected ten times less concentration after 4 h post administration []. In vitro metabolism studies indicate that xanthohumal in human and rat liver microsomes can be freely converted to glucuronides []. Gil-Izquierdo et al. (2001) studied the bioavailability of diversely processed juice of Citrus sinensis (L.) by mimicking in vitro digestion in stomach as well as the small intestine []. They have reported that in mild alkaline medium, 50–60% of the dissolved flavanones (mainly hesperidine) becomes converted to chalcones (hisperidin chalcone). Due to the poor solubility of these chalcones, the bioequivalence is not achieved to the expected level [,]. Another chalcone derivative is cardamonin, which is obtained from plants belonging to the Zingiberacea family, which has been reported to be poorly absorbed upon oral administration exhibiting 18% oral bioavailability in mice. It exhibited a high volume of distribution, short mean residence, high clearance, and was excreted in bile in its conjugated and unchanged form.
Zhao et al. (2020) studied the pharamacokinetics of phloretin, a naturally occurring dihydrochalcone flavonoid found in apple, pear, roots peels, and juicy fruits peels, by orally administering it to Sprague–Dawley rats. Absorption mechanisms have been investigated in a Caco-2 cell monolayer and by a single pass intestinal perfusion in rats []. Phloretin is transported through active transport, efflux protein transport, and by cell bypass. It has been reported to be a substrate of P-glycoprotein (P-gp) and multi-drug resistance protein (MRP2) and found to have low oral bioavailability (8.676%) with colon as the best absorption site.
Naturally occurring chalcones have also been found to affect the pharmacokinetic parameters of drugs when administered simultaneously. Choi et al. (2014) investigated the effect of licochalcone A on the pharmacokinetics of nifedipine and its metabolite dehydronifedipine in rats. Hepatic CYP3A4 metabolizes nifedipine. Oral administration of nifedipine with licochalcone A has been found to inhibit CYP3A4 as well as exhibit the cellular accumulation of rhodamine-123 in MCF-7/ADR cells overexpressing P-gp, leading to a higher peak plasma concentration (Cmaxs) []. Boonnop et al. (2017) proposed that the co-administration of Boesenbergia rotunda extract with therapeutic drug may cause herb–drug interaction, leading to an alteration of the efficacy and toxicity of the drug. Panduratin A isolated from the Boesenbergia rotunda has been reported to cause herb–drug interaction and alter renal cationic drug clearance by inhibiting organic cation transporters (OCT2), which are responsible for the renal excretion of cationic drugs [].
Recently, Qin et al. (2021) also studied the metabolic and inhibitory effects of isobavachalcone, a natural chalcone obtained from Psoralea corylifolia, on efflux transporters, cytochrome P450 and UDP-glucuronosyltransferase enzymes. The glucuronidation of isobavachlacone in the human liver microsome and human intestine microsome has been well characterized with the production of three glucuronides. Moreover, the main contributors for glucuronidation were UGT1 A9, 1A8, 1A7, 1A3, and 1A1. MRP1, MRP4, and BCRP transporters have been found to participate more in glucuronide excretion. Isobavachalcone has been recognized as a broad-spectrum inhibitor against UGT2B7, UGT1A9, UGT1A1, CYP2E1, CYP2D6, CYP2C19, CYP2C9, and CYP2B6 [].
In view of the above facts, to design a chalcone derivative with acceptable ADMET properties, the maximization of its physiochemical properties with modification in the chemical structure would play a crucial role.
6. Conclusions and Future Directions
Chalcone scaffolds considered as the key bioactive precursors of plant flavonoids possess huge chemical and biological potential with significance in medicinal chemistry and pharmacology in current times. The chemistry and biological importance of naturally occurring chalcones have not been extensively explored. However, regardless of its versatile medicinal importance, the pharmacokinetics of plant-derived/dietary chalcones is a major challenge. Moreover, there is a lack of preclinical or clinical data on naturally occurring chalcones in the current literature. Further in-depth research studies are required to be carried out to address the pharmacokinetic issues and toxicological aspects related to naturally occurring chalcones and chalcone-derived flavonoids. There are ample scopes for the discovery of lead molecules or drug candidates from naturally occurring bioactive chalcones. Therefore, the proper chemical derivatization of natural chalcones is necessary to obtain novel flavonoid molecules that would play a vital role in the chalcone scaffolds-based discovery of drug molecules.
Author Contributions
Conceptualization, M.R.; methodology, M.R. and E.I.A.; investigation, M.R. and E.I.A.; resources, E.I.A.; data curation, E.I.A.; writing—original draft preparation, M.R., E.I.A. and T.S.; writing—review and editing, M.R., E.I.A., T.S., S.J.K. and A.R.B.; visualization, J.K. and T.S.; supervision, M.R.; project administration, M.R.; funding acquisition, J.K., A.A.B.D. and R.M.I.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Deanship of Scientific Research, Majmaah University under Project number R-2021-276.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work under Project number R-2021-276.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ADMET: absorption, distribution, metabolism, excretion and toxicity, COX-2: cyclooxygenase-2, CHS: chalcone synthase, cAMP: 3′,5′-cyclic adenosine monophosphate, cGMP: guanosine 3′,5′-cyclic monophosphate, DFR: dihydroflavonol-4-reductase, DMAPP:dimethylallyl pyrophosphate, FLS: Flavonol synthase, F3H: flavanone-3-hydroxylase, GLUT2: glucose transporter 2, I-κBα: IκBα inhibitory-κBα, IFS: isoflavonone synthase, LPS: ipopolysaccharide, MAPKs: mitogen-activated protein kinases, MRP2: multidrug resisteace-associtaed protein 2, NF-κB: nuclear factor-κB, NO: nitric oxide, NOS: nitric oxide synthase, iNOS: inducible NOS, OCT2: organic cation transporter 2, OMTs: O-methyltransferase, PAL: Phenylalanine ammonia-lyase, PTBIB: protein tyrosine phosphatase IB, TNF: tumor necrosis factor, UF3GT: UDP-glucose, flavonoid-3-O-glucosyltransferase.
References
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.; Andrade, C.H.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Piya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic/therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.; Bottura, C.; Fonseca, M.J.; Vignoli, J.A.; Baracat, M.M.; et al. Trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Kostanecki, S.; Tambor, J. Ueber die sechs isomeren Monooxybenzalacetophenone (Monooxychalkone). Ber. Dtsch. Chem. 1899, 32, 1921–1926. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- García-Calderón, M.; Pérez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Márquez, A.J. Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants 2020, 9, 774. [Google Scholar] [CrossRef]
- Abe, I.; Morita, H. Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 2010, 27, 809–838. [Google Scholar] [CrossRef]
- Cheng, A.X.; Han, X.J.; Wu, Y.F.; Lou, H.X. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095. [Google Scholar] [CrossRef]
- Waki, T.; Mameda, R.; Nakano, T.; Yamada, S.; Terashita, M.; Ito, K.; Tenma, N.; Li, Y.; Fujino, N.; Uno, K.; et al. A conserved strategy of chalcone isomerase-like protein to rectify promiscuous chalcone synthase specificity. Nat. Commun. 2020, 11, 870. [Google Scholar] [CrossRef]
- Saleh, O.; Haagen, Y.; Seeger, K.; Heide, L. Prenyl transfer to aromatic substrates in the biosynthesis of aminocoumarins, meroterpenoids and phenazines: The ABBA prenyltransferase family. Phytochemistry 2009, 70, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhou, Y.; Peng, T.; Guo, Y.; Zhao, Y.; Zeng, Z. Crystal structure of a S-adenosyl-L-methionine-dependent O-methyltransferase-like enzyme from Aspergillus flavus. Proteins Struct. Funct. Bioinform. 2021, 89, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Barz, W. Cytochrome P450-dependent methylenedioxy bridge formation in Cicer arietinum. Phytochemistry 1996, 41, 457–460. [Google Scholar] [CrossRef]
- Davies, K.M.; Bloor, S.J.; Spiller, G.B.; Deroles, S.C. Production of yellow colour in flowers: Redirection of flavonoid biosynthesis in Petunia. Plant J. 1998, 13, 259–266. [Google Scholar] [CrossRef]
- Ayabe, S.L.; Furuya, T. Biosynthesis of a retrochalcone, echinatin: A feeding study with advanced precursors. Tetrahedron Lett. 1981, 22, 2097–2098. [Google Scholar] [CrossRef]
- Dixon, R.A.; Lamb, C.J.; Masoud, S.; Sewalt, V.J.; Paiva, N.L. Metabolic engineering: Prospects for crop improvement through the genetic manipulation of phenylpropanoid biosynthesis and defense responses—A review. Gene 1996, 179, 61–71. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Abbas, N.; Bukhari, S.; Jasamai, M.; Jantan, I.; Ahmad, W. Review of methods and various catalysts used for chalcone synthesis. Mini Rev. Org. Chem. 2013, 10, 73–83. [Google Scholar]
- Star, A.E.; Mabry, T.J.; Smith, D.M. Triangularin, a new chalcone from Pityrogramma triangularis. Phytochemistry 1978, 17, 586–587. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Lutomski, J.; Nieman, C. Liquorice, Glycyrrhiza glabra L.—Composition, uses and analysis. Food Chem. 1990, 38, 119–143. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, S. New type chalcones from licorice root. Tetrahedron Lett. 1975, 50, 4461–4462. [Google Scholar] [CrossRef]
- Furuya, T.; Matsumoto, K.; Hikichi, M. Echinatin, a new chalcone from tissue culture of Glycyrrhiza echinata. Tetrahedron Lett. 1971, 12, 2567–2569. [Google Scholar] [CrossRef]
- Miething, H.; Speicher-Brinker, A. Neolicurosid-ein neues Chalkonglykosid aus der Süßholzwurzel. Arch. Pharm. 1989, 322, 141–143. [Google Scholar] [CrossRef]
- Uyar, T.; Malterud, K.E.; Anthonsen, T. Two new dihydrochalcones from Myrica gale. Phytochemistry 1978, 17, 2011–2013. [Google Scholar] [CrossRef]
- Krohn, K.; Steingröver, K.; Rao, M.S. Isolation and synthesis of chalcones with different degrees of saturation. Phytochemistry 2002, 61, 931–936. [Google Scholar] [CrossRef]
- Suri, J.L.; Gupta, G.K.; Dhar, K.L.; Atal, C.K. Bavachromanol: A new chalcone from the seeds of Psoralea corylifolia. Phytochemistry 1980, 19, 336–337. [Google Scholar] [CrossRef]
- Yam-Puc, A.; Peña-Rodríguez, L.M. Isocordoin derivatives from the root extract of Lonchocarpus xuul. J. Mexican Chem. Soc. 2009, 53, 12–14. [Google Scholar] [CrossRef]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural chalcones in Chinese materia medica: Licorice. Evid. Based Complement. Alternat. Med. 2020, 16, 3821248. [Google Scholar] [CrossRef]
- Drewes, S.E.; Hudson, N.A. Brackenin, a dimeric dihydrochalcone from Brackenridgea zanguebarica. Phytochemistry 1983, 22, 2823–2825. [Google Scholar] [CrossRef]
- Shin, J.E.; Choi, E.J.; Jin, Q.; Jin, H.G.; Woo, E.R. Chalcones isolated from Angelica keiskei and their inhibition of IL-6 production in TNF-α-stimulated MG-63 cell. Arch. Pharm. Res. 2011, 34, 437–442. [Google Scholar] [CrossRef]
- Dominguez, X.A.; Tellez, O.; Ramirez, G. Mixtecacin, a prenylated flavanone and oaxacacin its chalcone from the roots of Tephrosia woodii. Phytochemistry 1983, 22, 2047–2049. [Google Scholar] [CrossRef]
- Gómez-Garibay, F.; Arciniega, M.D.; Céspedes, C.L.; Taboada, J.; Calderón, J.S. Chromene chalcones from Tephrosia carrollii and the revised structure of oaxacacin. Z. Naturforsch. C J. Biosci. 2001, 56, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Rezende-Júnior, L.M.; Andrade, L.M.; Leal, A.L.; Mesquita, A.B.; Santos, A.L.; Neto, J.D.; Siqueira-Junior, J.P.; Nogueira, C.E.; Kaatz, G.W.; Coutinho, H.D.; et al. Chalcones isolated from Arrabidaea brachypoda flowers as inhibitors of NorA and MepA multidrug efflux pumps of Staphylococcus aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef]
- Hailemariam, A.; Feyera, M.; Deyou, T.; Abdissa, N. Antimicrobial Chalcones from the Seeds of Persicaria lapathifolia. Biochem. Pharmacol. 2018, 7, 237. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Wu, G.; Yang, X.; Pan, S.; Wang, Y.; Ruan, J. Chalcone derivatives from the fern Cyclosorus parasiticus and their anti-proliferative activity. Food Chem. Toxicol. 2013, 60, 147–152. [Google Scholar] [CrossRef]
- Bohlmann, F.; Misra, L.N. New prenylflavanones and chalcones from Helichrysum rugulosum. Planta Med. 1984, 50, 271–272. [Google Scholar] [CrossRef]
- Aida, K.; Tawata, M.; Shindo, H.; Onaya, T.; Sasaki, H.; Yamaguchi, T.; Chin, M.; Mitsuhashi, H. Isoliquiritigenin: A new aldose reductase inhibitor from glycyrrhizae radix. Planta Med. 1990, 56, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Daikonya, A.; Katsuki, S.; Kitanaka, S. Antiallergic agents from natural sources 9. Inhibition of nitric oxide production by novel chalcone derivatives from Mallotus philippinensis (Euphorbiaceae). Chem. Pharm. Bull. 2004, 52, 1326–1329. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Balasooriya, B.A.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef]
- Drewes, S.E.; van Vuuren, S.F. Antimicrobial acylphloroglucinols and dibenzyloxy flavonoids from flowers of Helichrysum gymnocomum. Phytochemistry 2008, 69, 1745–1749. [Google Scholar] [CrossRef]
- Christensen, L.P.; Lam, J.; Thomasen, T. A chalcone and other constituents of Bidens tripartitus. Phytochemistry 1990, 29, 3155–3156. [Google Scholar] [CrossRef]
- Tanaka, T.; Iinuma, M.; Yuki, K.; Fujii, Y.; Mizuno, M. Two new β-hydroxychalcones from the root bark of Pongamia pinnata. Chem. Pharm. Bull. 1991, 39, 1473–1475. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.; Percivill, D.; Lewis, K.; Tegos, G.P.; Ekart, J. Phenolic metabolites of dalea versicolor that enhance antibiotic activity against model pathogenic bacteria. J. Nat. Prod. 2004, 67, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Offen, P.; Taylor, P.B.; Votta, B.J.; Johnson, R.K. A New Dimeric Dihydrochalcone and a New Prenylated Flavone from the Bud Covers of Artocarpus altilis: Potent Inhibitors of Cathepsin, K. J. Nat. Prod. 2002, 65, 624–627. [Google Scholar] [CrossRef]
- Bhatt, P.; Dayal, R. Stipulin, a prenylated chalcone from Dalbergia stipulacea. Phytochemistry 1992, 31, 719–721. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Shah, M.G.; Noorwala, M.; Mohammad, F.V. Isolation of 3, 3’-Dihydroxychalcone from Primula macrophylla. J. Nat. Prod. 1992, 55, 956–958. [Google Scholar] [CrossRef]
- Rao, E.V.; Prasad, Y.R. Two chalcones from Tephrosia spinosa. Phytochemistry 1992, 31, 2121–2122. [Google Scholar] [CrossRef]
- Cioffi, G.; Escobar, L.M.; Braca, A.; De Tommasi, N. Antioxidant Chalcone Glycosides and Flavanones from Maclura (Chlorophora) tinctoria. J. Nat. Prod. 2003, 66, 1061–1064. [Google Scholar] [CrossRef]
- Beutler, J.A.; Cardellina, J.H.; Gray, G.N.; Prather, T.R.; Shoemaker, R.H.; Boyd, M.R.; Lin, C.M.; Hamel, E.; Cragg, G.M. Two new cytotoxic chalcones from Calythropsis aurea. J. Nat. Prod. 1993, 56, 1718–1722. [Google Scholar] [CrossRef]
- Koorbanally, N.A.; Randrianarivelojosia, M.; Mulholland, D.A.; van Ufford, L.Q.; van den Berg, A.J. Chalcones from the seed of Cedrelopsis grevei (Ptaeroxylaceae). Phytochemistry 2003, 62, 1225–1229. [Google Scholar] [CrossRef]
- Huang, H.Y.; Ko, H.H.; Jin, Y.J.; Yang, S.Z.; Shih, Y.A.; Chen, I.S. Dihydrochalcone glucosides and antioxidant activity from the roots of Anneslea fragrans var. lanceolata. Phytochemistry 2012, 78, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Armstrong, J.A.; Waterman, P.G. Dihydrochalcones from the aerial parts of Boronia inconspicua. Phytochemistry 1994, 36, 799–801. [Google Scholar] [CrossRef]
- Portet, B.; Fabre, N.; Roumy, V.; Gornitzka, H.; Bourdy, G.; Chevalley, S.; Sauvain, M.; Valentin, A.; Moulis, C. Activity-guided isolation of antiplasmodial dihydrochalcones and flavanones from Piper hostmannianum var. berbicense. Phytochemistry 2007, 68, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Xie, B.F.; Zhou, J.M.; Feng, G.K.; Liu, Z.C.; Wei, X.Y.; Zhang, F.X.; Liu, M.F.; Zeng, Y.X. Blockade of vascular endothelial growth factor receptor signal pathway and antitumor activity of ON-III (2′, 4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone), a component from Chinese herbal medicine. Mol. Pharmacol. 2005, 67, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Fontenele, J.B.; Nobre Júnior, H.V.; De Sousa, F.C.; Silveira, E.R.; Nogueira, N.A.; De Moraes, M.O.; Viana, G.S.; Costa-Lotufo, L.V. Cytotoxic activity of chalcones isolated from Lonchocarpus sericeus (Pocr.) Kunth. Phytother. Res. 2003, 17, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Alias, Y.; Awang, K.; Hadi, A.H.; Thoison, O.; Sévenet, T.; Païs, M. An antimitotic and cyctotoxic chalcone from Fissistigma lanuginosum. J. Nat. Prod. 1995, 58, 1160–1166. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Cabrera, E.; Rodriguez-Garcia, I. Cytotoxic activity of flavonoids from Carthamus arborescens, Ononis natrix ssp. ramosissima and Centaurea malacitana. Fitoterapia 1997, 68, 281–283. [Google Scholar]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.T.; Luo, Y.P.; Zhao, J.F.; Yang, X.D.; Zhang, H.B. Novel cytotoxic chalcones from Litsea rubescens and Litsea pedunculata. Bioorg. Med. Chem. Lett. 2011, 21, 7431–7433. [Google Scholar] [CrossRef]
- Lai, S.L.; Wong, P.F.; Lim, T.K.; Lin, Q.; Mustafa, M.R. Cytotoxic mechanisms of panduratin A on A375 melanoma cells: A quantitative and temporal proteomics analysis. Proteomics 2015, 15, 1608–1621. [Google Scholar] [CrossRef]
- Yang, S.W.; Cordell, G.A.; Lotter, H.; Wagner, H.; Mouly, B.C.; Appa Rao, A.V.; Rao, P.S. Munchiwarin, a prenylated chalcone from Crotalaria trifoliastrum. J. Nat. Prod. 1998, 61, 1274–1276. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.; Ramadan, M.A.; Khalifa, A.A. Acetophenones, a chalcone, a chromone and flavonoids from Pancratium maritimum. Phytochemistry 1998, 49, 2579–2583. [Google Scholar] [CrossRef]
- Buckwold, V.E.; Wilson, R.J.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W., III; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef]
- Adianti, M.; Aoki, C.; Komoto, M.; Deng, L.; Shoji, I.; Wahyuni, T.S.; Lusida, M.I.; Fuchino, H.; Kawahara, N.; Hotta, H. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014, 58, 180–187. [Google Scholar] [CrossRef]
- Ersam, T.; Achmad, S.A.; Ghisalberti, E.L.; Hakim, E.H.; Makmur, L.; Syah, Y.M. A new isoprenylated chalcone, artoindonesianin J, from the root and tree bark of Artocarpus bracteata. J. Chem. Res. 2002, 2002, 186–187. [Google Scholar] [CrossRef]
- Da Silva Landim, E.M.; Ruiz, A.L.; de Carvalho, J.E.; Pomini, A.M.; Pastorini, L.H.; Oliveira Santin, S.M. Antiproliferative activity and chemical constituents of Lonchocarpus cultratus (Fabaceae). Nat. Prod. Res. 2021, 35, 2056–2059. [Google Scholar] [CrossRef]
- Chantrapromma, K.; Rat-A-pa, Y.; Karalai, C.; Lojanapiwatana, V.; Seechamnanturakit, V. A chalcone and a dihydrochalcone from Uvaria dulcis. Phytochemistry 2000, 53, 511–513. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Ngadjui, B.T.; Dongo, E.; Ngameni, B.; Nindi, M.N.; Bezabih, M. Chalcones and other constituents of Dorstenia prorepens and Dorstenia zenkeri. Phytochemistry 2002, 59, 877–883. [Google Scholar] [CrossRef]
- Escobar-Ramos, A.; Lobato-García, C.E.; Zamilpa, A.; Gómez-Rivera, A.; Tortoriello, J.; González-Cortazar, M. Homoisoflavonoids and chalcones isolated from Haematoxylum campechianum L.; with spasmolytic activity. Molecules 2017, 22, 1405. [Google Scholar] [CrossRef]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Perez-Gutierrez, R.M.; Garcia-Campoy, A.H.; Muñiz-Ramirez, A. Properties of flavonoids isolated from the bark of Eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid. Med. Cell. Longev. 2016, 2016, 9156510. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Brieger, L.; Strohmann, C.; Spiteller, M. Anti-inflammatory steroidal sapogenins and a conjugated chalcone-stilbene from Dracaena usambarensis Engl. Fitoterapia 2020, 146, 104717. [Google Scholar] [CrossRef]
- Rahman, M.A. Chalcone: A valuable insight into the recent advances and potential pharmacological activities. Chem. Sci. J. 2011. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The flavonoids of tomatoes. J. Agric. Food. Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef]
- Iijima, Y.; Suda, K.; Suzuki, T.; Aoki, K.; Shibata, D. Metabolite profiling of chalcones and flavanones in tomato fruit. J. Jpn. Soc. Hortic. Sci. 2008, 77, 94–102. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Properties of chalconaringenin and rutin isolated from cherry tomatoes. J. Agric. Food. Chem. 2011, 59, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lo, C.Y.; Ho, C.T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food. Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Miosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Ko, J.A.; Kim, D.W.; Kim, Y.M.; Kwon, H.J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2016, 31, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nikolic, D.; Van Breemen, R.B. Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS. J. Agric. Food. Chem. 2016, 64, 8062–8070. [Google Scholar] [CrossRef]
- Cheng, M.; Ding, L.; Kan, H.; Zhang, H.; Jiang, B.; Sun, Y.; Cao, S.; Li, W.; Koike, K.; Qiu, F. Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J. Nat. Med. 2019, 73, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-γ ligand-binding activity. Bioorg. Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Asada, Y.; Li, W.; Yoshikawa, T. Isoprenylated flavonoids from hairy root cultures of Glycyrrhiza glabra. Phytochemistry 1998, 47, 389–392. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Lv, C.; Du, Y.; Xu, H.; Wang, D.; Li, M.; Li, B.; Li, J.; Bi, K. Combination of the advantages of chromatographic methods based on active components for the quality evaluation of licorice. J. Sep. Sci. 2015, 38, 4180–4186. [Google Scholar] [CrossRef]
- Cai, L.N.; Zhang, R.Y.; Wang, B.; Qiao, L.; Huang, L.R.; Zhang, Z.L. Studies on the chemical constituents of Glycyrrhiza pallidiflora Maxim. Yao Xue Xue Bao Acta Pharm. Sin. 1992, 27, 748–751. [Google Scholar]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Antioxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food. Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Li, K.; Ji, S.; Song, W.; Kuang, Y.; Lin, Y.; Tang, S.; Cui, Z.; Qiao, X.; Yu, S.; Ye, M. Glycybridins A–K, bioactive phenolic compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar] [CrossRef]
- Trakoontivakorn, G.; Nakahara, K.; Shinmoto, H.; Takenaka, M.; Onishi-Kameyama, M.; Ono, H.; Yoshida, M.; Nagata, T.; Tsushida, T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J. Agric. Food. Chem. 2001, 49, 3046–3050. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xia, X.; He, J.; Liu, Y.; Shao, Z.; Hu, T.; Yu, C.; Liu, X.; Xu, Q.; Liu, B.; et al. ERα is a target for butein-induced growth suppression in breast cancer. Am. J. Cancer Res. 2020, 10, 3721. [Google Scholar]
- Wang, J.; Wu, M.; Zheng, D.; Zhang, H.; Lv, Y.; Zhang, L.; Tan, H.S.; Zhou, H.; Lao, Y.Z.; Xu, H.X. Garcinol inhibits esophageal cancer metastasis by suppressing the p300 and TGF-β1 signaling pathways. Acta Pharmacol. Sin. 2020, 41, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, R.; Nan, Y.; Li, W.; Wang, Q.; Jin, F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int. J. Oncol. 2016, 48, 843–853. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Croft, S.L.; Tan, A.L.; Go, M.L. Structure–activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem. 2003, 11, 2729–2738. [Google Scholar] [CrossRef]
- Jin, Y.S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Ogawa, H.; Okada, Y.; Kamisako, T.; Baba, K. Beneficial effect of xanthoangelol, a chalcone compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin. Exp. Physiol Pharmacol. 2007, 34, 238–243. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Sivakumar, P.M. Protein tyrosine phosphatase 1B inhibitors: A novel therapeutic strategy for the management of type 2 diabetes mellitus. Curr. Pharm. Des. 2019, 25, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłow, E. Microbial transformations of chalcones to produce food sweetener derivatives. J. Mol. Catal. B 2013, 98, 55–61. [Google Scholar] [CrossRef]
- Pandey, D.K.; Shekelle, R.; Selwyn, B.J.; Tangney, C.; Stamler, J. Dietary vitamin C and β-carotene and risk of death in middle-aged men: The Western Electric study. Am. J. Epidemiol. 1995, 142, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, S.A.; Bowen, K.; Garg, M.L. Tomato juice and platelet aggregation in type 2 diabetes. JAMA 2004, 292, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Grieb, S.M.; Theis, R.P.; Burr, D.; Benardot, D.; Siddiqui, T.; Asal, N.R. Food groups and renal cell carcinoma: Results from a case-control study. J. Am. Diet. Assoc. 2009, 109, 656–667. [Google Scholar] [CrossRef]
- Hirai, S.; Kim, Y.I.; Goto, T.; Kang, M.S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef]
- Gafner, F.R.; Schmid, D.A.; Lozza, J.A.; Zülli, F.R. Tetra-carboxy-methyl-naringenin-chalcone, a new active to treat rosacea. Household Pers. Care Today 2013, 8, 14–16. [Google Scholar]
- Horiba, T.; Nishimura, I.; Nakai, Y.; Abe, K.; Sato, R. Naringenin chalcone improves adipocyte functions by enhancing adiponectin production. Mol. Cell. Endocrinol. 2010, 323, 208–214. [Google Scholar] [CrossRef]
- Mah, S.H. Chalcones in diets. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2019; pp. 1–52. [Google Scholar]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef]
- Ongwisespaiboon, O.; Jiraungkoorskul, W. Fingerroot, Boesenbergia rotunda and its aphrodisiac activity. Pharmacogn. Rev. 2017, 11, 27. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Kim, C.; Hwang, J.K. Oral intake of Boesenbergia pandurata extract improves skin hydration, gloss, and wrinkling: A randomized, double-blind, and placebo-controlled study. J. Cosmet. Dermatol. 2017, 16, 512–519. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Lee, K.H.; Hwang, J.K. Activity of panduratin A isolated from Kaempferia pandurata Roxb. against multi-species oral biofilms in vitro. J. Oral Sci. 2009, 51, 87–95. [Google Scholar] [CrossRef]
- Kirana, C.; Jones, G.P.; Record, I.R.; McIntosh, G.H. Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J. Nat. Med. 2007, 61, 131–137. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Puripattanavong, J.; Panphadung, T. HIV-1 protease inhibitory substances from the rhizomes of Boesenbergia pandurata Holtt. Songklanakarin J. Sci. Technol. 2003, 25, 504–508. [Google Scholar]
- Tan, B.C.; Tan, S.K.; Wong, S.M.; Ata, N.; Rahman, N.A.; Khalid, N. Distribution of flavonoids and cyclohexenyl chalcone derivatives in conventional propagated and in vitro-derived field-grown Boesenbergia rotunda (L.) Mansf. Evid. Based Complement. Altern. Med. 2015, 2015, 451870. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Park, J.H.; Kim, D.H.; Kim, Y.H.; Park, J.H.; Shin, H.K.; Kim, J.K. Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264. 7 cells and endotoxin shock in mice. J. Mol. Med. 2008, 86, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, J.I.; Funakoshi-Tago, M.; Mashino, T.; Tago, K.; Inoue, H.; Sonoda, Y.; Kasahara, T. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-κB p65 in LPS signaling pathway. Int. Immunopharmacol. 2009, 9, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Bak, E.J.; Woo, G.H.; Kim, J.M.; Quan, Z.; Kim, J.M.; Yoon, H.K.; Cheon, S.H.; Yoon, G.; Yoo, Y.J.; et al. Licochalcone E has an antidiabetic effect. J. Nutr. Biochem. 2012, 23, 759–767. [Google Scholar] [CrossRef]
- Zhai, L.; Blom, J.; Chen, M.; Christensen, S.B.; Kharazmi, A. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 1995, 39, 2742–2748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef]
- Tsukiyama, R.I.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother. 2002, 46, 1226–1230. [Google Scholar] [CrossRef]
- Ramirez-Ambrosi, M.; Abad-Garcia, B.; Viloria-Bernal, M.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B. A new ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry analytical strategy for fast analysis and improved characterization of phenolic compounds in apple products. J. Chromatogr. A 2013, 1316, 78–91. [Google Scholar] [CrossRef]
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Kuo, S.C.; Chan, S.C.; Ko, F.N.; Teng, C.M. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1998, 1392, 291–299. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Lin, C.N.; Hwang, T.L.; Teng, C.M. Broussochalcone A, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages. Biochem. Pharmacol. 2001, 61, 939–946. [Google Scholar] [CrossRef]
- Kabanda, M.M.; Gbashi, S.; Madala, N.E. Proportional coexistence of okanin chalcone glycoside and okanin flavanone glycoside in Bidens pilosa leaves and theoretical investigation on the antioxidant properties of their aglycones. Free Radic. Res. 2021, 55, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Arfan, M.; Amin, H.; Khan, N.; Khan, I.; Saeed, M.; Khan, M.A. Analgesic and anti-inflammatory activities of 11-O-galloylbergenin. J. Ethnopharmacol. 2010, 131, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Nyandoro, S.S.; Nkunya, M.H.; Josepha, C.C.; Odalo, J.O.; Sattler, I. New glucopyranosylglyceryl-N-octenyl adipate and bioactivity of retro and condensed chalcones from Toussaintia orientalis. Tanz. J. Sci. 2012, 38, 108–126. [Google Scholar]
- Cui, Y.; Ao, M.; Hu, J.; Yu, L. Anti-inflammatory activity of licochalcone A isolated from Glycyrrhiza inflata. Z. Naturforsch. C 2008, 63, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull. 2003, 26, 61–65. [Google Scholar] [CrossRef]
- Kim, D.H.; Li, H.; Han, Y.E.; Jeong, J.H.; Lee, H.J.; Ryu, J.H. Modulation of inducible nitric oxide synthase expression in LPS-Stimulated BV-2 microglia by prenylated chalcones from Cullen corylifolium (L.) Medik. through inhibition of I-κBα degradation. Molecules 2018, 23, 109. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.Y.; Ryu, J.H. Prenylflavones from Psoralea corylifolia inhibit nitric oxide synthase expression through the inhibition of I-κB-α degradation in activated microglial cells. Biol. Pharm. Bull. 2005, 28, 2253–2257. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chang, F.R.; Tseng, P.Y.; Chen, Y.F.; El-Shazly, M.; Du, Y.C.; Fang, S.C. Geranyl flavonoid derivatives from the fresh leaves of Artocarpus communis and their anti-inflammatory activity. Planta Med. 2012, 78, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Han, S.; Yong, D.; Hwang, J.K. In vitro antibacterial activity of panduratin A against enterococci clinical isolates. Biol. Pharm. Bull. 2010, 33, 1489–1493. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsusita, Y.I.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Tupe, S.G.; Gample, S.P.; Chandgude, M.G.; Sarkar, D.; Deshpande, M.V.; Joshi, S.P. Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Nat. Prod. Res. 2014, 28, 245–250. [Google Scholar] [CrossRef]
- ElSohly, H.N.; Joshi, A.S.; Nimrod, A.C.; Walker, L.A.; Clark, A.M. Antifungal chalcones from Maclura tinctoria. Planta Med. 2001, 67, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Svetaz, L.; Agüero, M.B.; Alvarez, S.; Luna, L.; Feresin, G.; Derita, M.; Tapia, A.; Zacchino, S. Antifungal activity of Zuccagnia punctata Cav.: Evidence for the mechanism of action. Planta Med. 2007, 73, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Z.H.; Liu, J.K.; Zheng, Y.T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006, 14, 1710–1714. [Google Scholar] [CrossRef]
- Dao, T.T.; Nguyen, P.H.; Lee, H.S.; Kim, E.; Park, J.; Lim, S.I.; Oh, W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg. Med. Chem. Lett. 2011, 21, 294–298. [Google Scholar] [CrossRef]
- Yenesew, A.; Induli, M.; Deres, S.; Midiwo, J.O.; Heydenreich, M.; Peter, M.G.; Akala, H.; Wangui, J.; Liyala, P.; Waters, N.C. Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry 2004, 65, 3029–3032. [Google Scholar] [CrossRef]
- Narender, T.; Tanvir, K.; Rao, M.S.; Srivastava, K.; Puri, S.K. Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2005, 15, 2453–2455. [Google Scholar] [CrossRef]
- Kharazmi, A.; Chen, M.; Theander, T.; Christensen, S.B. Discovery of oxygentade chalcones as novel antimalarial agents. Ann. Trop. Med. Parasitol. 1997, 91, S91–S95. [Google Scholar] [CrossRef]
- Kuo, Y.F.; Su, Y.Z.; Tseng, Y.H.; Wang, S.Y.; Wang, H.M.; Chueh, P.J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Takaya, K.; Mitsui, T.; Kido, M.; Kakumoto, K.; Hayashi, K.I.; Kuboki, A.; Tani, H.; Ikeda, S.; Iinuma, M.; et al. New chalcone dimers from Caesalpinia ferrea Mart act as potent inhibitors of DNA topoisomerase II. Tetrahedron Lett. 2013, 54, 5052–5055. [Google Scholar] [CrossRef]
- Lai, S.L.; Cheah, S.C.; Wong, P.F.; Noor, S.M.; Mustafa, M.R. In vitro and in vivo anti-angiogenic activities of Panduratin, A. PLoS ONE 2012, 7, e38103. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Ohno, M.; Baba, K. Hypotensive and lipid regulatory actions of 4-hydroxyderricin, a chalcone from Angelica keiskei, in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2005, 32, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Logendra, S.; Ribnicky, D.M.; Yang, H.; Poulev, A.; Ma, J.; Kennelly, E.J.; Raskin, I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry 2006, 67, 1539–1546. [Google Scholar] [CrossRef]
- Fontenele, J.B.; Leal, L.K.; Ferreira, M.A.; Silveira, E.R.; Viana, G.S. Antiplatelet Effect of Lonchocarpin and Derricin Isolated from Lonchocarpus sericeus. Pharm. Biol. 2005, 43, 726–731. [Google Scholar] [CrossRef]
- Birari, R.B.; Gupta, S.; Mohan, C.G.; Bhutani, K.K. Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: Experimental and computational studies. Phytomedicine 2011, 18, 795–801. [Google Scholar] [CrossRef]
- Kramer, C.; Ting, A.; Zheng, H.; Hert, J.; Schindler, T.; Stahl, M.; Robb, G.; Crawford, J.J.; Blaney, J.; Montague, S.; et al. Learning medicinal chemistry absorption, distribution, metabolism, excretion, and toxicity (ADMET) rules from cross-company matched molecular pairs analysis (MMPA) miniperspective. J. Med. Chem. 2017, 61, 3277–3292. [Google Scholar] [CrossRef]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Yilmazer, M.; Stevens, J.F.; Deinzer, M.L.; Buhler, D.R. In vitro biotransformation of xanthohumol, a flavonoid from hops (Humulus lupulus), by rat liver microsomes. Drug Metab. Dispos. 2001, 29, 223–231. [Google Scholar] [PubMed]
- Yilmazer, M.; Stevens, J.F.; Buhler, D.R. In vitro glucuronidation of xanthohumol, a flavonoid in hop and beer, by rat and human liver microsomes. FEBS Lett. 2001, 491, 252–256. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Cermak, R.; Durazzo, A.; Maiani, G.; Böhm, V.; Kammerer, D.R.; Carle., R.; Wiczkowski, W.; Piskula, M.K.; Galensa, R. The influence of postharvest processing and storage of foodstuffs on the bioavailability of flavonoids and phenolic acids. Mol. Nutr. Food Res. 2009, 53, S184–S193. [Google Scholar] [CrossRef]
- Jaiswal, S.; Shukla, M.; Sharma, A.; Rangaraj, N.; Vaghasiya, K.; Malik, M.Y.; Lal, J. Preclinical pharmacokinetics and ADME characterization of a novel anticancer chalcone, cardamonin. Drug Test. Anal. 2017, 9, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.; bo Zou, J.; Zhang, X.; Shi, Y.; yan Guo, D. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Choi, J.S.; Choi, J.S.; Choi, D.H. Effects of licochalcone A on the bioavailability and pharmacokinetics of nifedipine in rats: Possible role of intestinal CYP3A4 and P-gp inhibition by licochalcone A. Biopharm. Drug Dispos. 2014, 35, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Boonnop, R.; Soodvilai, S. Interaction of Compounds Isolated from Boesenbergia rotunda with Human Renal Organic Anion and Cation Transporters. J. Physiol. Biomed. Sci. 2017, 30, 52–56. [Google Scholar]
- Qin, Z.; Wang, P.; Duan, S.; Wan, X.; Xing, H.; Yang, J.; Zhang, X.; Yao, Z.; Yao, X. Potential Determinants for Metabolic Fates and Inhibitory Effects of Isobavachalcone Involving in Human Cytochrome P450, UDP-Glucuronosyltransferase Enzymes, and Efflux Transporters. J. Pharm. Sci. 2021, 110, 2285–2294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).