Towards a Synthetic Biology Toolset for Metallocluster Enzymes in Biosynthetic Pathways: What We Know and What We Need
Abstract
:1. Introduction
2. Biotechnological Relevance of Metallocluster Enzymes: Potential and Challenges
2.1. Many Industrially Important Pathways Rely on Metallocluster Enzyme Activity
2.2. Challenges Related to Recombinant Pathways That Contain Metallocluster Enzymes
2.2.1. The Functional Expression of Metallocluster Enzymes Requires Specific Maturation Pathways
2.2.2. Metalloenzyme Catalysis Often Requires Specific Electron Transfer Proteins
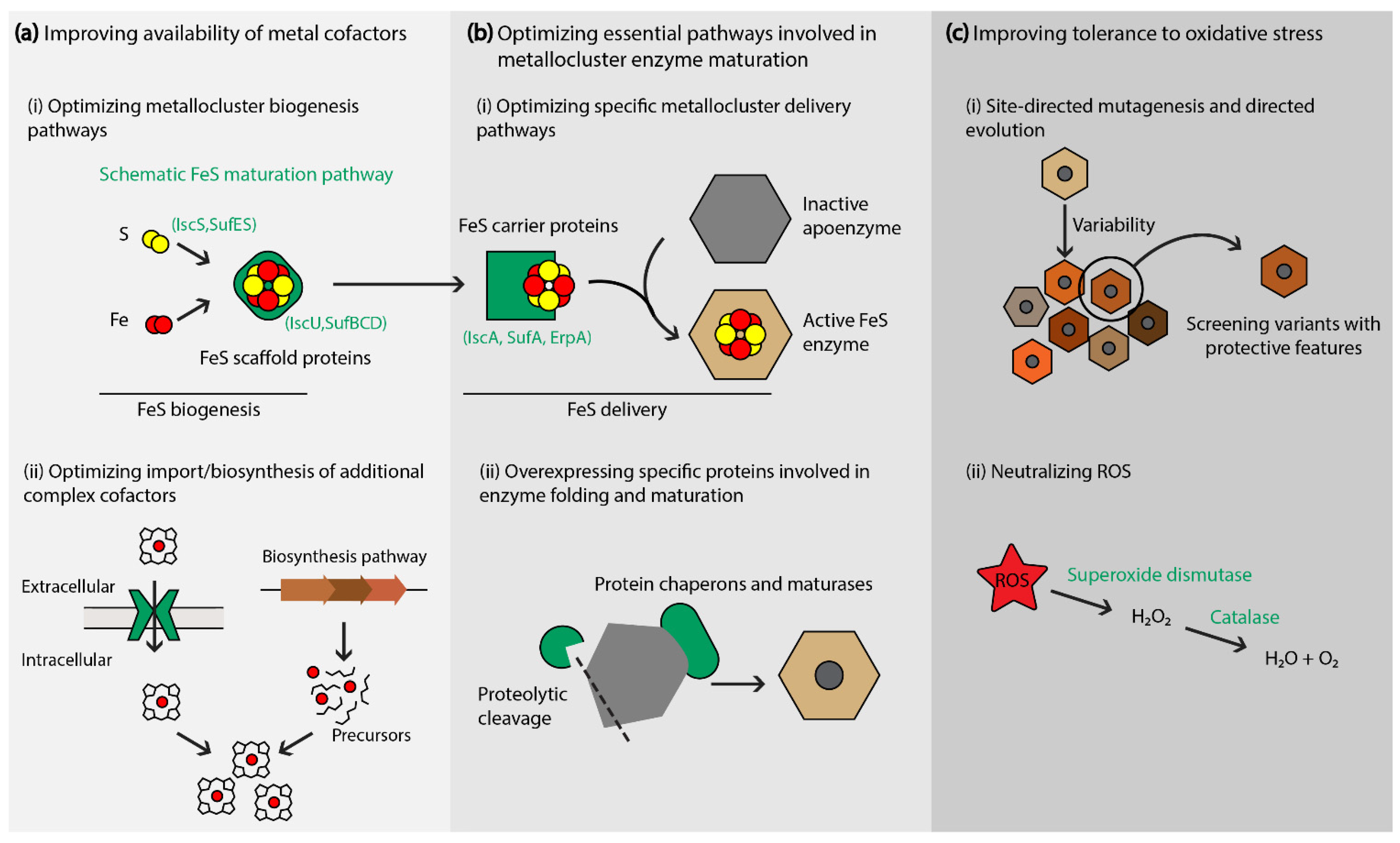
3. Strategies to the Promote Abundance of Functional Metallocluster Enzymes in Production Hosts
3.1. Optimizing Specific Maturation Pathways
3.2. Increasing the Synthesis of Additional Essential Metal Cofactors
3.3. Improving the Tolerance of Metallic Clusters to Oxidative Stress
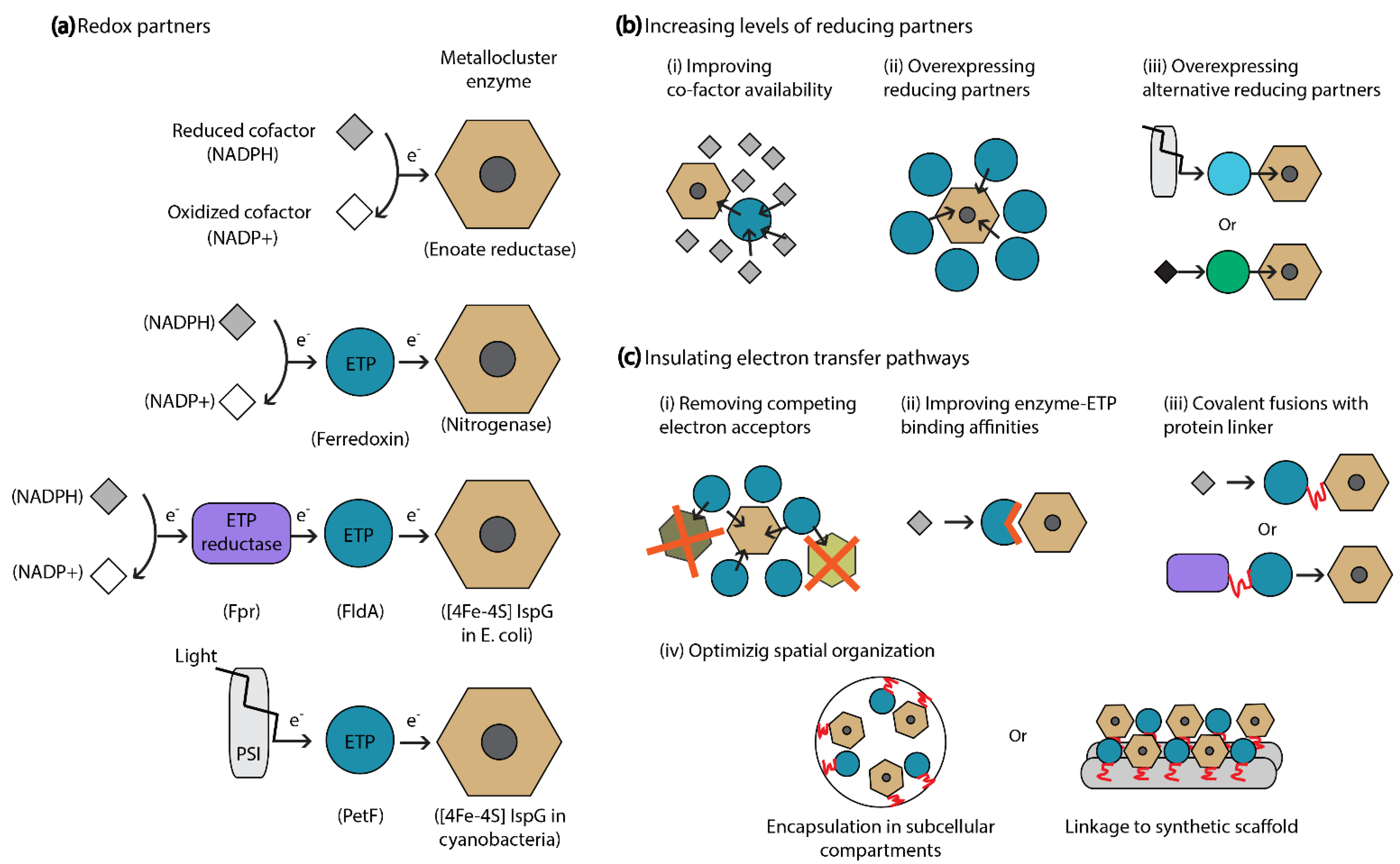
4. Optimizing Electron Transfer Pathways to Boost Redox Active Metallocluster Enzymes
4.1. Identification of Redox Partners Required for Metallocluster Enzyme Activity
4.2. Increasing the Amount of Redox Partners Enhances Electron Supply and Catalytic Activity
4.2.1. Improving Availability of Small-Molecule Cofactors
4.2.2. Overexpression of Partner ETPs to Direct Electron Flow to Metallocluster Enzymes
4.3. Insulation Strategies for Electron Transfer Pathways
4.3.1. Removing Competing Reactions
4.3.2. Covalent Fusions with Redox Partner
4.3.3. Spatial Organization of Enzymes and Partner ETPs
5. Towards Transferring Metallocluster Enzymes from Bacteria to Eukaryotes
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Enzyme | Origin | Uniprot ID | PDB ID |
|---|---|---|---|
| Fe-Fe hydrogenase | Clostridium pasteurianum | P29166 | 1FEH |
| Ni-Fe hydrogenase | Desulfovibrio fructosovorans | P18187 | 1FRF |
| [2Fe-2S] ferredoxin FdxB | Pseudomonas putida | Q76CS9 | 3AH7 |
| D-xylonate dehydratase | Caulobacter vibrioides CB15 | Q9A9Z2 | 5OYN |
| [2Fe-2S] and [4Fe-4S] rSAM enzyme BioB | Escherichia coli | P12996 | 1R30 |
| Nitrogenase molybdenum-iron protein | Azotobacter vinelandii | P07328 | 6UG0 |
| [4Fe-4S] Viperin | Nematostella vectensis | A7RNF3 | 7N7H |
| [4Fe-4S] enzyme IspG | Aquifex aeolicus | O67496 | 3NOY |
References
- Nielsen, J.; Keasling, J.D. Engineering Cellular Metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jullesson, D.; David, F.; Pfleger, B.; Nielsen, J. Impact of synthetic biology and metabolic engineering on industrial production of fine chemicals. Biotechnol. Adv. 2015, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, M.; Lee, S.Y. Prospects of microbial cell factories developed through systems metabolic engineering. Microb. Biotechnol. 2016, 9, 610–617. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. Systems metabolic engineering of Escherichia coli for the heterologous production of high value molecules—A veteran at new shores. Curr. Opin. Biotechnol. 2016, 42, 178–188. [Google Scholar] [CrossRef]
- Robertsen, H.L.; Weber, T.; Kim, H.U.; Lee, S.Y. Toward Systems Metabolic Engineering of Streptomycetes for Secondary Metabolites Production. Biotechnol. J. 2018, 13, 1700465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsui, R.; Yamada, R. Saccharomyces cerevisiae as a microbial cell factory. In Microbial Cell Factories Engineering for Production of Biomolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 319–333. [Google Scholar] [CrossRef]
- Stephanopoulos, G. Synthetic Biology and Metabolic Engineering. ACS Synth. Biol. 2012, 1, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C. Great Metalloclusters in Enzymology. Annu. Rev. Biochem. 2002, 71, 221–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R. A comparison of types of catalyst: The quality of metallo-enzymes. J. Inorg. Biochem. 2008, 102, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.S.; Drennan, C.L. Metalloenzymes in natural product biosynthetic pathways. Nat. Prod. Rep. 2018, 35, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.E.; Ryu, M.-H.; Ozaydin, B.; Broglie, R. Harnessing atmospheric nitrogen for cereal crop production. Curr. Opin. Biotechnol. 2020, 62, 181–188. [Google Scholar] [CrossRef]
- Shen, L.; Kohlhaas, M.; Enoki, J.; Meier, R.; Schönenberger, B.; Wohlgemuth, R.; Kourist, R.; Niemeyer, F.; Van Niekerk, D.; Bräsen, C.; et al. A combined experimental and modelling approach for the Weimberg pathway optimisation. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Chang, W.-C.; Xiao, Y.; Liu, H.-W.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Zhou, J.; Zhang, Z.; Kim, C.H.; Jiang, B.; Shi, J.; Hao, J. Isobutanol and 2-ketoisovalerate production by Klebsiella pneumoniae via a native pathway. Metab. Eng. 2017, 43, 71–84. [Google Scholar] [CrossRef]
- Streit, W.R.; Entcheva, P. Biotin in microbes, the genes involved in its biosynthesis, its biochemical role and perspectives for biotechnological production. Appl. Microbiol. Biotechnol. 2003, 61, 21–31. [Google Scholar] [CrossRef]
- Marous, D.R.; Lloyd, E.P.; Buller, A.R.; Moshos, K.A.; Grove, T.L.; Blaszczyk, A.J.; Booker, S.J.; Townsend, C.A. Consecutive radical S-adenosylmethionine methylations form the ethyl side chain in thienamycin biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 10354–10358. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.F.; Vagstad, A.L.; Piel, J. Polytheonamide biosynthesis showcasing the metabolic potential of sponge-associated uncultivated ‘Entotheonella’ bacteria. Curr. Opin. Chem. Biol. 2016, 31, 8–14. [Google Scholar] [CrossRef]
- Sato, S.; Kudo, F.; Kim, S.-Y.; Kuzuyama, T.; Eguchi, T. Methylcobalamin-Dependent Radical SAM C-Methyltransferase Fom3 Recognizes Cytidylyl-2-hydroxyethylphosphonate and Catalyzes the Nonstereoselective C-Methylation in Fosfomycin Biosynthesis. Biochemistry 2017, 56, 3519–3522. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Reva, A.; Huang, F.; Xiong, B.; Liu, Y.; Deng, Z.; Leadlay, P.F.; Sun, Y. Methyltransferases of gentamicin biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, 1340–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Xu, H.; Li, Y.; Zhang, F.; Chen, X.-Y.; He, Q.-L.; Igarashi, Y.; Tang, G.-L. Characterization of Yatakemycin Gene Cluster Revealing a Radical S-Adenosylmethionine Dependent Methyltransferase and Highlighting Spirocyclopropane Biosynthesis. J. Am. Chem. Soc. 2012, 134, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Millman, A.; Ofir, G.; Meitav, G.; Avraham, C.; Shomar, H.; Rosenberg, M.M.; Tal, N.; Melamed, S.; Amitai, G.; et al. Prokaryotic viperins produce diverse antiviral molecules. Nat. Cell Biol. 2021, 589, 120–124. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B12: A review and future perspectives. Microb. Cell Factories 2017, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.C.; Khusnutdinova, A.N.; Flick, R.; Kim, T.; Bornscheuer, U.T.; Yakunin, A.F.; Mahadevan, R. Alkene hydrogenation activity of enoate reductases for an environmentally benign biosynthesis of adipic acid. Chem. Sci. 2017, 8, 1406–1413. [Google Scholar] [CrossRef] [Green Version]
- Micallef, M.L.; Sharma, D.; Bunn, B.M.; Gerwick, L.; Viswanathan, R.; Moffitt, M.C. Comparative analysis of hapalindole, ambiguine and welwitindolinone gene clusters and reconstitution of indole-isonitrile biosynthesis from cyanobacteria. BMC Microbiol. 2014, 14, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.W.; Schut, G.J.; Boyd, E.S.; Mulder, D.W.; Shepard, E.M.; Broderick, J.B.; King, P.W.; Adams, M.W.W. [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta—Mol. Cell Res. 2015, 1853, 1350–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldron, K.; Robinson, N. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef]
- Roche, B.; Aussel, L.; Ezraty, B.; Mandin, P.; Py, B.; Barras, F. Iron/sulfur proteins biogenesis in prokaryotes: Formation, regulation and diversity. Biochim. Biophys. Acta—Bioenerg. 2013, 1827, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Rubio, L.M.; Ludden, P.W. Maturation of Nitrogenase: A Biochemical Puzzle. J. Bacteriol. 2005, 187, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Shomar, H.; Garcia, P.S.; Fernández-Fueyo, E.; D’angelo, F.; Pelosse, M.; Manuel, R.R.; Büke, F.; Liu, S.; van den Broek, N.; Duraffourg, N.; et al. Resolving phylogenetic and biochemical barriers to functional expression of heterologous iron-sulphur cluster enzymes. bioRxiv 2021. bioRxiv:2021.02.02.429153. [Google Scholar]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef]
- Yokoyama, N.; Nonaka, C.; Ohashi, Y.; Shioda, M.; Terahata, T.; Chen, W.; Sakamoto, K.; Maruyama, C.; Saito, T.; Yuda, E.; et al. Distinct roles for U-type proteins in iron–sulfur cluster biosynthesis revealed by genetic analysis of the Bacillus subtilis sufCDSUB operon. Mol. Microbiol. 2018, 107, 688–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinella, D.; Brochier-Armanet, C.; Loiseau, L.; Talla, E.; Barras, F. Iron-Sulfur (Fe/S) Protein Biogenesis: Phylogenomic and Genetic Studies of A-Type Carriers. PLoS Genet. 2009, 5, e1000497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, P.C.; Johnson, D.C.; Ragle, B.E.; Unciuleac, M.C.; Dean, D.R. Controlled expression of nif and isc iron-sulfur protein maturation components reveals target specificity and limited functional replacement between the two systems. J. Bacteriol. 2007, 189, 2854–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Ribbe, M.W. Nitrogenase assembly. Biochim. Biophys. Acta—Bioenerg. 2013, 1827, 1112–1122. [Google Scholar] [CrossRef] [Green Version]
- Biphenyls, C.P. Structure and Function of Four Classes of the 4Fe-4S Protein, IspH. Biochemistry 2016, 55, 4119–4129. [Google Scholar]
- Atkinson, J.; Campbell, I.; Bennett, G.N.; Silberg, J.J. Cellular Assays for Ferredoxins: A Strategy for Understanding Electron Flow through Protein Carriers That Link Metabolic Pathways. Biochemistry 2016, 55, 7047–7064. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.; Wang, C.; Choi, E.-S.; Kim, S.-W. Enhanced performance of the methylerythritol phosphate pathway by manipulation of redox reactions relevant to IspC, IspG, and IspH. J. Biotechnol. 2017, 248, 1–8. [Google Scholar] [CrossRef]
- Chotani, G.K.; Mcauliffe, J.C.; Miller, M.C.; Muir, R.E.; Vavlline, D.V.; Weyler, W. Isoprene Production Using the DXP and MVA Pathway. U.S. Patent 8,507,235 B2, 20 January 2011. [Google Scholar]
- Vickers, C.E.; Sabri, S. Isoprene. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 289–317. [Google Scholar]
- Poudel, S.; Colman, D.R.; Fixen, K.R.; Ledbetter, R.N.; Zheng, Y.; Pence, N.; Seefeldt, L.C.; Peters, J.W.; Harwood, C.S.; Boyd, E.S. Electron Transfer to Nitrogenase in Different Genomic and Metabolic Backgrounds. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [Green Version]
- Kuchar, J.; Hausinger, R.P. Biosynthesis of Metal Sites. Chem. Rev. 2004, 104, 509–526. [Google Scholar] [CrossRef]
- Senger, M.; Stripp, S.T.; Soboh, B. Proteolytic cleavage orchestrates cofactor insertion and protein assembly in [NiFe]-hydrogenase biosynthesis. J. Biol. Chem. 2017, 292, 11670–11681. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Hopkins, R.C.; Jenney, F.E.; McTernan, P.M.; Adams, M.W.W. Heterologous expression and maturation of an NADP-Dependent [NiFe]-Hydrogenase: A key enzyme in biofuel production. PLoS ONE 2010, 5, e10526. [Google Scholar] [CrossRef] [Green Version]
- Weyman, P.D.; Vargas, W.A.; Chuang, R.-Y.; Chang, Y.; Smith, H.O.; Xu, Q. Heterologous expression of Alteromonas macleodii and Thiocapsa roseopersicina [NiFe] hydrogenases in Escherichia coli. Microbiology 2011, 157, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Agapakis, C.M.; Ducat, D.C.; Boyle, P.M.; Wintermute, E.H.; Way, J.C.; Silver, P.A. Insulation of a synthetic hydrogen metabolism circuit in bacteria. J. Biol. Eng. 2010, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Colunga, L.M.; Rodríguez, A.D.L. Escherichia coli and its application to biohydrogen production. Rev. Environ. Sci. Bio/Technol. 2015, 14, 123–135. [Google Scholar] [CrossRef]
- Dixon, R.A.; Postgate, J.R. Transfer of Nitrogen-fixation Genes by Conjugation in Klebsiella pneumoniae. Nature 1971, 234, 47–48. [Google Scholar] [CrossRef]
- Yang, J.; Xie, X.; Wang, X.; Dixon, R.; Wang, Y. Reconstruction and minimal gene requirements for the alternative iron-only nitrogenase in Escherichia coli. Proc. Natl. Acad. Sci. USA 2014, 111, E3718–E3725. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, M.; Li, J.; Wang, G.; Li, F.; Xiong, M. Chaperone-assisted maturation of the recombinant Fe-type nitrile hydratase is insufficient for fully active expression in Escherichia coli. Process. Biochem. 2017, 56, 37–44. [Google Scholar] [CrossRef]
- Zheng, L.; Cash, V.L.; Flint, D.H.; Dean, D.R. Assembly of Iron-Sulfur Clusters—Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 1998, 273, 13264–13272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zepeck, F.; Gräwert, T.; Kaiser, J.; Schramek, N.; Eisenreich, W.; Bacher, A.A.; Rohdich, F. Biosynthesis of Isoprenoids. Purification and Properties of IspG Protein from Escherichia coli. J. Org. Chem. 2005, 70, 9168–9174. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, A.B.; Hyeon, J.E.; Han, S.O. Improved catalytic activities of a dye-decolorizing peroxidase (DyP) by overexpression of ALA and heme biosynthesis genes in Escherichia coli. Process. Biochem. 2015, 50, 1272–1276. [Google Scholar] [CrossRef]
- Lanz, N.D.; Blaszczyk, A.J.; McCarthy, E.L.; Wang, B.; Wang, R.X.; Jones, B.S.; Booker, S.J. Enhanced Solubilization of Class B Radical S-Adenosylmethionine Methylases by Improved Cobalamin Uptake in Escherichia coli. Biochemistry 2018, 57, 1475–1490. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Li, D.; Kang, J.; Jiang, P.; Sun, J.; Zhang, D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kirby, J.; Dietzel, K.L.; Wichmann, G.; Chan, R.; Antipov, E.; Moss, N.; Baidoo, E.E.; Jackson, P.; Gaucher, S.P.; Gottlieb, S.; et al. Engineering a functional 1-deoxy-D-xylulose 5-phosphate (DXP) pathway in Saccharomyces cerevisiae. Metab. Eng. 2016, 38, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imlay, J.A.; Sethu, R.; Rohaun, S.K. Evolutionary adaptations that enable enzymes to tolerate oxidative stress. Free Radic. Biol. Med. 2019, 140, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kalms, J.; Schmidt, A.; Frielingsdorf, S.; Utesch, T.; Gotthard, G.; von Stetten, D.; van der Linden, P.; Royant, A.; Mroginski, M.A.; Carpentier, P.; et al. Tracking the route of molecular oxygen in O2-tolerant membrane-bound [NiFe] hydrogenase. Proc. Natl. Acad. Sci. USA 2018, 115, E2229–E2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.H.; Jo, B.H.; Cha, H.J. Production of biohydrogen by heterologous expression of oxygen-tolerant Hydrogenovibrio marinus [NiFe]-hydrogenase in Escherichia coli. J. Biotechnol. 2011, 155, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Liebgott, P.-P.; de Lacey, A.L.; Burlat, B.; Cournac, L.; Richaud, P.; Brugna, M.; Fernandez, V.M.; Guigliarelli, B.; Rousset, M.; Léger, C.; et al. Original Design of an Oxygen-Tolerant [NiFe] Hydrogenase: Major Effect of a Valine-to-Cysteine Mutation near the Active Site. J. Am. Chem. Soc. 2010, 133, 986–997. [Google Scholar] [CrossRef]
- Schlesier, J.; Rohde, M.; Gerhardt, S.; Einsle, O. A Conformational Switch Triggers Nitrogenase Protection from Oxygen Damage by Shethna Protein II (FeSII). J. Am. Chem. Soc. 2016, 138, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Kabasakal, B.V.; Cotton, C.A.R.; Schumacher, J.; Rutherford, A.W.; Fantuzzi, A.; Murray, J.W. A low-potential terminal oxidase associated with the iron-only nitrogenase from the nitrogen-fixing bacterium Azotobacter vinelandii. J. Biol. Chem. 2019, 294, 9367–9376. [Google Scholar] [CrossRef] [Green Version]
- Biz, A.; Mahadevan, R. Overcoming Challenges in Expressing Iron–Sulfur Enzymes in Yeast. Trends Biotechnol. 2021, 39, 665–677. [Google Scholar] [CrossRef]
- Wang, Y.; San, K.-Y.; Bennett, G.N. Cofactor engineering for advancing chemical biotechnology. Curr. Opin. Biotechnol. 2013, 24, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Englund, E.; Shabestary, K.; Hudson, E.; Lindberg, P. Systematic overexpression study to find target enzymes enhancing production of terpenes in Synechocystis PCC 6803, using isoprene as a model compound. Metab. Eng. 2018, 49, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Agapakis, C.M.; Silver, P.A. Modular electron transfer circuits for synthetic biology. Bioeng. Bugs 2010, 1, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, S.; Siebel, J.F.; Farès, C.; Duan, J.; Reijerse, E.; Happe, T.; Lubitz, W.; Winkler, M. Enhancing hydrogen production of microalgae by redirecting electrons from photosystem I to hydrogenase. Energy Environ. Sci. 2014, 7, 3296–3301. [Google Scholar] [CrossRef]
- Lamont, C.M.; Kelly, C.; Pinske, C.; Buchanan, G.; Palmer, T.; Sargent, F. Expanding the substrates for a bacterial hydrogenlyase reaction. Microbiology 2017, 163, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Agapakis, C.M.; Boyle, P.M.; Silver, P.A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 2012, 8, 527–535. [Google Scholar] [CrossRef]
- Delebecque, C.J.; Lindner, A.B.; Silver, P.A.; Aldaye, F.A. Organization of Intracellular Reactions with Rationally Designed RNA Assemblies. Science 2011, 333, 470–474. [Google Scholar] [CrossRef]
- Lee, H.; DeLoache, W.C.; Dueber, J.E. Spatial organization of enzymes for metabolic engineering. Metab. Eng. 2012, 14, 242–251. [Google Scholar] [CrossRef]
- Carlsen, S.; Ajikumar, P.K.; Formenti, L.R.; Zhou, K.; Phon, T.H.; Nielsen, M.L.; Lantz, A.E.; Kielland-Brandt, M.C.; Stephanopoulos, G. Heterologous expression and characterization of bacterial 2-C-methyl-d-erythritol-4-phosphate pathway in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 5753–5769. [Google Scholar] [CrossRef] [Green Version]
- Benisch, F.; Boles, E. The bacterial Entner–Doudoroff pathway does not replace glycolysis in Saccharomyces cerevisiae due to the lack of activity of iron–sulfur cluster enzyme 6-phosphogluconate dehydratase. J. Biotechnol. 2014, 171, 45–55. [Google Scholar] [CrossRef]
- Wasserstrom, L.; Portugal-Nunes, D.; Almqvist, H.; Sandström, A.G.; Lidén, G.; Gorwa-Grauslund, M.F. Exploring d-xylose oxidation in Saccharomyces cerevisiae through the Weimberg pathway. AMB Express 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Partow, S.; Siewers, V.; Daviet, L.; Schalk, M.; Nielsen, J. Reconstruction and Evaluation of the Synthetic Bacterial MEP Pathway in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e52498. [Google Scholar] [CrossRef] [Green Version]
- Anthony, L.C.; Maggio-Hall, L.A.; Rothman, S.C.; Tomb, J.-F. Increased Heterologous Fe-S Enzyme Activity in Yeast. U.S. Patent US20,160,024,534A1, 28 January 2016. [Google Scholar]
- Salusjärvi, L.; Toivari, M.; Vehkomäki, M.-L.; Koivistoinen, O.; Mojzita, D.; Niemelä, K.; Penttilä, M.; Ruohonen, L. Production of ethylene glycol or glycolic acid from D-xylose in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 8151–8163. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.J.; Dean, D.R. Keeping the nitrogen-fixation dream alive. Proc. Natl. Acad. Sci. USA 2017, 114, 3009–3011. [Google Scholar] [CrossRef] [Green Version]
- López-Torrejón, G.; Jiménez-Vicente, E.; Buesa, J.M.; Hernandez, J.A.; Verma, H.K.; Rubio, L.M. Expression of a functional oxygen-labile nitrogenase component in the mitochondrial matrix of aerobically grown yeast. Nat. Commun. 2016, 7, 11426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Xie, X.; Yang, M.; Dixon, R.; Wang, Y. Modular electron-transport chains from eukaryotic organelles function to support nitrogenase activity. Proc. Natl. Acad. Sci. USA 2017, 114, E2460–E2465. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Payá-Tormo, L.; Coroian, D.; García-Rubio, I.; Castellanos-Rueda, R.; Eseverri, Á.; López-Torrejón, G.; Burén, S.; Rubio, L.M. Exploiting genetic diversity and gene synthesis to identify superior nitrogenase NifH protein variants to engineer N2-fixation in plants. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwizer, F.; Okamoto, Y.; Heinisch, T.; Gu, Y.; Pellizzoni, M.M.; Lebrun, V.; Reuter, R.; Köhler, V.; Lewis, J.C.; Ward, T.R. Artificial Metalloenzymes: Reaction Scope and Optimization Strategies. Chem. Rev. 2018, 118, 142–231. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Yu, Y.; Wang, J. Improving artificial metalloenzymes’ activity by optimizing electron transfer. Chem. Commun. 2017, 53, 4173–4186. [Google Scholar] [CrossRef]
- Artero, V.; Berggren, G.; Atta, M.; Caserta, G.; Roy, S.; Pecqueur, L.; Fontecave, M. From Enzyme Maturation to Synthetic Chemistry: The Case of Hydrogenases. Acc. Chem. Res. 2015, 48, 2380–2387. [Google Scholar] [CrossRef]
- Atkinson, J.T.; Campbell, I.; Thomas, E.E.; Bonitatibus, S.C.; Elliott, S.J.; Bennett, G.N.; Silberg, J.J. Metalloprotein switches that display chemical-dependent electron transfer in cells. Nat. Chem. Biol. 2019, 15, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mutter, A.C.; Tyryshkin, A.M.; Campbell, I.; Poudel, S.; Bennett, G.N.; Silberg, J.J.; Nanda, V.; Falkowski, P.G. De novo design of symmetric ferredoxins that shuttle electrons in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 14557–14562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.J.; Margolis, P.S.; Trias, J.; Yuan, Z. Targeting metalloenzymes: A strategy that works. Curr. Opin. Pharmacol. 2003, 3, 502–507. [Google Scholar] [CrossRef]

| Name/Type | Metal Cluster Composition | Compound or Compound Family | Native Pathway | Biotechnological Relevance | Ref. |
|---|---|---|---|---|---|
| Nitrogenase | Complex FeS cluster [X–7Fe–C-9S] where “X” is molybdenum, vanadium, or iron | Reduction of N2 to bioavailable NH3 | Nitrogen fixation | Agriculture; plant-associated nitrogen-fixing bacteria | [11] |
| D-xylonate dehydratase | [2Fe-2S] cluster | Xylose catabolism, 1,2,4 butanetriol production | Weimberg pathway | Renewable carbon sources | [12] |
| IspG, IspH | [4Fe-4S] cluster | Isoprenoid precursors | MEP pathway | Pharmaceuticals, fragances, flavors, biofuels, polymers | [13] |
| IlvD | [4Fe-4S] cluster | Isobutyraldehyde isobutanol | Isoleucine and valine pathway | Biofuels | [14] |
| BioB | B12-Radical SAM [2Fe-2S] and [4Fe-4S] clusters | Biotin | Biotin biosynthesis | Food supplements, pharmaceuticals, cosmetics, animal feed | [15] |
| ThnK, ThnL, ThnP | B12-Radical SAM [4Fe-4S] clusters | Thienamycin and carbapenem derivatives | Thienamycin pathway | Antibiotics | [16] |
| PoyB, PoyC, PoyD | Radical SAM [4Fe-4S] clusters | Polytheonamide cytotoxins | Polytheonamide biosynthesis | Antibiotics | [17] |
| Fom3 | B12-Radical SAM [4Fe-4S] cluster | Fosfomycin | Fosfomycin biosynthesis | Antibiotics | [18] |
| GenK, GenD1 | B12-Radical SAM [4Fe-4S] cluster | Gentamicin | Gentamicin biosynthesis | Antibiotics | [19] |
| YtkT (radical SAM/FeS) | Radical SAM [4Fe-4S] cluster | Yatakemycin | Yatakemycin biosynthesis | Antitumor | [20] |
| Viperin | Radical SAM [4Fe-4S] cluster | Antiviral ribonucleotides | Antiviral defense | Antivirals | [21] |
| Cob enzymes | Contain [4Fe-4S] clusters | Vitamin B12 | Cobalamin biosynthesis | Medical and food industries | [22] |
| Enoate reductases | [4Fe-4S] cluster | Adipic acid, precursor for Nylon-6,6 polymer | Phenylalanine pathway | Commodity chemicals | [23] |
| [2Fe-2S] Rieske-type oxygenases | Iron-sulfur Rieske domain and non-heme Fe(II)-binding motif | Hapalindole-type products | Hapalindole biosynthesis | Antimycotic insecticidal | [24] |
| Hydrogenases | [FeFe]- or [NiFe] active site. Contain multiple Fe-S subclusters | Hydrogen (H2) gas | Hydrogen production | Biofuels | [25,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomar, H.; Bokinsky, G. Towards a Synthetic Biology Toolset for Metallocluster Enzymes in Biosynthetic Pathways: What We Know and What We Need. Molecules 2021, 26, 6930. https://doi.org/10.3390/molecules26226930
Shomar H, Bokinsky G. Towards a Synthetic Biology Toolset for Metallocluster Enzymes in Biosynthetic Pathways: What We Know and What We Need. Molecules. 2021; 26(22):6930. https://doi.org/10.3390/molecules26226930
Chicago/Turabian StyleShomar, Helena, and Gregory Bokinsky. 2021. "Towards a Synthetic Biology Toolset for Metallocluster Enzymes in Biosynthetic Pathways: What We Know and What We Need" Molecules 26, no. 22: 6930. https://doi.org/10.3390/molecules26226930






