Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer
Abstract
:1. Introduction
2. Results and Discussion
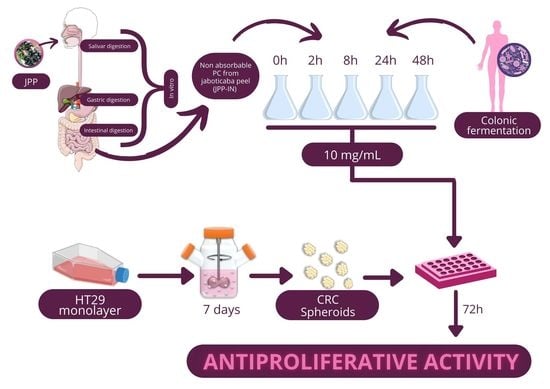
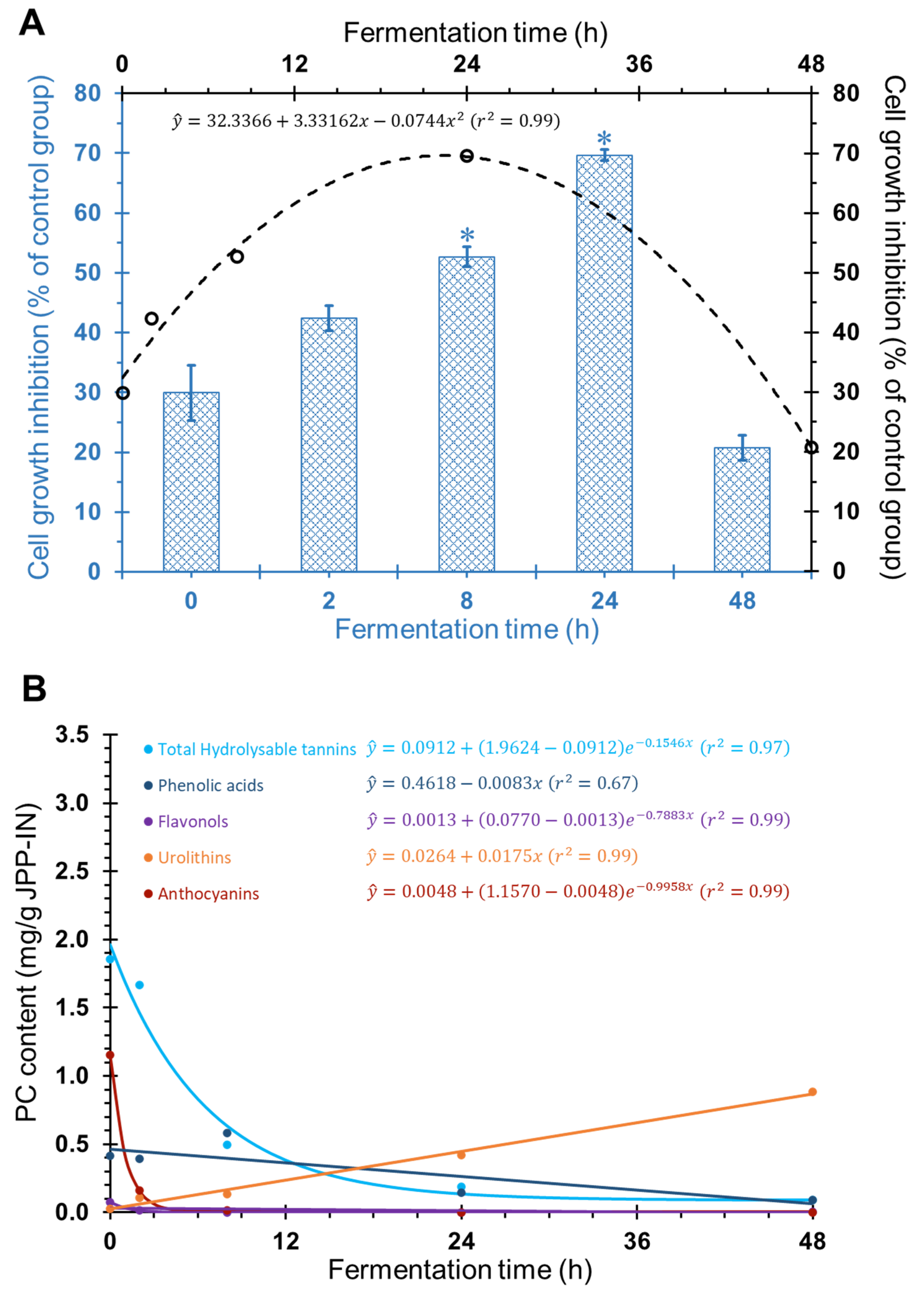
2.1. Antiproliferative Effect of FJPP in Monolayer Cultures and Spheroids of HT29 Cell Line
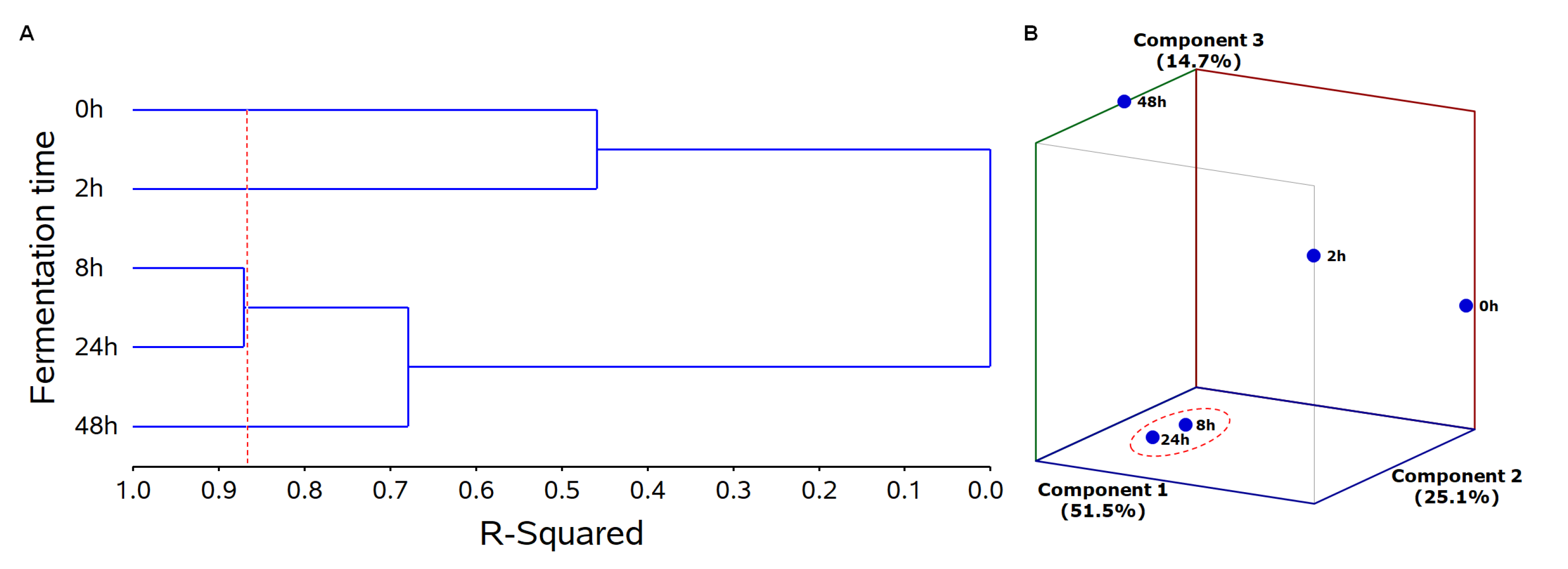
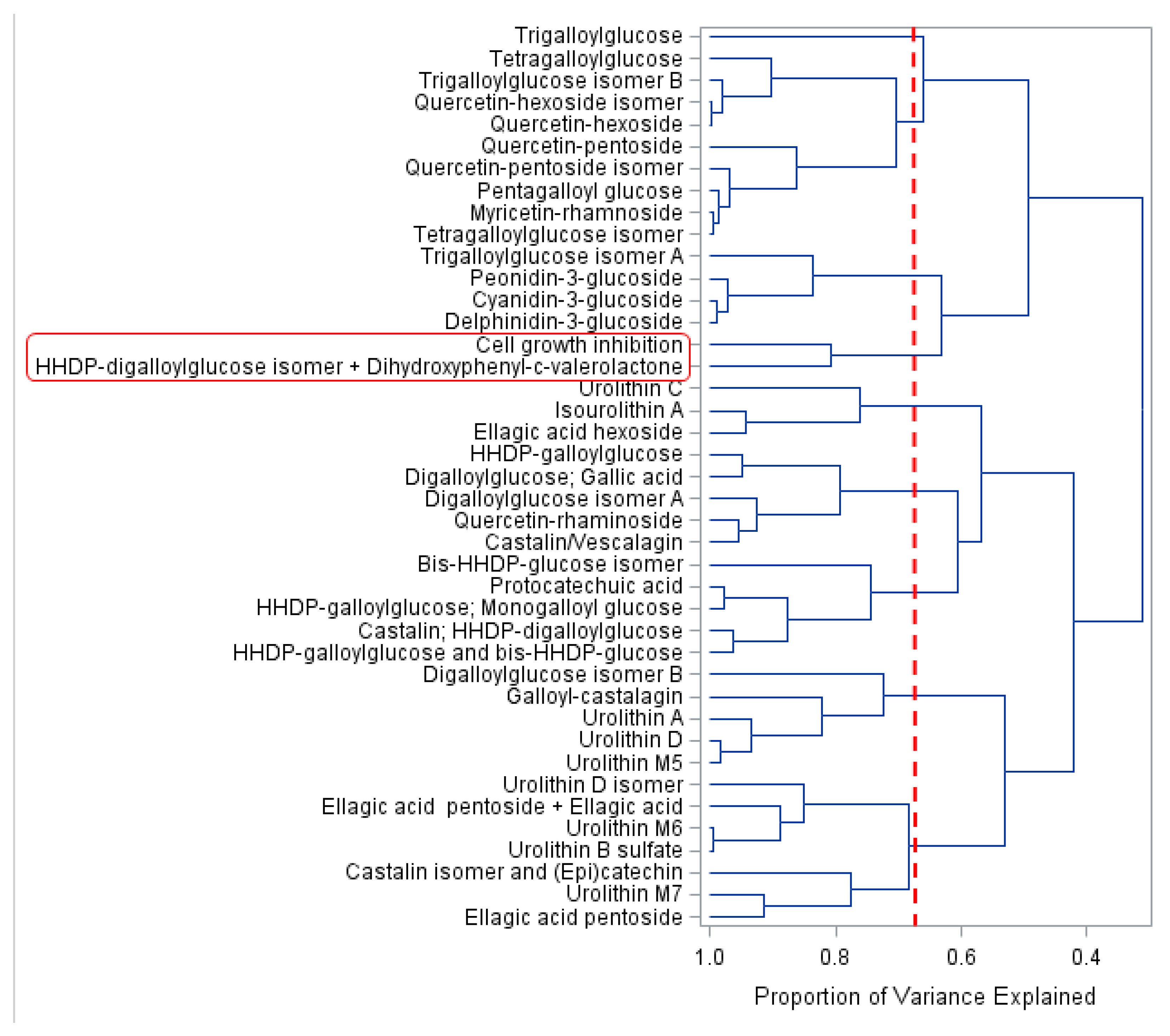
2.2. Chemometric Analyses to Identify Bioactive PC Metabolites
2.2.1. Treatment Grouping
2.2.2. Variable Grouping
3. Materials and Methods
3.1. FJPP Samples
3.2. Cell-Based Assays
3.2.1. Cell Lines and Culture
3.2.2. 3D Cell Culture Using a Stirred-Tank Culture System
3.2.3. Cytotoxicity Assay in Caco-2 Cells
3.2.4. Antiproliferative Assay in HT29 Cell Monolayers
3.2.5. Antiproliferative Assay in HT29 Cell Spheroids (3D Cells)
3.3. HPLC-Q-TOF-MS/MS Analysis of PC during Colonic Fermentation
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Agency for Research on Cancer, Global Cancer Observatory. Cancer Today. 2018. Available online: https.//gco.iarc.fr (accessed on 12 June 2021).
- Jaganathan, S.K.; Vellayappan, M.V.; Narasimhan, G.; Supriyanto, E.; Dewi, D.E.O.; Narayanan, A.L.T.; Balaji, A.; Subramanian, A.P.; Yusof, M. Chemopreventive effect of apple and berry fruits against colon cancer. World J. Gastroenterol. 2014, 20, 17029–17036. [Google Scholar] [CrossRef]
- Costea, T.; Hudită, A.; Ciolac, O.-A.; Gălăteanu, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.M. Chemoprevention of colorectal cancer by dietary compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Alves, S.C.; Pereira, R.S.; Pereira, A.B.; Ferreira, A.; Mecha, E.; Silva, A.B.; Serra, A.T.; Bronze, M.R. Identification of functional compounds in baru (Dipteryx alata Vog.) nuts. Nutritional value, volatile and phenolic composition, antioxidant activity and antiproliferative effect. Food Res. Int. 2020, 131, 109026. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Serra, M.; Silva, A.C.; Brckalo, T.; Seshire, A.; Brito, C.; Wolf, M.; Alves, P.M. Scalable culture strategies for the expansion of patient-derived cancer stem cell lines. Stem Cells Int. 2019, 2019, 8347595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, I.; Estrada, M.F.; Pereira, V.C.; da Silva, A.B.; Bronze, M.R.; Alves, P.M.; Duarte, C.M.M.; Brito, C.; Serra, A.T. polymethoxylated flavones from orange peels inhibit cell proliferation in a 3D cell model of human colorectal Ccncer. Nutr. Cancer 2018, 70, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Duarte, M.; Silva, P.; Bento da Silva, A.; Duarte, C.; Cifuentes, A.; García-Cañas, V.; Bronze, M.R.; Albuquerque, C.; Serra, A.T. Polymethoxylated flavones target cancer stemness and improve the antiproliferative effect of 5-fluorouracil in a 3D cell model of colorectal cancer. Nutrients 2019, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.; Albuquerque, C.; Serra, A. Targeting colorectal cancer proliferation, stemness and metastatic potential using Brassicaceae extracts enriched in isothiocyanates. A 3D cell model-based study. Nutrients 2017, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A.; Bertea, C.M.; Gentile, C. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits. A new source of nutraceuticals. Food Chem. 2020, 307, 125515. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica Junior, M.R.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel. A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Wallace, A.M.; Adachi, S.; Gil, R.R.; Yang, H.; Basile, M.J.; D’Armiento, J.; Weinstein, I.B.; Kennelly, E.J. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora). J. Nat. Prod. 2006, 69, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Junior, M.R.M.; Fonseca, B.d.S.; Ragagnin de Menezes, C.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2020, 65, 103714. [Google Scholar] [CrossRef]
- Frauches, N.S.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, Bioavailability, and Gut Microbiota Transformations of Dietary Phenolic Compounds. Implications for Covid-19. J. Nutr. Biochem. 2021, 108787. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Attri, S.; Goel, G. Influence of polyphenol rich seabuckthorn berries juice on release of polyphenols and colonic microbiota on exposure to simulated human digestion model. Food Res. Int. 2021, 111, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Castro, A.P.; Zamora-Gasga, V.M.; Alvarez-Parrilla, E.; Ruíz-Valdiviezo, V.M.; Venema, K.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of tomato (Solanum lycopersicum L.) and husk tomato (Physalis ixocarpa Brot.). Phenolic compounds released and bioconverted by gut microbiota. Food Chem. 2021, 360, 130051. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Mohajeri, M.H. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Benef. Microbes. 2018, 9, 725–742. [Google Scholar] [CrossRef]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M.; et al. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Ma, G.; Khan, S.I.; Ferreira, D. Colon cancer chemopreventive activities of pomegranate ellagitannins and Urolithins. J. Agric. Food Chem. 2010, 58, 2180–2187. [Google Scholar] [CrossRef]
- Vieira do Carmo, M.A.; Fidelis, M.; Francielli de Oliveira, P.; Feitoza, L.Q.; Marques, M.J.; Ferreira, E.B.; Oh, W.Y.; Shahidi, F.; Hellström, J.; Almeida, L.A.; et al. Ellagitannins from jabuticaba (Myrciaria jaboticaba) seeds attenuated inflammation, oxidative stress, aberrant crypt foci, and modulated gut microbiota in rats with 1, 2 dimethyl hydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2021, 154, 112287. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Tamemoto, K.; Yayabe, F.; Kakuda, T. Urinary excretion of 5-(3′, 4′-Dihydroxyphenyl)-γ -valerolactone, a ring-fission metabolite of (-)-epicatechin, in rats and its in vitro antioxidant activity. J. Agric. Food Chem. 2003, 51, 6893–6898. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Carregosa, D.; Domenech-Coca, C.; Savi, M.; Figueira, I.; Brindani, N.; Jang, S.; Lakshman, S.; Molokin, A.; Urban, J.F., Jr.; et al. 5-(Hydroxyphenyl)-γ-valerolactone-sulfate, a key microbial metabolite of flavan-3-ols, is able to reach the brain. Evidence from different in silico, in vitro and in vivo experimental models. Nutrients 2019, 11, 2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal-Pan, A.; Dudonné, S.; Bourassa, P.; Bourdoulous, M.; Tremblay, C.; Desjardins, Y.; Calon, F. Neurophenols consortium, Cognitive-enhancing effects of a polyphenols-rich extract from fruits without changes in neuropathology in an animal model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 55, 115–135. [Google Scholar] [CrossRef] [Green Version]
- Holkem, A.T.; Robichaud, V.; Favaro-Trindade, C.S.; Lacroix, M. Chemopreventive properties of extracts obtained from blueberry (Vaccinium myrtillus L.) and jabuticaba (Myrciaria cauliflora Berg.) in combination with probiotics. Nutr. Cancer 2021, 73, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, M.; Santos, J.S.; Escher, G.B.; Rocha, R.S.; Cruz, A.G.; Cruz, T.M.; Marques, M.B.; Nunes, J.B.; do Carmo, M.A.V.; de Almeida, L.A.; et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O.Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1, 2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021, 334, 127565. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells. Food Chem. Toxicol. 2020, 139, 111260. [Google Scholar] [CrossRef]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusti, P.R.; Quatrin, A.; Mello, R.; Bochi, V.C.; Rodrigues, E.; Prazeres, I.D.; Macedo, A.C.; Oliveira-Alves, S.C.; Emanuelli, T.; Bronze, M.R.; et al. Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer. Molecules 2021, 26, 4469. https://doi.org/10.3390/molecules26154469
Augusti PR, Quatrin A, Mello R, Bochi VC, Rodrigues E, Prazeres ID, Macedo AC, Oliveira-Alves SC, Emanuelli T, Bronze MR, et al. Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer. Molecules. 2021; 26(15):4469. https://doi.org/10.3390/molecules26154469
Chicago/Turabian StyleAugusti, Paula Rossini, Andréia Quatrin, Renius Mello, Vivian Caetano Bochi, Eliseu Rodrigues, Inês D. Prazeres, Ana Catarina Macedo, Sheila Cristina Oliveira-Alves, Tatiana Emanuelli, Maria Rosário Bronze, and et al. 2021. "Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer" Molecules 26, no. 15: 4469. https://doi.org/10.3390/molecules26154469