New Coumarin Derivatives as Cholinergic and Cannabinoid System Modulators
Abstract
:1. Introduction
- the carbamate function is the key fragment for the interaction with the catalytic sites of ChEs and FAAH where it is supposed to carbamoylate the catalytic serine residue;
- the N,N’-benzylmethylamino group gave potent enzyme inhibition and was proposed as the recognition element for the anionic sites of both AChE and BuChE;
- a 3-methylene units linker between the coumarin and the amino function provided high and balanced inhibition activity.
2. Results
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Predicted Pharmacokinetic Parameters
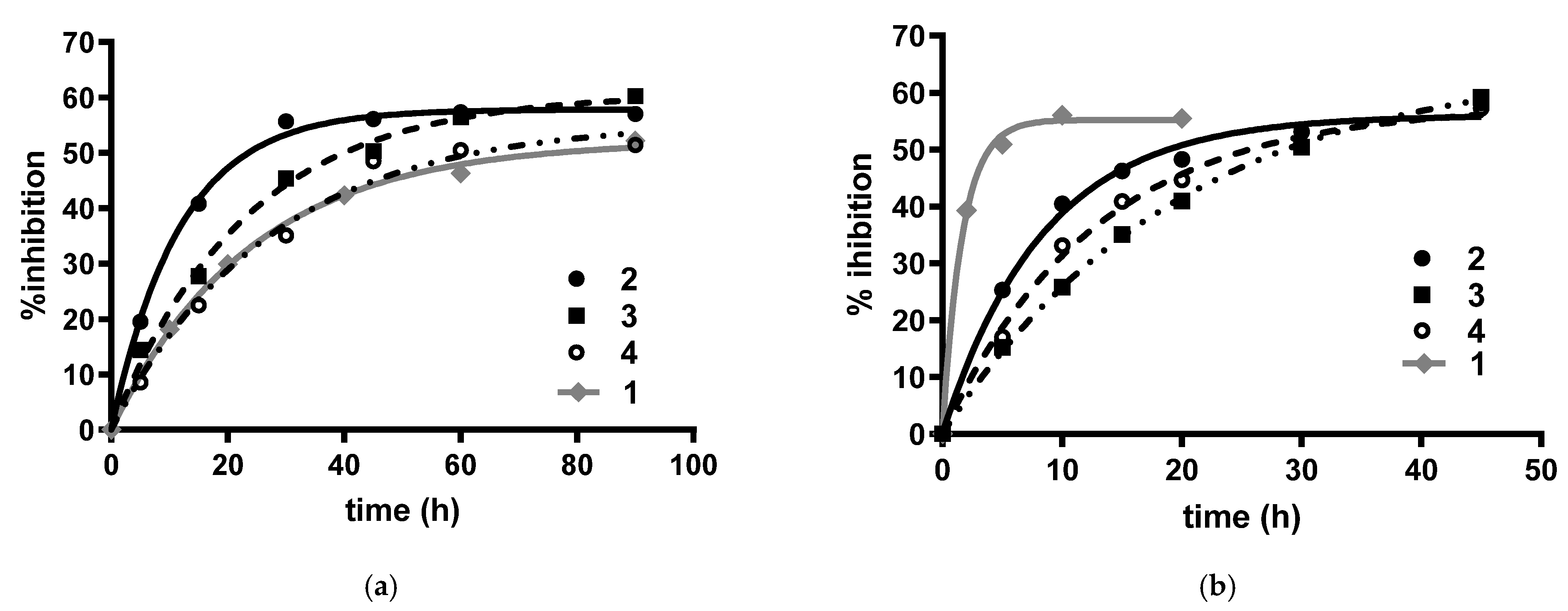
2.2.2. Cholinesterases and FAAH Inhibition by Compounds 2–7
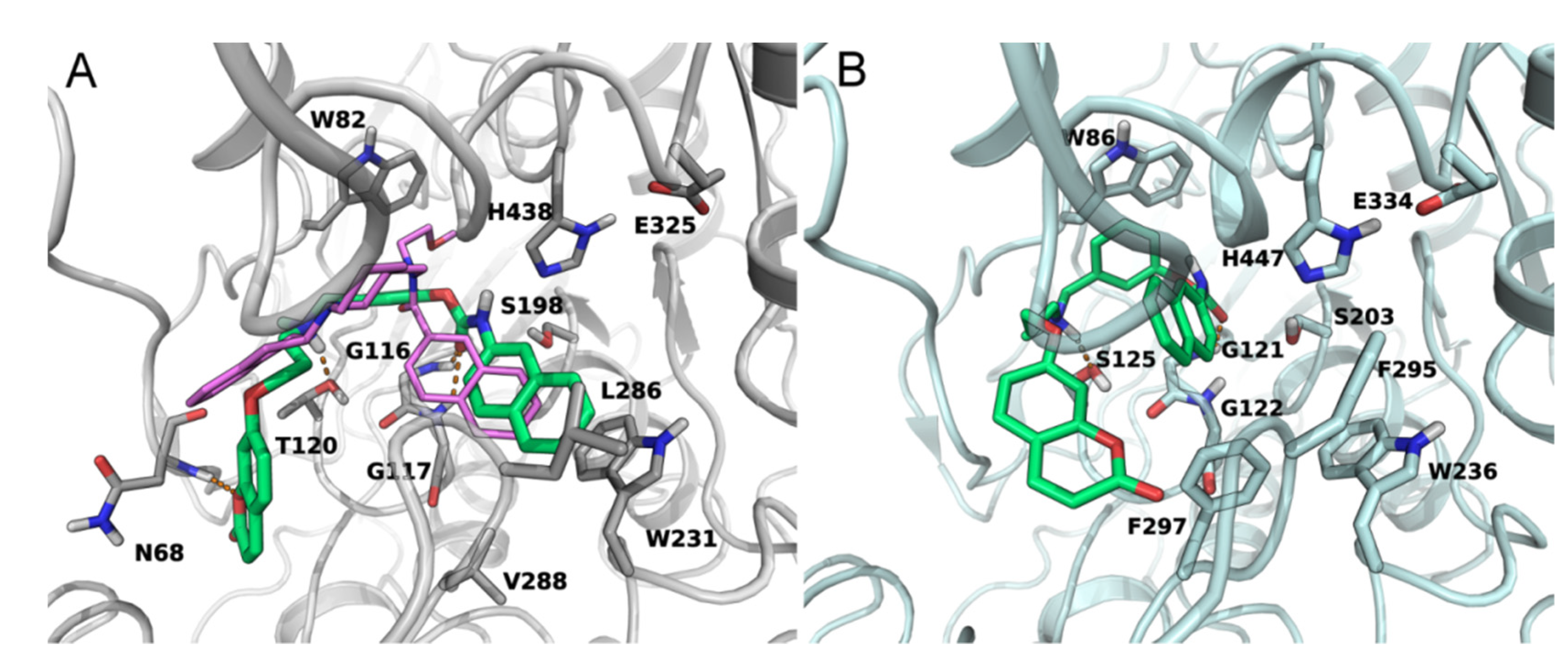
2.2.3. BuChE and FAAH Docking Studies
2.2.4. CB Receptors Binding Assay for Compounds 5–7
2.2.5. Inhibition of Aβ42 Self-Aggregation for Compounds 2–7
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Methods
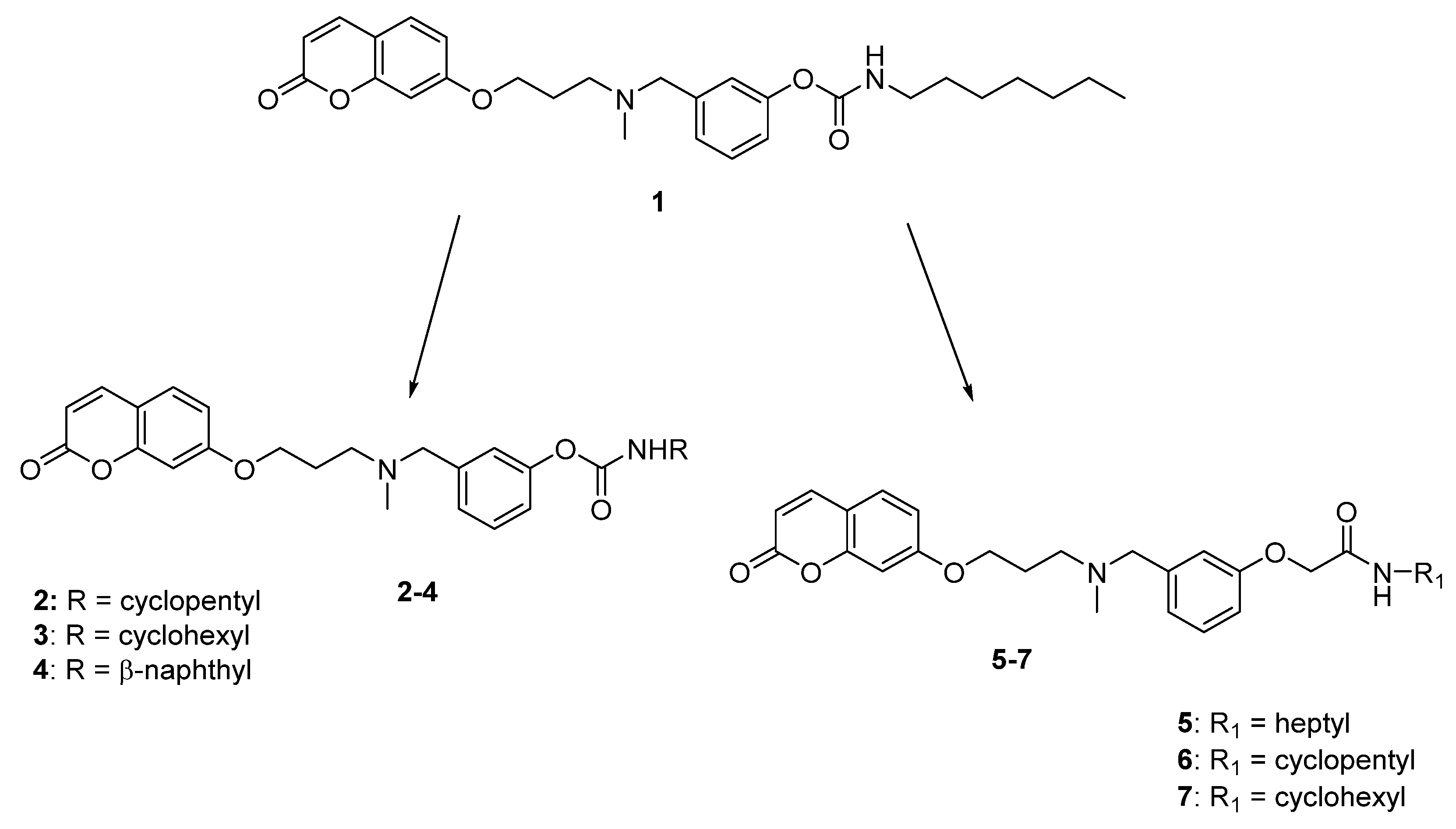
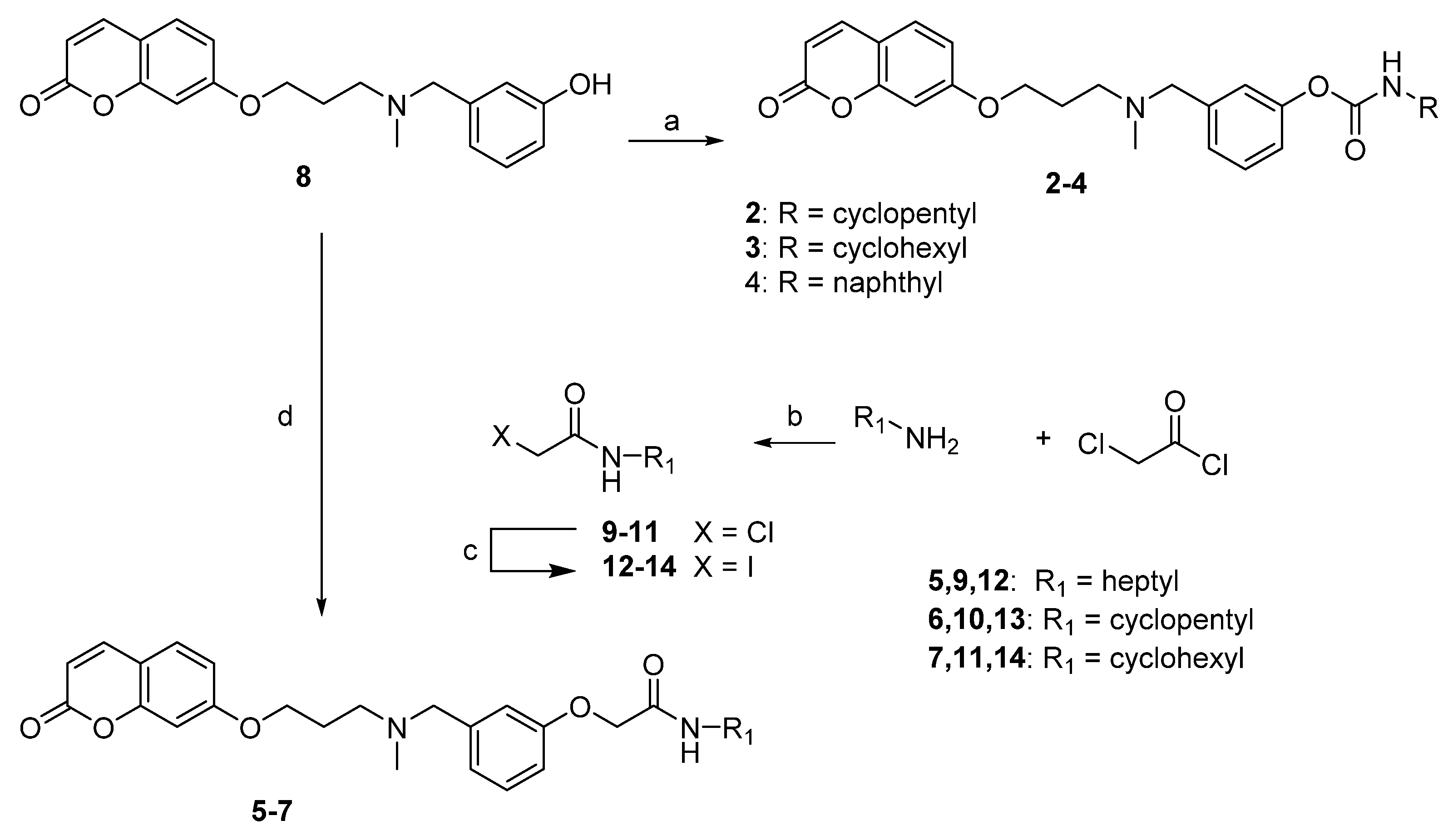
4.1.2. General Method for the Preparation of Carbamates 2–4
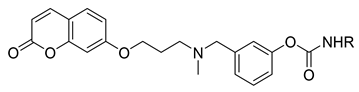
3-((Methyl(3-((2-oxo-2H-chromen-7-yl)oxy)propyl)amino)methyl)phenyl cyclopentylcarbamate (2)
3-((Methyl(3-((2-oxo-2H-chromen-7-yl)oxy)propyl)amino)methyl)phenyl cyclohexylcarbamate (3)
3-((Methyl(3-((2-oxo-2H-chromen-7-yl)oxy)propyl)amino)methyl)phenyl naphthalen-2-ylcarbamate (4)
4.1.3. General Method for the Preparation of Acetamides 5–7
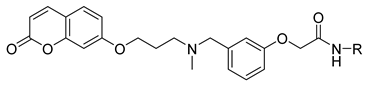
N-Heptyl-2-(3-((methyl(3-((2-oxo-2H-chromen-7-yl)oxy)propyl)amino)methyl)phenoxy)acetamide (5)
N-Cyclopentyl-2-(3-((methyl(3-((2-oxo-2H-chromen-7yl)oxy)propyl)amino)methyl)phenoxy)-acetamide (6)
N-Cyclohexyl-2-(3-((methyl(3-((2-oxo-2H-chromen-7-yl)oxy)propyl)amino)methyl)phenoxy)-acetamide (7)
4.1.4. General Method for the Preparation of Amide Derivatives 9–11
2-Chloro-N-heptylacetamide (9)
2-Chloro-N-cyclopentylacetamide (10)
2-Chloro-N-cyclohexylacetamide (11)
4.1.5. General Method for the Preparation of Iodo Derivatives 12–14
4.1.6. General Method for the Preparation of Isocyanates
4.2. Biological Evaluation Methods
4.2.1. Determination of the Inhibitory Potency towards Human AChE and BuChE
4.2.2. Determination of Carbamoylation Events for Carbamic Derivatives 2–4
4.2.3. Inhibition of Aβ42 Self-Aggregation
4.2.4. FAAH Assay
4.2.5. Competition Binding Assay
4.2.6. Computational Studies
4.2.7. Protein Preparation
4.2.8. rFAAH Induced Fit Docking Studies
4.2.9. hBuChE and hAChE Induced Fit Docking Studies
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- World Health Organisation. Dementia Fact Sheet. December 2017. Available online: http://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 1 October 2018).
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Contestabile, A. The history of the cholinergic hypothesis. Behav. Brain Res. 2011, 221, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Massoud, F.; Gauthier, S. Update on the pharmacological treatment of Alzheimers disease. Curr. Neuropharmacol. 2010, 8, 69–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H. Reconsideration of Anticholinesterase Therapeutic Strategies against Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 852–862. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Khachaturian., A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the cholinergic hypothesis in Alzheimer’s Disease: Emerging evidence from translation and clinical research. J. Prev. Alzheimer Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2006, 160, 1–24. [Google Scholar] [CrossRef]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Costa, M.A.; Almada, M.; Correia-da-Silva, G.; Teixeira, N.A. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Lipid Mediat. 2013, 102, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Mazzola, C.; Drago, F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007, 56, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Talarico, G.; Trebbastoni, A.; Bruno, G.; de Lena, C. Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr. Neuropharmacol. 2019, 17, 176–183. [Google Scholar] [CrossRef]
- Ahn, K.; Johnson, D.S.; Cravatt, B.F. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opin. Drug Discov. 2009, 4, 763–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designing multiple ligands–medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef]
- Rampa, A.; Bartolini, M.; Bisi, A.; Belluti, F.; Gobbi, S.; Andrisano, V.; Ligresti, A.; Di Marzo, V. The first dual ChE/FAAH inhibitors: New perspective for Alzheimer’s disease? ACS Med. Chem. Lett. 2012, 3, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Montanari, S.; Scalvini, L.; Bartolini, M.; Belluti, F.; Gobbi, S.; Andrisano, V.; Ligresti, A.; Di Marzo, V.; Rivara, S.; Mor, M.; et al. Fatty Acid Amide Hydrolase (FAAH), Acetylcholinesterase (AChE), and Butyrylcholinesterase (BuChE): Networked Targets for the Development of Carbamates as Potential Anti-Alzheimer’s Disease Agents. J. Med. Chem. 2016, 59, 6387–6406. [Google Scholar] [CrossRef]
- Supuran, C.T. Coumarin carbonic anhydrase inhibitors from natural sources. J. Enzym. Inhib. Med. Chem. 2020, 35, 1462–1470. [Google Scholar] [CrossRef]
- Menezes, J.; Diederich, M. Translational role of natural coumarins and their derivatives as anticancer agents. Fut. Med. Chem. 2019, 11, 1057–1082. [Google Scholar] [CrossRef]
- Mor, M.; Rivara, S.; Lodola, A.; Plazzi, P.V.; Tarzia, G.; Duranti, A.; Tontini, A.; Piersanti, G.; Kathuria, S.; Piomelli, D. Cyclohexylcarbamic acid 3′- or 4′-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: Synthesis, quantitative structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2004, 47, 4998–5008. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Main, A.R.; Hastings, F.L. Carbamylation and Binding Constants for the Inhibition of Acetylcholinesterase by Physostigmine. Science 1966, 154, 400–402. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Bartolini, M.; Cavalli, A.; Andrisano, V.; Rosini, M.; Minarini, A.; Melchiorre, C. Design, synthesis, and biological evaluation of conformationally restricted rivastigmine analogues. J. Med. Chem. 2004, 47, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel Tacrine–Benzofuran Hybrids as Potent Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography. J. Med. Chem. 2016, 59, 114–131. [Google Scholar] [CrossRef]
- Tuo, W.; Leleu-Chavain, N.; Spencer, J.; Sansook, S.; Millet, R.; Chavatte, P. Therapeutic Potential of Fatty Acid Amide Hydrolase, Monoacylglycerol Lipase, and N-Acylethanolamine Acid Amidase Inhibitors. J. Med. Chem. 2017, 60, 4–46. [Google Scholar] [CrossRef]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Bracey, M.H.; Hanson, M.A.; Masuda, K.R.; Stevens, R.C.; Cravatt, B.F. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science 2002, 298, 1793–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mileni, M.; Kamtekar, S.; Wood, D.C.; Benson, T.E.; Cravatt, B.F.; Stevens, R.C. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: Discovery of a deacylating water molecule and insight into enzyme inactivation. J. Mol. Biol. 2010, 400, 743–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, C.; Nunez, E.; Tolon, R.M.; Carrier, E.J.; Rabano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.-M.; Astarita, G.; Yasar, S.; Vasilevko, V.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. An amyloid β42-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1522–1532. [Google Scholar] [CrossRef] [Green Version]
- Barricklow, J.; Blatnik, M. 2-Arachidonoylglycerol is a substrate for butyrylcholinesterase: A potential mechanism for extracellular endocannabinoid regulation. Arch. Biochem. Biophys. 2013, 536, 1–5. [Google Scholar] [CrossRef]
- Schweda, S.I.; Alder, A.; Gilberger, T.; Kunick, C. 4-Arylthieno[2,3-b]pyridine-2-carboxamides Are a New Class of Antiplasmodial Agents. Molecules 2020, 25, 3187. [Google Scholar] [CrossRef]
- Jöst, C.; Nitsche, C.; Scholz, T.; Roux, L.; Klein, C.D. Promiscuity and Selectivity in Covalent Enzyme Inhibition: A Systematic Study of Electrophilic Fragments. J. Med. Chem. 2014, 57, 7590–7599. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-D.; Song, S.-Y.; Park, Y.-D.; Kim, J.-J.; Joo, W.-H.; Shiro, M.; Falck, J.R.; Shin, D.-S.; Yoon, Y.-J. Novel synthesis of pyridazino[4,5-b][1,4]oxazin-3,8-diones. Tetrahedron Lett. 2003, 44, 8995–8998. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid beta (1-42) peptide self-aggregation: Elucidation of inhibitors’ mechanism of action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Nakakuki, K.; Takeda, T. Kinetic analysis of amyloid fibril polymerization in vitro. Lab. Investig. 1991, 65, 104–110. [Google Scholar]
- Mugnaini, C.; Kostrzewa, M.; Bryk, M.; Mahmoud, A.M.; Brizzi, A.; Lamponi, S.; Giorgi, G.; Ferlenghi, F.; Vacondio, F.; Maccioni, P.; et al. Design, Synthesis, and Physicochemical and Pharmacological Profiling of 7-Hydroxy-5-oxopyrazolo[4,3-b]pyridine-6-carboxamide Derivatives with Antiosteoarthritic Activity In Vivo. J. Med. Chem. 2020, 63, 7369–7391. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Suite. 2018-2 Protein Preparation Wizard. Available online: https://www.schrodinger.com/products/protein-preparation-wizard (accessed on 1 October 2018).
- Epik, version 4.4; Schrödinger, LLC: New York, NY, USA, 2018.
- Impact, version 7.9; Schrödinger, LLC: New York, NY, USA, 2018.
- Prime, version 5.2; Schrödinger, LLC: New York, NY, USA, 2018.
- Maestro, version 11.6; Schrödinger, LLC: New York, NY, USA, 2018.
- LigPrep, version 4.2; Schrödinger, LLC: New York, NY, USA, 2018.
- Glide, version 7.9; Schrödinger, LLC: New York, NY, USA, 2018.
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Macromodel, version 12.0; Schrödinger, LLC: New York, NY, USA, 2018.

| Comp | QPlogPo/w a | QPlogS a | QPlogBB a,b |
|---|---|---|---|
| 1 | 5.46 | −7.55 | −1.65 |
| 2 | 4.16 | −5.74 | −1.01 |
| 3 | 4.47 | −6.17 | −1.02 |
| 4 | 5.27 | −6.94 | −1.11 |
| Riva | 2.46 | −2.25 | 0.41 |
| 5 | 4.89 | −6.07 | −1.72 |
| 6 | 3.85 | −5.14 | −1.22 |
| 7 | 4.15 | −5.52 | −1.22 |
 | ||||||
|---|---|---|---|---|---|---|
| Comp. | R | IC50 hAChE (±SEM) nM a | IC50 hBuChE (±SEM) nM a | IC50 FAAH (m ± sd) μM | IC50 FAAH with 20 min Pre-incubation μM | % Inhibition Aβ42 self Aggreg. b (±SEM) |
| 1 | 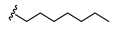 | 37.4 ± 1.9 c | 1.36 ± 0.07 c | 0.161 ± 0.027 c | 0.029 ± 0.001 c | nd |
| 2 | 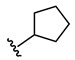 | 521 ± 14 d | 8.42 ± 0.13 e | 6.09 ± 1.55 | 2.53 ± 0.55 | 22.9 ± 1.3 |
| 3 |  | 104 ± 21 f | 34.9 ± 4.2 d | 2.55 ± 0.33 | 1.33 ± 0.28 | 18.9 ± 2.1 |
| 4 | 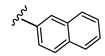 | 264 ± 0.05 f | 2.00 ± 0.20 d | 1.58 ± 0.26 | 0.63 ± 0.08 | 13.9 ± 3.8 |
| Riva | 1535 ± 64 g | 301 ± 14 g | nd | nd | nd | |
 | |||||
|---|---|---|---|---|---|
| Comp. | R | hAChE % Inhibition (±SEM) a [I] = 40 µM | hBuChE IC50 (±SEM) µM a | IC50 FAAH (m ± sd) μM | % Inhibition Aβ42 Self-Aggregation b (±SEM) |
| 5 | 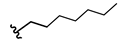 | 13.6 ± 5.1 | 182 ± 28 | >1000 | n.a. |
| 6 | 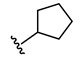 | 41.7 ± 0.5 c | 11.6 ± 0.8 | >1000 | 20.3 ± 1.9 |
| 7 | 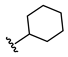 | 34.0 ± 3.2 | 12.8 ± 2.1 | >1000 | 13.5 ± 5.0 |
| Comp. | Ki on CB1 (m ± sd) µM | Max Tested on CB1 (% Displacement) (m ± sd) µM | Ki on CB2 (m ± sd) µM | Max Tested on CB2 (% Displacement) (m ± sd) µM |
|---|---|---|---|---|
| 5 | >10 | 10 (35.57 ± 0.43) | >10 | 10 (41.41 ± 0.87) |
| 6 | >10 | 10 (35.75 ± 0.65) | >10 | 10 (48.33 ± 1.73) |
| 7 | >10 | 10 (39.62 ± 10.48) | >10 | 10 (14.3 ±3.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanari, S.; Allarà, M.; Scalvini, L.; Kostrzewa, M.; Belluti, F.; Gobbi, S.; Naldi, M.; Rivara, S.; Bartolini, M.; Ligresti, A.; et al. New Coumarin Derivatives as Cholinergic and Cannabinoid System Modulators. Molecules 2021, 26, 3254. https://doi.org/10.3390/molecules26113254
Montanari S, Allarà M, Scalvini L, Kostrzewa M, Belluti F, Gobbi S, Naldi M, Rivara S, Bartolini M, Ligresti A, et al. New Coumarin Derivatives as Cholinergic and Cannabinoid System Modulators. Molecules. 2021; 26(11):3254. https://doi.org/10.3390/molecules26113254
Chicago/Turabian StyleMontanari, Serena, Marco Allarà, Laura Scalvini, Magdalena Kostrzewa, Federica Belluti, Silvia Gobbi, Marina Naldi, Silvia Rivara, Manuela Bartolini, Alessia Ligresti, and et al. 2021. "New Coumarin Derivatives as Cholinergic and Cannabinoid System Modulators" Molecules 26, no. 11: 3254. https://doi.org/10.3390/molecules26113254






