Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds?
Abstract
:1. Introduction
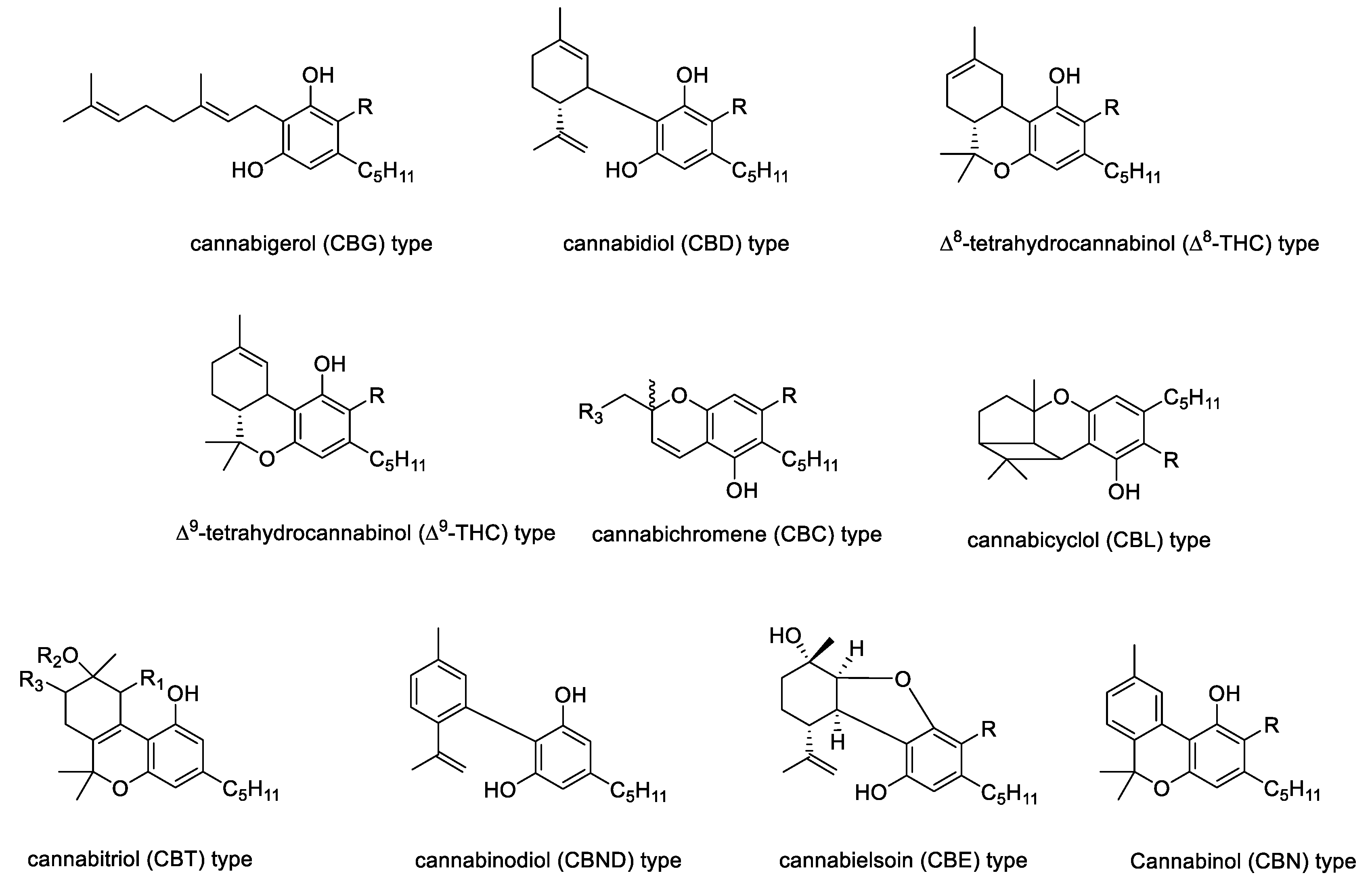
2. The Chemistry of Hemp
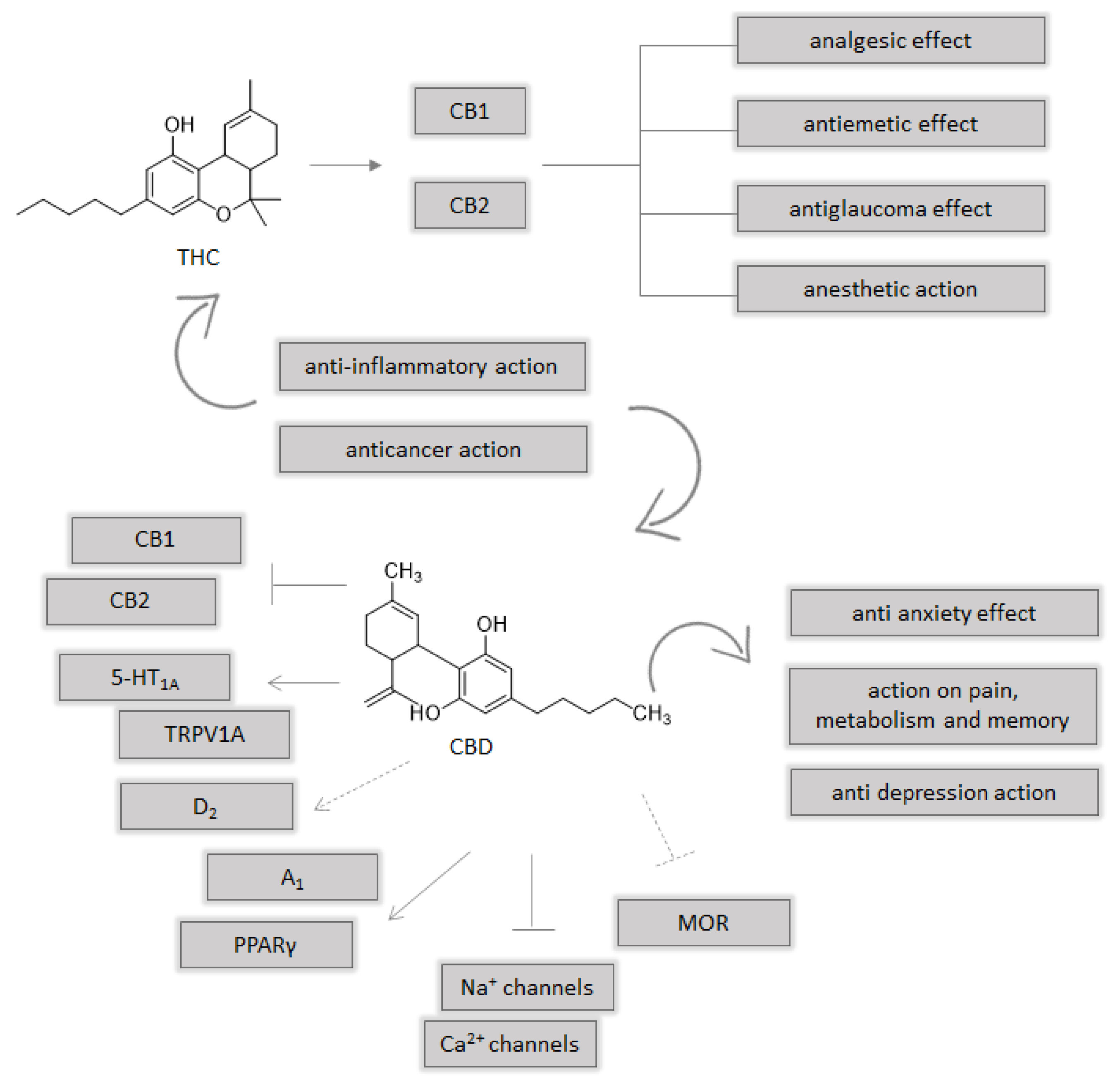
3. A Brief Focus on Endocannabinoid System
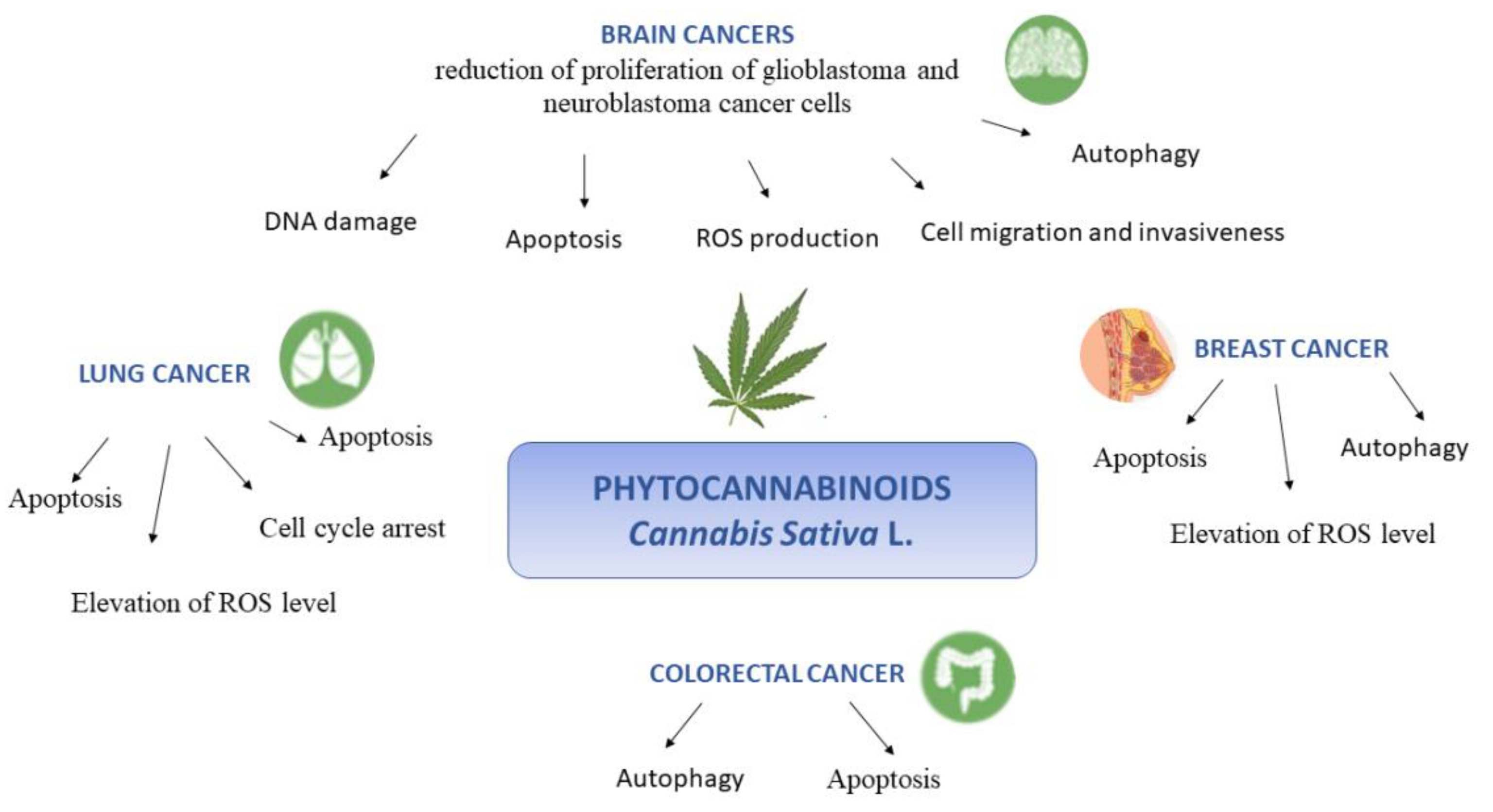
4. Molecular Effects of Cannabis sativa L.
4.1. Brain Cancer
4.2. Lung Cancer
4.3. Breast Cancer
4.4. Colorectal Cancer
5. Cannabis sativa L. in Cancer Clinical Trials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| THC | Δ9-tetrahydrocannabinol |
| CBD | cannabidiol |
| CBDA | cannabidiolic acid |
| THCA | tetrahydrocannabinol acid |
| CBN | cannabinol |
| AC | adenylyl cyclase |
| CB1 and CB2 | cannabinoid receptor 1 and 2 |
| cAMP | adenosine monophosphate |
| PKA | protein kinase A |
| MAPK | protein kinases |
| PI3K/AKT | phosphoinositide-3-kinase/protein kinase B |
| BBB | blood–brain barrier |
| OA | olivetolic acid |
| CBGAS | cannabigerolic acid synthase |
| CBGA | cannabigerolic acid |
| CBDAS | cannabidiolic acid synthase |
| Δ9-THCAS | Δ9-tetrahydrocannabinolic acid synthase |
| CBF-C5 | cannabifuran |
| DCBF-C5 | dehydrocannabifuran |
| CBCON-C5 | cannabicoumaronone-C5 |
| CBGVA | cannabigerovarinic acid |
| PUFA | polyunsaturated fatty acids |
| ALA | α-linolenic |
| GLA | γ-linolenic acid |
| SDA | stearidonic acid |
| ES | endocannabinoid system |
| eCBs | endogenous cannabinoid ligands |
| NAPE | N-acyl-phosphatidylethanolamine |
| NAPE-PLD | NAPE-specific phospholipase D |
| DAG | diacylglycerol |
| DAGL | DAG lipase |
| FAAH | fatty acid amide hydrolase |
| MAGL | monoacylglycerol lipase |
| TRPV1 | transient receptor potential vanilloid 1 |
| PPAR | peroxisome proliferator-activated receptor |
| TRP | transient receptor potential |
| TNF-𝛼 | Tumor Necrosis Factor alpha |
| IL | Interleukin |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| COX-2 | Cyclooxygenase 2 |
| PGE2 | Prostaglandin E2 |
| NO | nitric oxide |
| TLR4 | Toll like receptos 4 |
| MMP | matrix metallopeptidase |
| VEGF | vascular endothelial growth factor |
| MDR | multidrug resistance |
| GBM | glioblastoma multiforme |
| GSC | glioma stem cell lines |
| ERK | extracellular signal-related kinase |
| PI3K | phosphoinositide3-kinase |
| p38MAPK | p38mitogen-activated protein kinase |
| ER | estrogen receptors |
| SOD | Superoxide dismutase |
| GPX | glutathione peroxidase |
| CINV | chemotherapy-induced nausea and vomiting |
References
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diviant, J.P.; Vigil, J.M.; Stith, S.S. The Role of Cannabis within an Emerging Perspective on Schizophrenia. Medicines 2018, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [Green Version]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (‒)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Formato, M.; Pacifico, S. A Cup of Hemp Coffee by Moka Pot from Southern Italy: An UHPLC-HRMS Investigation. Foods 2020, 9, 1123. [Google Scholar] [CrossRef]
- Chousidis, I.; Chatzimitakos, T.; Leonardos, D.; Filiou, M.D.; Stalikas, C.D.; Leonardos, I.D. Cannabinol in the spotlight: Toxicometabolomic study and behavioral analysis of zebrafish embryos exposed to the unknown cannabinoid. Chemosphere 2020, 252, 126417. [Google Scholar] [CrossRef]
- Lewis, M.A.; Russo, E.B.; Smith, K.M. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2017, 84, 225–233. [Google Scholar] [CrossRef]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Cell Cytotoxic Activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef] [Green Version]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ9-THC at Human CB1and CB2Receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9 -tetrahydrocannabinol, cannabidiol and Δ9 -tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobovišek, L.; Krstanović, F.; Borštnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Organotypic Models Drug Dev. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Neuropharmacology and therapeutic potential of cannabinoids. Addict. Biol. 2000, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zettl, U.K.; Rommer, P.; Hipp, P.; Patejdl, R. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther. Adv. Neurol. Disord. 2015, 9, 9–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2009, 147, S163–S171. [Google Scholar] [CrossRef] [Green Version]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Endocannabinoids and their pharmacological actions. In Endocannabinoids; Pertwee, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 231, pp. 1–37. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Pan, C.-H.; Hishimoto, A.; Li, C.-Y.; Xi, Z.-X.; Llorente-Berzal, A.; Viveros, M.-P.; Ishiguro, H.; Arinami, T.; Onaivi, E.S.; et al. Species differences in cannabinoid receptor 2 (CNR2gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; LaViolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, F.; Chi, M.; Eamens, A.L.; Duchatel, R.J.; Douglas, A.M.; Schneider, J.; Gedye, C.; Woldu, A.S.; Dun, M.D. Can Hemp Help? Low-THC Cannabis and Non-THC Cannabinoids for the Treatment of Cancer. Cancers 2020, 12, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, Â.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017, 17, fox037. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. PKS Activities and Biosynthesis of Cannabinoids and Flavonoids in Cannabis sativa L. Plants. Plant. Cell Physiol. 2008, 49, 1767–1782. [Google Scholar] [CrossRef] [Green Version]
- Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, M.A.; Sultana, G.N.N.; Mehmedic, Z.; Hossain, C.F.; Chandra, S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem. Anal. 2005, 16, 45–48. [Google Scholar] [CrossRef]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef]
- Marzo, V.; Petrocellis, L. Endocannabinoids as Regulators of Transient Receptor Potential (TRP)Channels: A Further Opportunity to Develop New Endocannabinoid-Based Therapeutic Drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Nahas, G.; Harvey, D.J.; Sutin, K.; Turndorf, H.; Cancro, R. A molecular basis of the therapeutic and psychoactive properties of cannabis (Δ9-tetrahydrocannabinol). Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 721–730. [Google Scholar] [CrossRef]
- De Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013, 52, 80–109. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer Mechanisms of Cannabinoids. Curr. Oncol. 2016, 23, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Soboci, A.A.; Szczylik, C.; Czarnecka, A.M.; Król, M.; Botta, B. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Jamontt, J.; Molleman, A.; Pertwee, R.G.; Parsons, M.E. The effects of Δ9-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation andin vitromotility disturbances in rat colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Sido, J.M.; Jackson, A.R.; Nagarkatti, P.S.; Nagarkatti, M. Marijuana-derived Δ-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through microRNA regulation. J. Mol. Med. 2016, 94, 1039–1051. [Google Scholar] [CrossRef]
- McCoy, K.L. Interaction between Cannabinoid System and Toll-Like Receptors Controls Inflammation. Mediat. Inflamm. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, B.; Colleoni, M.; Conti, S.; Parolaro, D.; Franke, C.; Trovato, A.E.; Giagnoni, G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn. Schmiedebergs. Arch. Pharmacol. 2004, 369, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-activated NF-κB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growthin vitroandin vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2012, 168, 79–102. [Google Scholar] [CrossRef] [Green Version]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract en-riched in cannabidiol. Pharmacol. Pharm. 2015, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2020, 1542. [Google Scholar] [CrossRef]
- Miller, H.P.; Bonawitz, S.C.; Ostrovsky, O. The effects of delta-9-tetrahydrocannabinol (THC) on inflammation: A review. Cell. Immunol. 2020, 352, 104111. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.F.K.F.K.; Miranda, K.; Wilson, K.; Singh, N.P.P.; Cai, G.; Putluri, N.; Nagarkatti, P.; Nagarkatti, M. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int. J. Mol. Sci. 2020, 21, 6244. [Google Scholar] [CrossRef] [PubMed]
- Kusher, D.I.; Dawson, L.O.; Taylor, A.C.; Djeu, J.Y. Effect of the Psychoactive Metabolite of Marijuana, Δ9-Tetrahydrocannabinol (THC), on the Synthesis of Tumor Necrosis Factor by Human Large Granular Lymphocytes. Cell. Immunol. 1994, 154, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.A.; Klein, T.W.; Friedman, H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect. Immun. 1994, 62, 4015–4020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SanGiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.L.; Atwal, N.; Vaughan, C.W. Cannabis constituent synergy in a mouse neuropathic pain model. Pain 2017, 158, 2452–2460. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, E.; et al. In vivo and in vitro anti-inflammatory activity evaluation of Lebanese Cannabis sativa L. ssp. indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. [Google Scholar] [CrossRef]
- Downer, E.J. Anti-inflammatory Potential of Terpenes Present in Cannabis sativa L. ACS Chem. Neurosci. 2020, 11, 659–662. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef] [Green Version]
- Romano, B.; Borrelli, F.; Pagano, E.; Cascio, M.G.; Pertwee, R.G.; Izzo, A.A. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 2014, 21, 631–639. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Sato, T. Novel Antitumor Invasive Actions of p-Cymene by Decreasing MMP-9/TIMP-1 Expression Ratio in Human Fibrosarcoma HT-1080 Cells. Biol. Pharm. Bull. 2016, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, B.O.; Hamani, C.; Ferreira, E.; Miranda, M.F.; Fernandes, M.J.S.; Rodrigues, A.M.; De Almeida, A.-C.G.; Covolan, L. Effects of A1 receptor agonist/antagonist on spontaneous seizures in pilocarpine-induced epileptic rats. Epilepsy Behav. 2016, 61, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Glass, J.C.A.A.M. The Cannabinoid CB2 Receptor as a Target for Inflammation-Dependent Neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Noonan, J.; Tanveer, R.; Klompas, A.; Gowran, A.; McKiernan, J.; Campbell, V.A. Endocannabinoids Prevent β-Amyloid-mediated Lysosomal Destabilization in Cultured Neurons. J. Biol. Chem. 2010, 285, 38543–38554. [Google Scholar] [CrossRef] [Green Version]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Huffman, J.W.; Csiszar, A.; Ungvari, Z.; Mackie, K.; Chatterjee, S.; et al. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Circ. Physiol. 2007, 293, H2210–H2218. [Google Scholar] [CrossRef] [Green Version]
- Majdi, F.; Taheri, F.; Salehi, P.; Motaghinejad, M.; Safari, S. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol may be effective against methamphetamine induced mitochondrial dysfunction and inflammation by modulation of Toll-like type-4(Toll-like 4) receptors and NF-κB signaling. Med. Hypotheses 2019, 133, 109371. [Google Scholar] [CrossRef]
- Hunter, S.A.; Burstein, S.H. Receptor mediation in cannabinoid stimulated arachidonic acid mobilization and anandamide synthesis. Life Sci. 1997, 60, 1563–1573. [Google Scholar] [CrossRef]
- Henriquez, J.E.; Bach, A.P.; Matos-Fernandez, K.M.; Crawford, R.B.; Kaminski, N.E. Δ9-Tetrahydrocannabinol (THC) Impairs CD8+ T Cell-Mediated Activation of Astrocytes. J. Neuroimmune Pharmacol. 2020, 15, 863–874. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of Cannabinoids, Δ9- Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1921. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid Receptor Activation Induces Apoptosis through Tumor Necrosis Factor α–Mediated Ceramide De novo Synthesis in Colon Cancer Cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Anis, O.; Vinayaka, A.; Shalev, N.; Namdar, D.; Nadarajan, S.; Anil, S.; Cohen, O.; Belausov, E.; Ramon, J.; Gati, E.M.; et al. Cannabis-Derived Compounds Cannabichromene and Δ9-Tetrahydrocannabinol Interact and Exhibit Cytotoxic Activity against Urothelial Cell Carcinoma Correlated with Inhibition of Cell Migration and Cytoskeleton Organization. Molecules 2021, 26, 465. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Karimi, I.; Yousofvand, N. Molecular docking study of lignanamides from Cannabis sativa against P-glycoprotein. Silico Pharmacol. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Bornhäuser, M.; Metzler, M.; Millot, F.; Schleyer, E. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Expert Rev. Clin. Pharmacol. 2017, 11, 219–231. [Google Scholar] [CrossRef]
- Molnár, J.; Szabó, D.; Pusztai, R.; Mucsi, I.; Berek, L.; Ocsovszki, I.; Kawata, E.; Shoyama, Y. Membrane associated antitumor effects of crocine-, ginsenoside- and cannabinoid derivates. Anticancer Res. 2000, 20, 861–867. [Google Scholar]
- Yan, X.; Tang, J.; Passos, C.D.S.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of Lignanamides from Hemp (Cannabis sativa L.) Seed and Their Antioxidant and Acetylcholinesterase Inhibitory Activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ng, L.; Ozawa, T.; Stella, N. Quantitative Analyses of Synergistic Responses between Cannabidiol and DNA-Damaging Agents on the Proliferation and Viability of Glioblastoma and Neural Progenitor Cells in Culture. J. Pharmacol. Exp. Ther. 2017, 360, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Shah, V. Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Patents Anti-Cancer Drug Discov. 2018, 13, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.C.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.D.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosgodage, U.S.; Uysal-Onganer, P.; MacLatchy, A.; Mould, R.; Nunn, A.V.; Guy, G.W.; Kraev, I.; Chatterton, N.P.; Thomas, E.L.; Inal, J.M.; et al. Cannabidiol Affects Extracellular Vesicle Release, miR21 and miR126, and Reduces Prohibitin Protein in Glioblastoma Multiforme Cells. Transl. Oncol. 2019, 12, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells via Endoplasmic Reticulum Stress–Related Genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef] [Green Version]
- Mc Allister, S.D.; Chan, C.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J. Neuro-Oncol. 2005, 74, 31–40. [Google Scholar] [CrossRef]
- Blázquez, C.; Salazar, M.; Carracedo, A.; Lorente, M.; Egia, A.; González-Feria, L.; Haro, A.; Velasco, G.; Guzmán, M. Cannabinoids Inhibit Glioma Cell Invasion by Down-regulating Matrix Metalloproteinase-2 Expression. Cancer Res. 2008, 68, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef] [Green Version]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [Green Version]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, V.N.; Wu, J.; Wang, T.J.C.; Hei, T.K. Inhibition of ATM kinase upregulates levels of cell death induced by cannabidiol and γ-irradiation in human glioblastoma cells. Oncotarget 2019, 10, 825–846. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2003, 308, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; De Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of Plasminogen Activator Inhibitor-1 May Contribute to the Anti-Invasive Action of Cannabidiol on Human Lung Cancer Cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Shenoy, A.K.; Padia, R.; Fang, D.; Jing, Q.; Yang, P.; Su, S.-B.; Huang, S. Suppression of lung cancer progression by isoliquiritigenin through its metabolite 2, 4, 2’, 4’-Tetrahydroxychalcone. J. Exp. Clin. Cancer Res. 2018, 37, 243. [Google Scholar] [CrossRef] [Green Version]
- Hosami, F.; Manayi, A.; Salimi, V.; Khodakhah, F.; Nourbakhsh, M.; Nakstad, B.; Tavakoli-Yaraki, M. The pro-apoptosis effects of Echinacea purpurea and Cannabis sativa extracts in human lung cancer cells through caspase-dependent pathway. BMC Complement. Med. Ther. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer Lett. 2009, 285, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5- HT 1A receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef] [Green Version]
- McAllister, S.D.; Glass, M. CB(1) and CB(2) receptor-mediated signalling: A focus on endocannabinoids. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 161–171. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef]
- McAllister, S.D.; Soroceanu, L.; Desprez, P.-Y. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.M.; Alotaibi, M.R.; Tao, Q.; Selley, D.E.; Lichtman, A.H.; Gewirtz, D.A. Combined Antiproliferative Effects of the Aminoalkylindole WIN55,212-2 and Radiation in Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2014, 348, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.; Ganju, R.K. Crosstalk between Chemokine Receptor CXCR4 and Cannabinoid Receptor CB2 in Modulating Breast Cancer Growth and Invasion. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Archana, A.; Dutta, D.; Kumar, V.; Kim, J.; Jan, A.T.; Minakshi, R. The onus of cannabinoids in interrupting the molecular odyssey of breast cancer: A critical perspective on UPRER and beyond. Saudi Pharm. J. 2019, 27, 437–445. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.-Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-L.; Yeung, C.-M.; Chiu, L.C.M.; Cen, Y.-Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother. Res. 2009, 23, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Haag, J.D.; Lindstrom, M.J.; Gould, M.N. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992, 52, 4021–4026. [Google Scholar]
- Gould, M.N.; Moore, C.J.; Zhang, R.; Wang, B.; Kennan, W.S.; Haag, J.D. Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Res. 1994, 54, 3540–3543. [Google Scholar]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Regression of Rat Primary Mammary Tumors Following Dietary d-Limonene2. J. Natl. Cancer Inst. 1986, 76, 323–325. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential Expression of Cannabinoid Receptors in the Human Colon: Cannabinoids Promote Epithelial Wound Healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhamoruni, A.; Lee, A.C.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Pharmacological Effects of Cannabinoids on the Caco-2 Cell Culture Model of Intestinal Permeability. J. Pharmacol. Exp. Ther. 2010, 335, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Alhamoruni, A.; Wright, K.; Larvin, M.; O’Sullivan, S.E. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br. J. Pharmacol. 2012, 165, 2598–2610. [Google Scholar] [CrossRef] [Green Version]
- Watzl, B.; Scuderi, P.; Watson, R.R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int. J. Immunopharmacol. 1991, 13, 1091–1097. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.; Novak, M.; Almidon, M.P.; Marinelli, O.; Baškovič, B.Ž.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [Green Version]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; De Sousa, D.P. Antitumor Activity of Monoterpenes Found in Essential Oils. Sci. World J. 2014, 2014, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, S.L.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2012, 29, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Arboleda, M.F.; Prosk, E.; Cyr, C.; Gamaoun, R.; Vigano, A. Medical cannabis in supportive cancer care: Lessons from Canada. Support. Care Cancer 2020, 28, 2999–3001. [Google Scholar] [CrossRef]
- Zolotov, Y.; Vulfsons, S.; Zarhin, D.; Sznitman, S. Medical cannabis: An oxymoron? Physicians’ perceptions of medical cannabis. Int. J. Drug Policy 2018, 57, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Cyr, C.; Arboleda, M.F.; Aggarwal, S.K.; Balneaves, L.G.; Daeninck, P.; Néron, A.; Prosk, E.; Vigano, A. Cannabis in palliative care: Current challenges and practical recommendations. Ann. Palliat. Med. 2018, 7, 463–477. [Google Scholar] [CrossRef]
- Pauli, C.S.; Conroy, M.; Heuvel, B.D.V.; Park, S.-H. Cannabidiol Drugs Clinical Trial Outcomes and Adverse Effects. Front. Pharmacol. 2020, 11, 63. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Bar-Sela, G.; Zalman, D.; Semenysty, V.; Ballan, E. The Effects of Dosage-Controlled Cannabis Capsules on Cancer-Related Cachexia and Anorexia Syndrome in Advanced Cancer Patients: Pilot Study. Integr. Cancer Ther. 2019, 18, 1534735419881498. [Google Scholar] [CrossRef]
- Strasser, F.; Lüftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.-D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of Orally Administered Cannabis Extract and Delta-9-Tetrahydrocannabinol in Treating Patients With Cancer-Related Anorexia-Cachexia Syndrome: A Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial From the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amna, M.; Salti, T.; Khoury, M.; Cohen, I.; Bar-Sela, G. Medical Cannabis in Oncology: A Valuable Unappreciated Remedy or an Undesirable Risk? Curr. Treat. Options Oncol. 2021, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Liu, W.; Dalgleish, A. Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol. Anticancer Res. 2018, 38, 5831–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press (US): Cambridge, MA, USA, 2017. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.M.; Morasco, B.J.; O’Neil, M.E.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’Apuaka, M.; Paynter, R.; et al. The Effects of Cannabis Among Adults With Chronic Pain and an Overview of General Harms. Ann. Intern. Med. 2017, 167, 319–331. [Google Scholar] [CrossRef]
- Darkovska-Serafimovska, M.; Serafimovska, T.; Arsova-Sarafinovska, Z.; Stefanoski, S.; Keskovski, Z.; Balkanov, T. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J. Pain Res. 2018, 11, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Dai, T.; Hanlon, J.G.; Downar, J.; Alibhai, S.M.; Clarke, H. Cannabis and cannabinoids in cancer pain management. Curr. Opin. Support. Palliat. Care 2020, 14, 87–93. [Google Scholar] [CrossRef]
- Levy, C.; Galenbeck, E.; Magid, K. Cannabis for Symptom Management in Older Adults. Med. Clin. N. Am. 2020, 104, 471–489. [Google Scholar] [CrossRef]
- Lossignol, D. Cannabinoids: A new approach for pain control? Curr. Opin. Oncol. 2019, 31, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Noyes, R.; Brunk, S.F.; Baram, D.A.; Canter, A. Analgesic Effect of Delta-9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1975, 15, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Noyes, R.; Brunk, S.F.; Avery, D.H.; Canter, A. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin. Pharmacol. Ther. 1975, 18, 84–89. [Google Scholar] [CrossRef]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients With Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Staquet, M.; Gantt, C.; Machin, D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin. Pharmacol. Ther. 1978, 23, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, P.R.; Lawton, R.L.; Versteeg, K.; Noyes, R. Effect of benzopyranoperidine, a Δ-9-THC congener, on pain. Clin. Pharmacol. Ther. 1978, 24, 223–227. [Google Scholar] [CrossRef]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal THC Spray in Patients With Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Oland, L.D.; Ho, W.; Hillard, C.J.; Mackie, K.; Davison, J.S.; Sharkey, K.A. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 2001, 121, 767–774. [Google Scholar] [CrossRef]
- Turgeman, I.; Bar-Sela, G. Cannabis Use in Palliative Oncology: A Review of the Evidence for Popular Indications. Isr. Med. Assoc. J. IMAJ 2017, 19, 85–88. [Google Scholar]
- Ben Amar, M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Pérez, E.; Abanades, S.; Vidal, X.; Saura, C.; Majem, M.; Arriola, E.; Rabanal, M.; Pastor, A.; Farré, M.; et al. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br. J. Clin. Pharmacol. 2010, 70, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallan, S.E.; Zinberg, N.E.; Frei, E. Antiemetic Effect of Delta-9-Tetrahydrocannabinol in Patients Receiving Cancer Chemotherapy. N. Engl. J. Med. 1975, 293, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, E.; Formato, M.; Crescente, G.; Daniele, A. Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds? Molecules 2021, 26, 2668. https://doi.org/10.3390/molecules26092668
Nigro E, Formato M, Crescente G, Daniele A. Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds? Molecules. 2021; 26(9):2668. https://doi.org/10.3390/molecules26092668
Chicago/Turabian StyleNigro, Ersilia, Marialuisa Formato, Giuseppina Crescente, and Aurora Daniele. 2021. "Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds?" Molecules 26, no. 9: 2668. https://doi.org/10.3390/molecules26092668





