Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus
Abstract
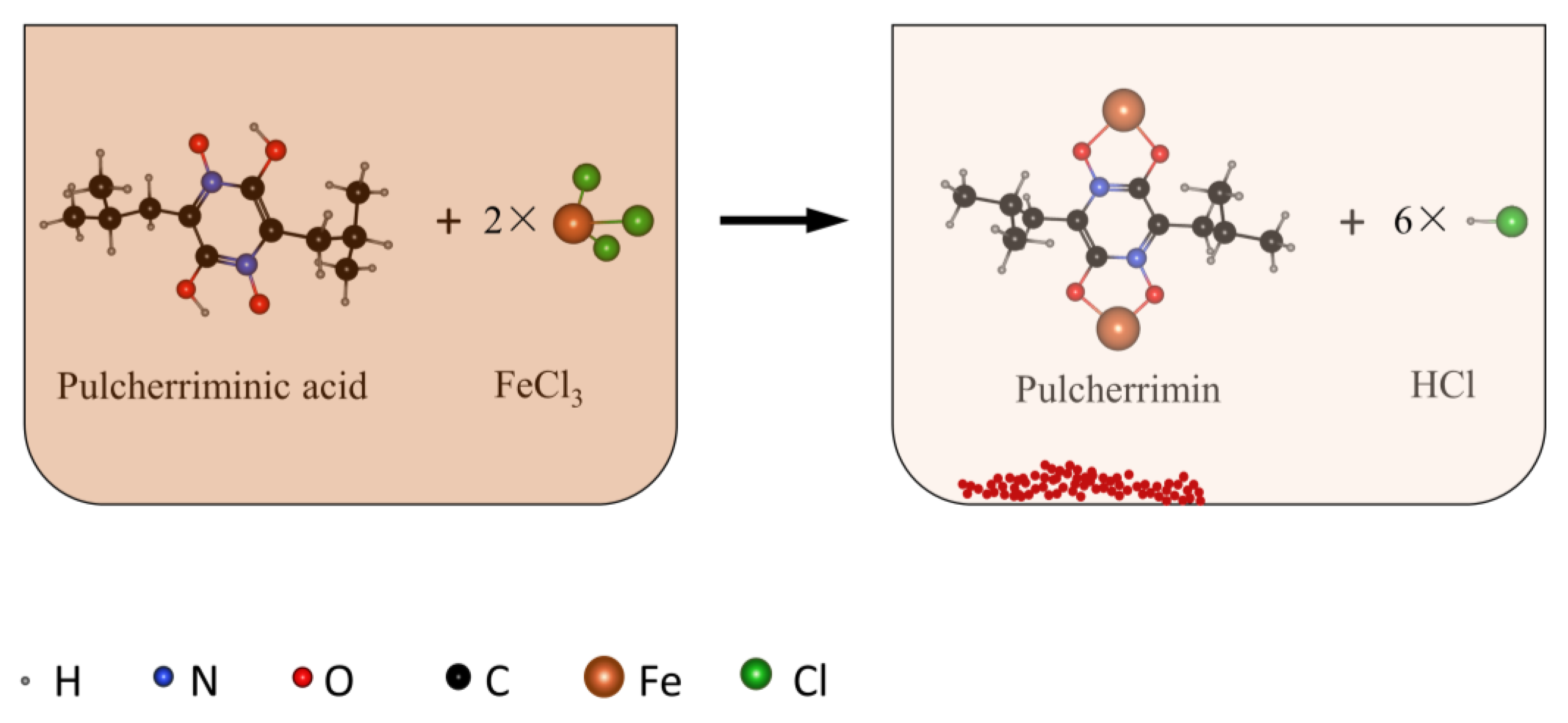
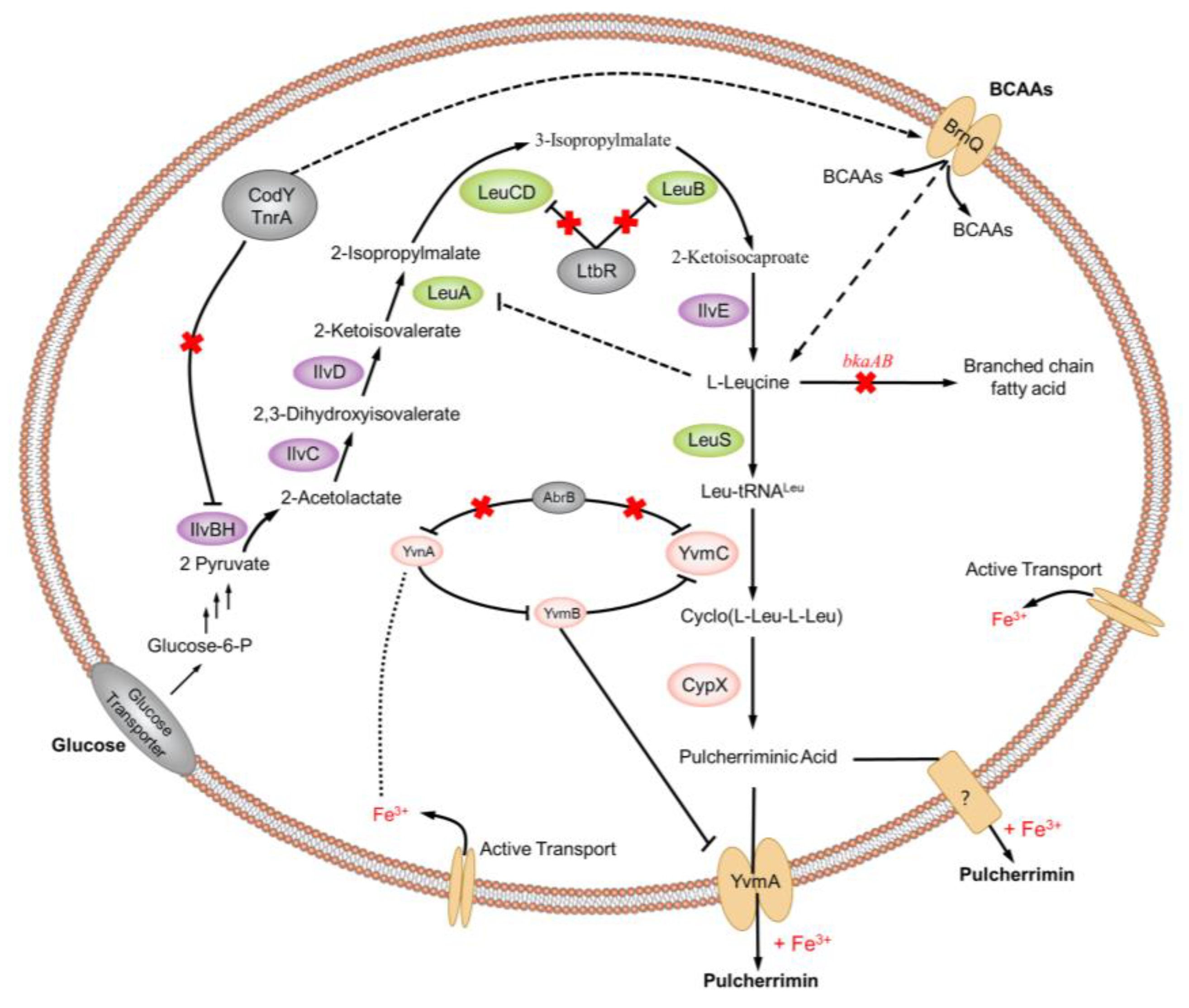
1. Introduction
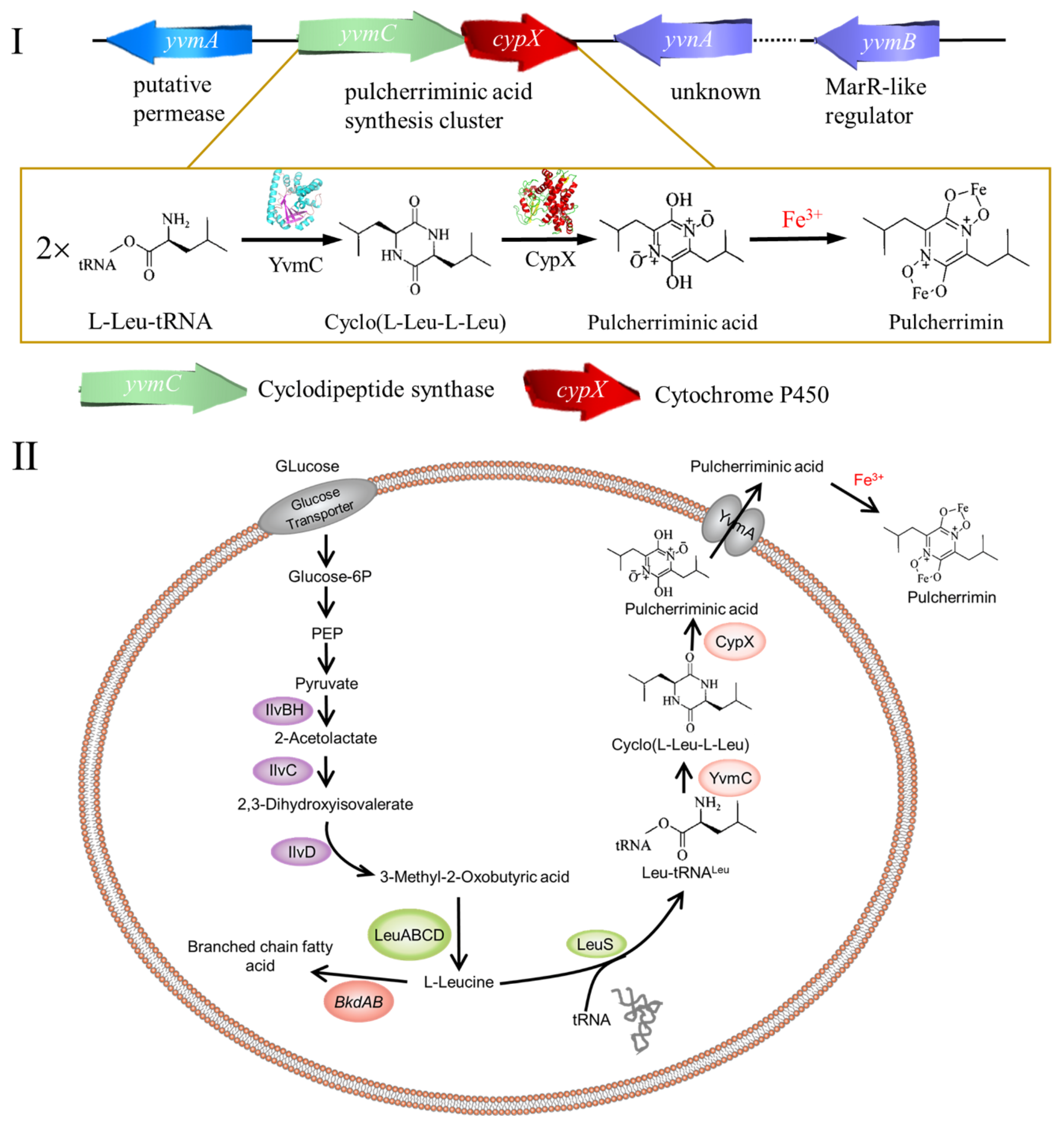
2. Cyclic Dileucine Synthase is Used to Synthesize the Precursor cLL of Pulcherriminic Acid
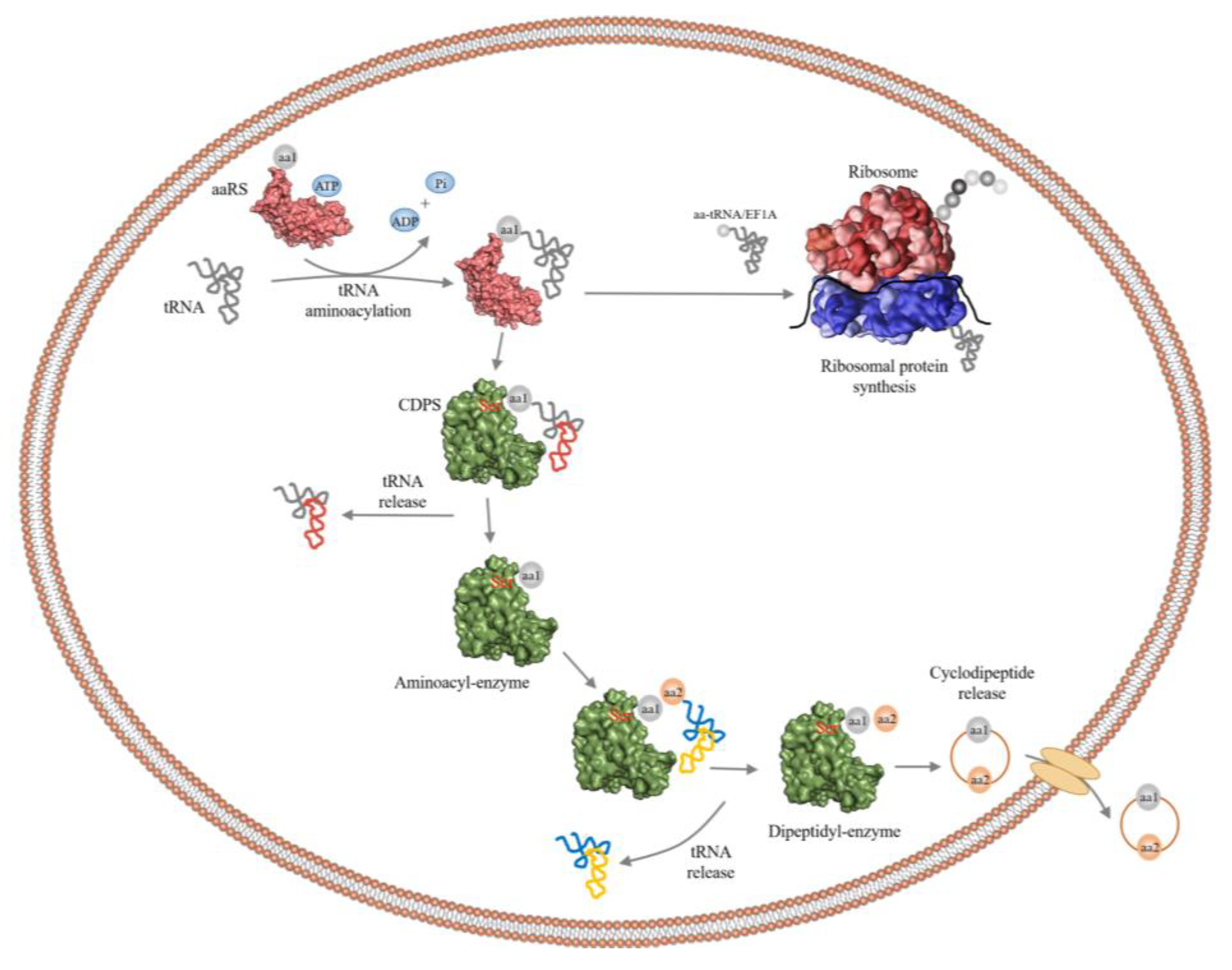
2.1. Cyclic Dipeptides
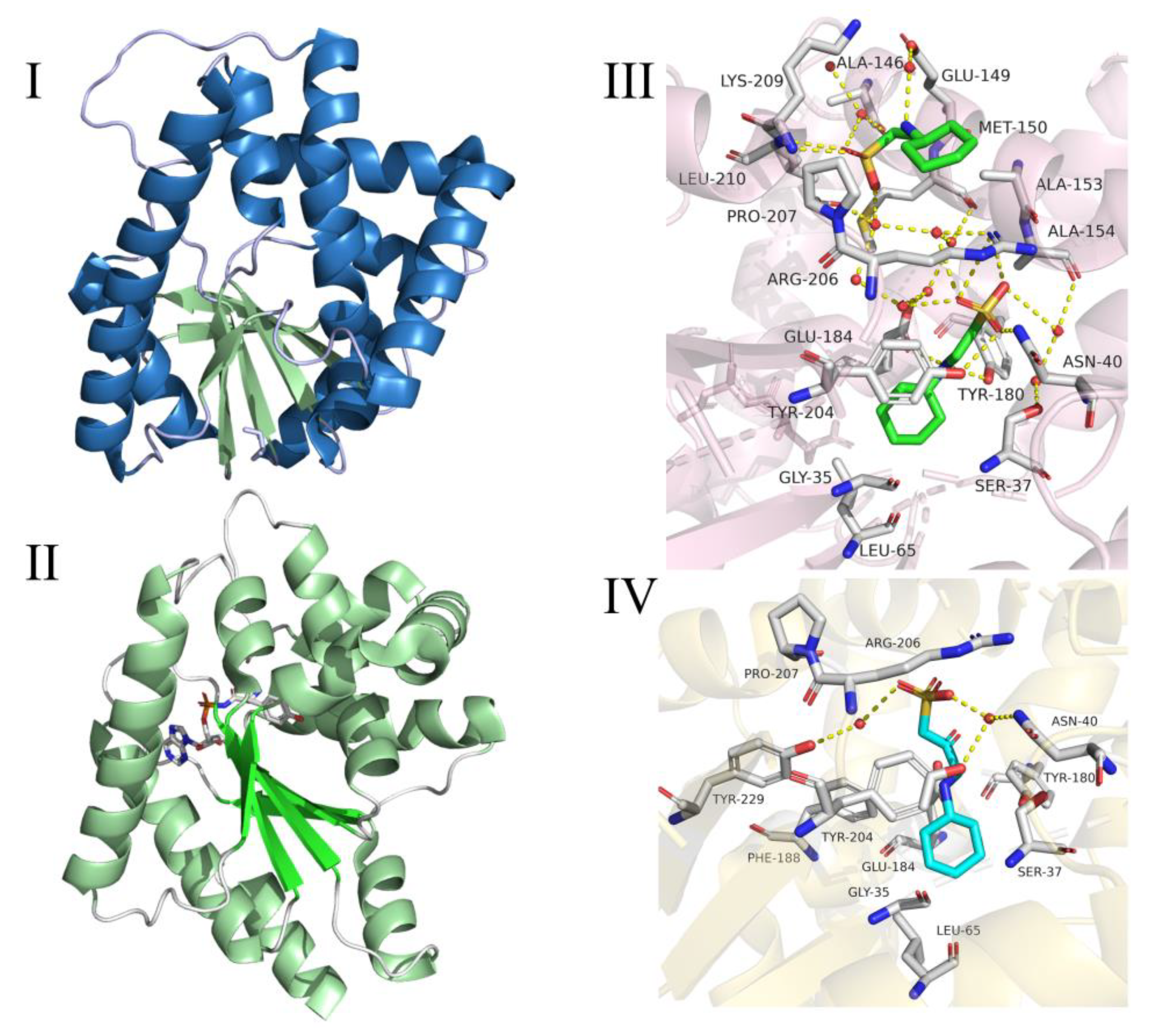
2.2. Cyclic Dipeptide Synthases (CDPSs)
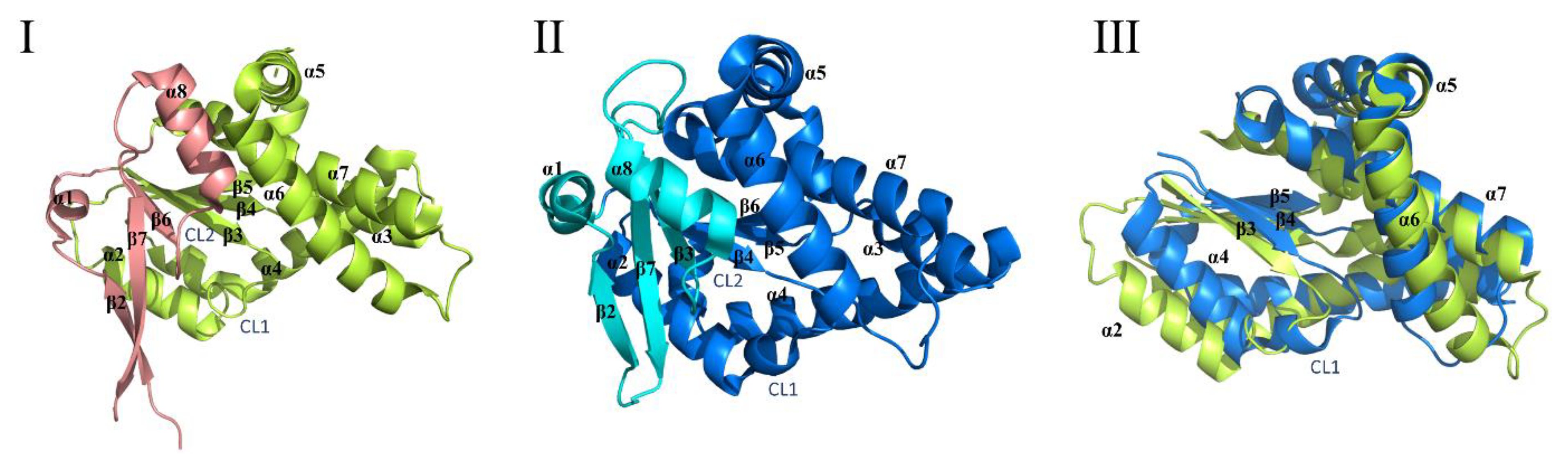
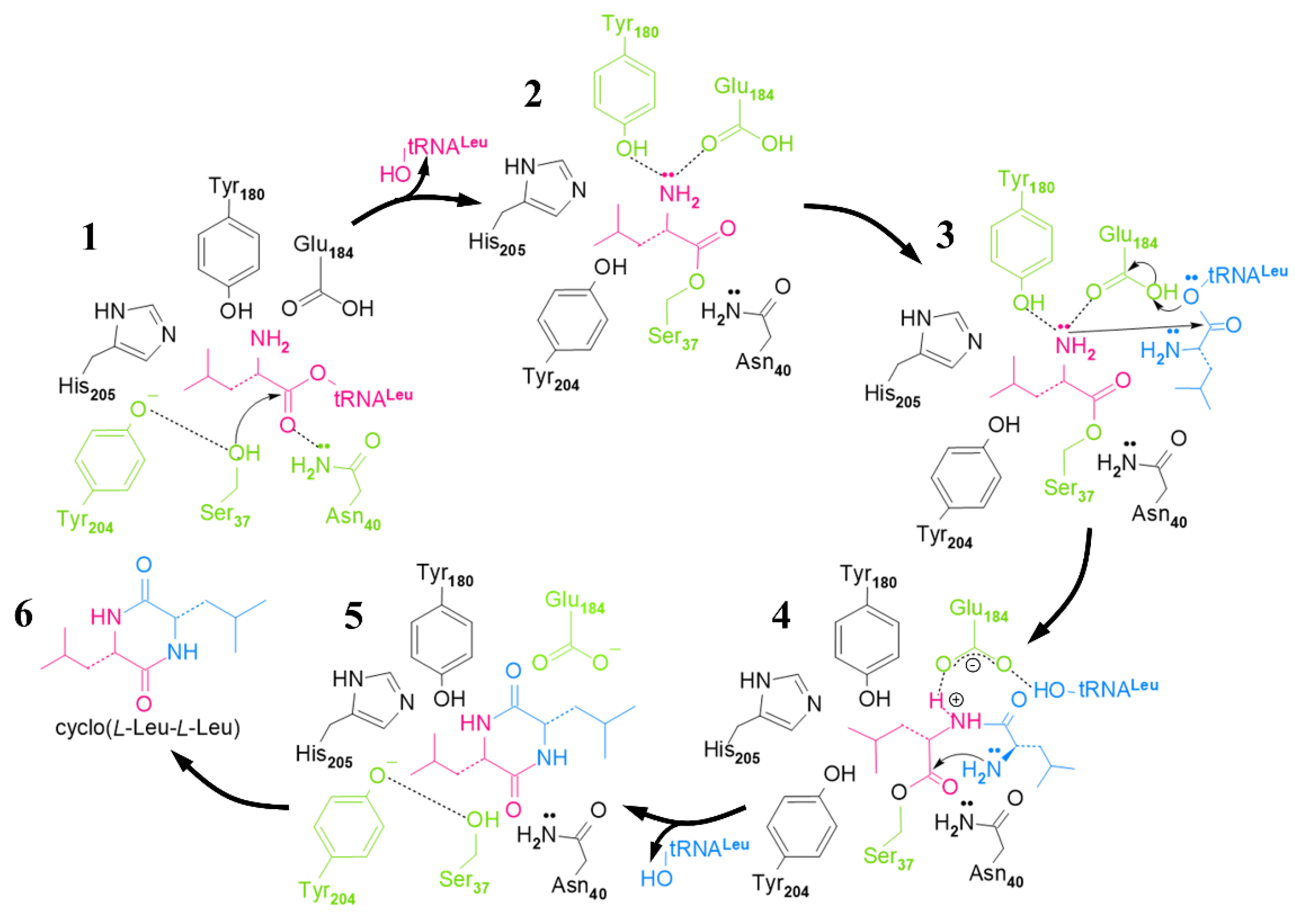
2.3. Cyclic Dileucine Synthase (YvmC)
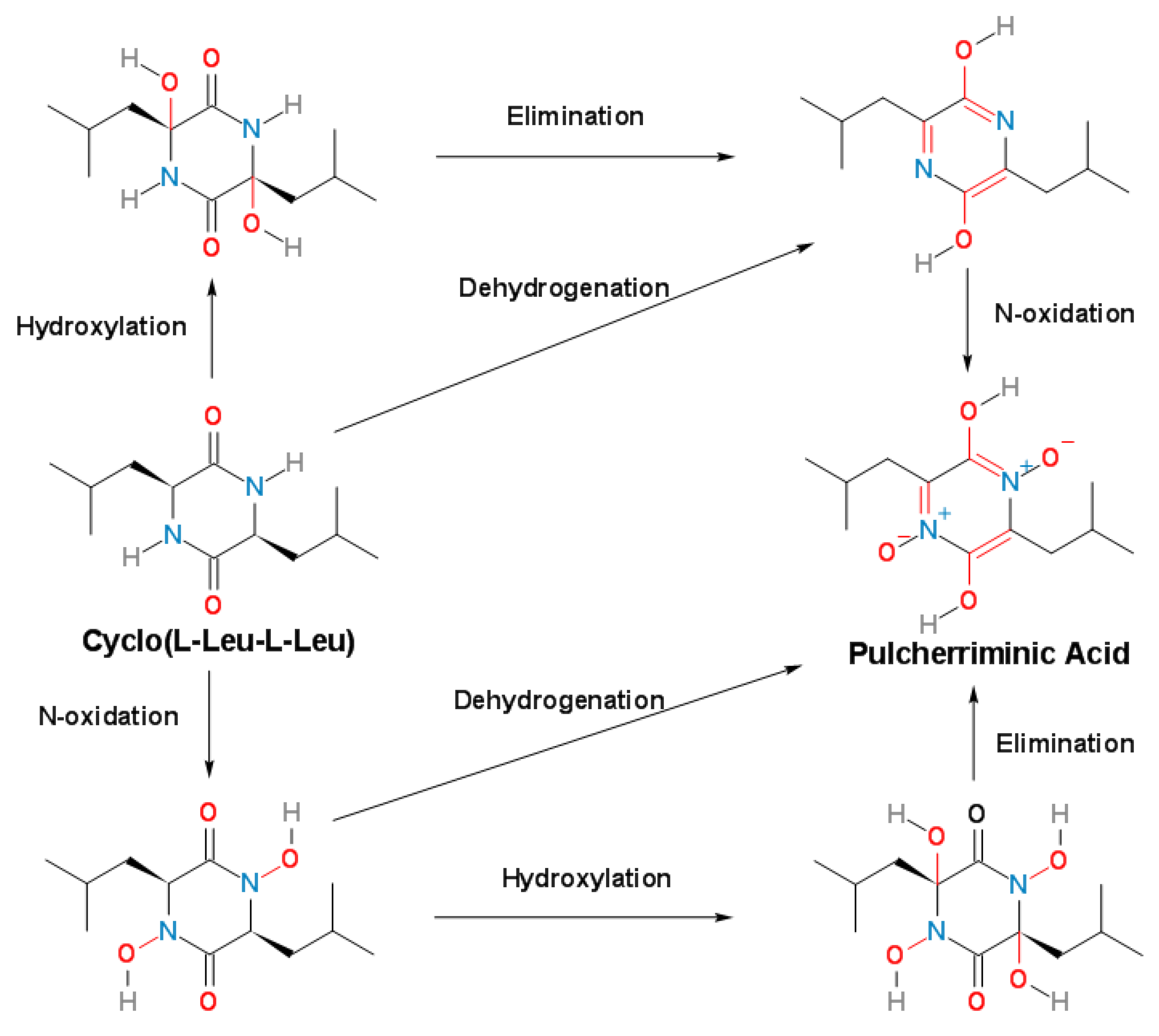
3. Cytochrome P450-oxidizing cLL to form pulcherriminic acid
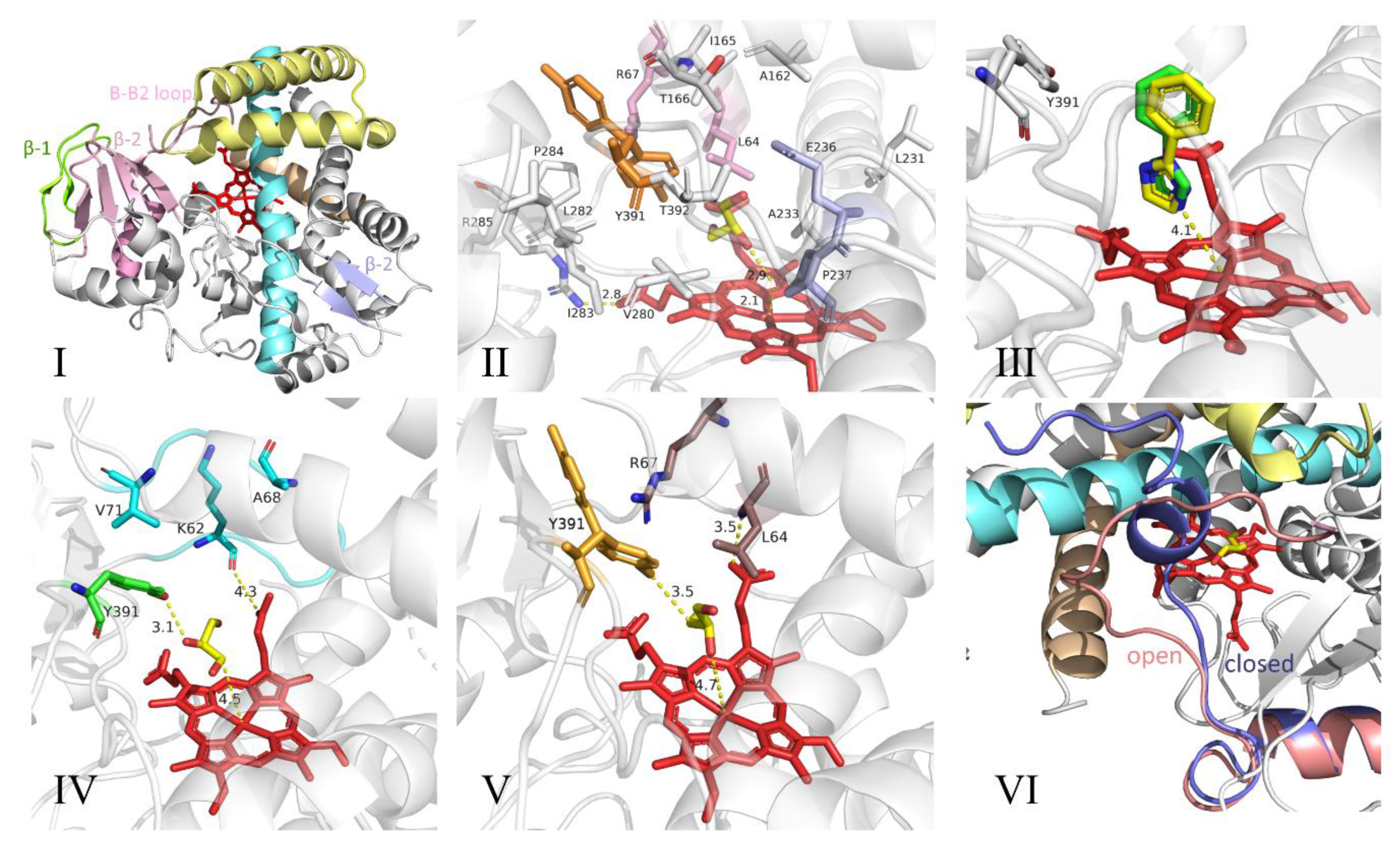
3.1. Cytochrome P450
3.2. Structure and Catalytic Mechanism of Cytochrome P450 Oxidase-CypX (CYP134A1)
4. Promotion of Pulcherriminic Acid Production by Metabolic Engineering
5. Conclusions
- With the development of synthetic biology and metabolic engineering, biological elements from different sources are introduced into target strains to reconstruct the synthesis pathway of pulcherriminic acid, realize the heterologous expression of YvmC and CypX, and regulate the biosynthesis pathway. When the alien pathway breaks the original metabolic network of the target strain, how to maintain the metabolic balance in the cell and how to regulate the new metabolic network to achieve the best state.
- In order to achieve the heterogeneous and high yield of pulcherriminic acid, and meet the demands of industrialization, how to screen high activity YvmC and CypX enzyme is critical to the future research. (The catalytic mechanism of Bacillus cyclodipeptide synthetase YvmC and CypX is still unclear, so far).
- When the YvmA gene is knocked out, the pulcherriminic acid can also be transported out of the cell. Little knowledge is known about the underlying transmembrane mechanism has not been elucidated for pulcherriminic acid. How does pulcherriminic acid get outside of the cell very quickly? It is an urgent problem. What’s the balance mechanism of pulcherriminic acid and the pulcherrimin inside and outside the cell?
- The directed evolution technique is applied to the enzymes in the synthesis pathway of pulcherriminic acid for the screening of the key synthetases with high catalytic activity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sipiczki, M. Metschnikowia Strains Isolated from Botrytized Grapes Antagonize Fungal and Bacterial Growth by Iron Depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef]
- Uffen, R.L.; Canale-Parola, E. Synthesis of Pulcherriminic Acid by Bacillus subtilis. J. Bacteriol. 1972, 111, 86–93. [Google Scholar] [CrossRef]
- Cryle, M.J.; Bell, S.G.; Schlichting, I. Structural and biochemical characterization of the cytochrome P450 CypX (CYP134A1) from Bacillus subtilis: A cyclo-L-leucyl-L-leucyl dipeptide oxidase. Biochemistry 2010, 49, 7282–7296. [Google Scholar] [CrossRef]
- Melvydas, V.; Staneviciene, R.; Balynaite, A.; Vaiciuniene, J.; Garjonyte, R. Formation of self-organized periodic patterns around yeasts secreting a precursor of a red pigment. Microbiol. Res. 2016, 193, 87–93. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J.; Hleba, L.; Kačániová, M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 282–285. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Kluyver, A.J.; Walt, J.P.V.D.; Triet, A.J.V. Pulcherrimin, the Pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 1953, 39, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.C. Biosynthesis of pulcherriminic acid. Biochem. J. 1965, 96, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.G.; Uffen, R.L.; Canale-Parola, E. The role of iron and molecular oxygen in pulcherrimin synthesis by bacteria. Arch. Microbiol. 1967, 56, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.J.; Kominek, J.; Opulente, D.A.; Shen, X.X.; Zhou, X.; Langdon, Q.K.; DeVirgilio, J.; Hulfachor, A.B.; Kurtzman, C.P.; Rokas, A.; et al. Functional and evolutionary characterization of a secondary metabolite gene cluster in budding yeasts. Proc. Natl. Acad. Sci. USA 2018, 115, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.R.; Sternberg, D.; Behr, R.K.; Sloma, A.; Berka, R.M. Use of transcriptional profiling & bioinformatics to solve production problems: Eliminating red pigment production in a Bacillus subtilis strain producing hyaluronic acid. Ind. Biotechnol. 2006, 2, 66–74. [Google Scholar]
- Gondry, M.; Sauguet, L.; Belin, P.; Thai, R.; Amouroux, R.; Tellier, C.; Tuphile, K.; Jacquet, M.; Braud, S.; Courçon, M.; et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond–forming enzymes. Nat. Chem. Biol. 2009, 5, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, L.; Arai, T.; Sakaguchi, Y.; Suzuki, T.; Ishitani, R.; Nureki, O. Structural basis for nonribosomal peptide synthesis by an aminoacyl-tRNA synthetase paralog. Proc. Natl. Acad. Sci. USA 2011, 108, 3912–3917. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, W.; Nomura, C.T.; Li, J.; Chen, S.; Yang, Y.; Wang, Q. Increased flux through the TCA cycle enhances bacitracin production by Bacillus licheniformis DW2. Appl. Microbiol. Biotechnol. 2018, 102, 6935–6946. [Google Scholar] [CrossRef]
- Sauguet, L.; Moutiez, M.; Li, Y.; Belin, P.; Seguin, J.; Le Du, M.-H.; Thai, R.; Masson, C.; Fonvielle, M.; Pernodet, J.-L. Cyclodipeptide synthases, a family of class-I aminoacyl-tRNA synthetase-like enzymes involved in non-ribosomal peptide synthesis. Nucleic Acids Res. 2011, 39, 4475–4489. [Google Scholar] [CrossRef]
- Randazzo, P.; Aubert-Frambourg, A.; Guillot, A.; Auger, S. The MarR-like protein PchR (YvmB) regulates expression of genes involved in pulcherriminic acid biosynthesis and in the initiation of sporulation in Bacillus subtilis. BMC Microbiol. 2016, 16, 190. [Google Scholar] [CrossRef]
- Wang, D.; Zhan, Y.; Cai, D.; Li, X.; Wang, Q.; Chen, S. Regulation of the Synthesis and Secretion of the Iron Chelator Cyclodipeptide Pulcherriminic Acid in Bacillus licheniformis. Appl. Environ. Microbiol. 2018, 84, e00262-18. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Zhang, D.; Li, X.; Zhu, J.; Zhan, Y.; Cai, D.; Wang, Q.; Ma, X.; Wang, D.; et al. Multistep metabolic engineering of Bacillus licheniformis to improve pulcherriminic acid production. Appl. Environ. Microbiol. 2020, 86, e03041-19. [Google Scholar] [CrossRef]
- Yao, T.; Liu, J.; Liu, Z.; Li, T.; Li, H.; Che, Q.; Zhu, T.; Li, D.; Gu, Q.; Li, W. Genome mining of cyclodipeptide synthases unravels unusual tRNA-dependent diketopiperazine-terpene biosynthetic machinery. Nat. Commun. 2018, 9, 4091. [Google Scholar] [CrossRef]
- Belin, P.; Moutiez, M.; Lautru, S.; Seguin, J.; Pernodet, J.-L.; Gondry, M. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat. Prod. Rep. 2012, 29, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Giessen, T.; Marahiel, M. The tRNA-Dependent Biosynthesis of Modified Cyclic Dipeptides. Int. J. Mol. Sci. 2014, 15, 14610–14631. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Gusain, Y.S.; Kumar, V.; Sharma, A.K. Disease management through biological control agents: An eco-friendly and cost effective approach for sustainable agriculture-A Review. Agric. Rev. 2015, 36, 37. [Google Scholar] [CrossRef]
- Abraham, W.R.; De Carvalho, M.P. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar]
- Kohn, H.; Widger, W. The Molecular Basis for the Mode of Action of Bicyclomycin. Curr. Drug Target Infect. Disord. 2005, 5, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Strom, K.; Sjogren, J.; Broberg, A.; Schnurer, J. Lactobacillus plantarum MiLAB 393 Produces the Antifungal Cyclic Dipeptides Cyclo(L-Phe-L-Pro) and Cyclo(L-Phe-trans-4-OH-L-Pro) and 3-Phenyllactic Acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Carrasco, L. Gliotoxin: Inhibitor of poliovirus RNA synthesis that blocks the viral RNA polymerase 3Dpol. J. Virol. 1992, 66, 1971–1976. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Katada, J.; Hayashi, Y.; Uno, I. Biochemistry, Antitumor Activity of Phenylahistin in Vitro and in Vivo. Biosci. Biotechnol. Biochem. 1999, 63, 1130–1133. [Google Scholar] [CrossRef]
- Kanzaki, H.; Yanagisawa, S.; Nitoda, T. Enzymatic synthesis of dehydro cyclo(His-Phe)s, analogs of the potent cell cycle inhibitor, dehydrophenylahistin, and their inhibitory activities toward cell division. Biosci. Biotechnol. Biochem. 2004, 68, 2341–2345. [Google Scholar] [CrossRef]
- Waring, P.; Beaver, J. Gliotoxin and related epipolythiodioxopiperazines. Gen. Pharmacol. 1996, 27, 1311–1316. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. 2,5-diketopiperazines as neuroprotective agents. Mini Rev. Med. Chem. 2012, 12, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Picaso, L.; Olivo, H.F.; Argotte-Ramos, R.; Rodríguez-Gutiérrez, M.; Rios, M.Y. Linear and cyclic dipeptides with antimalarial activity. Bioorg. Med. Chem. Lett. 2012, 22, 7048–7051. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Ai Tran, H.N.; Staderini, M.; Monaco, A.; López-Cobeñas, A.; Bongarzone, S.; Biarnés, X.; López-Alvarado, P.; Cabezas, N.; Caramelli, M.; et al. Discovery of a Class of Diketopiperazines as Antiprion Compounds. ChemMedChem 2010, 5, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Hwang, I.K.; Rosenthal, M.J.; Harris, D.M.; Go, V.L.W. Anti-Hyperglycemic Activity of Zinc Plus Cyclo (His-Pro) in Genetically Diabetic Goto-Kakizaki and Aged Rats. Exp. Biol. Med. 2004, 228, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Akutagawa, T.; Funatani, H.; Uchida, T.; Hotta, Y.; Niwa, M.; Takaya, Y. Cyclic dipeptides exhibit potency for scavenging radicals. Bioorganic Med. Chem. 2012, 20, 2002–2009. [Google Scholar] [CrossRef]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Moutiez, M.; Belin, P.; Gondry, M. Aminoacyl-tRNA-Utilizing Enzymes in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5578–5618. [Google Scholar] [CrossRef]
- Giessen, T.W.; Marahiel, M.A. Rational and combinatorial tailoring of bioactive cyclic dipeptides. Front. Microbiol. 2015, 6, 785. [Google Scholar] [CrossRef]
- Kopp, F.; Marahiel, M.A. Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis. Nat. Prod. Rep. 2007, 24, 735–749. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, T.; Liu, J.; Li, H.; Li, W. Recent Advances in Cyclodipeptide Synthases-Dependent Biosynthetic Pathway. Chin. J. Org. Chem. 2019, 39, 328–338. [Google Scholar] [CrossRef]
- Mishra, A.; Choi, J.; Choi, S.; Baek, K. Cyclodipeptides: An Overview of Their Biosynthesis and Biological Activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Zhuang, S.W.; Fu, J.S.; Wang, C.Y.; Sun, Y.H.; Wang, Y.F. Analysis of aroma compounds of different yeast strains & its molecular identification by bioinformatics. In Proceedings of the 2010 International Conference on Computer and Communication Technologies in Agriculture Engineering, Chengdu, China, 12 June 2010; pp. 25–30. [Google Scholar]
- Borthwick, D.A. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Lautru, S.; Gondry, M.; Genet, R.; Pernodet, J.L. The Albonoursin Gene Cluster of S. noursei: Biosynthesis of Diketopiperazine Metabolites Independent of Nonribosomal Peptide Synthetases. Chem. Biol. 2002, 9, 1355–1364. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Blanchard, J.S. The structure and mechanism of the Mycobacterium tuberculosis cyclodityrosine synthetase. Nat. Chem. Biol. 2010, 6, 797–799. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Johnston, C.W.; Merwin, N.J.; Dejong, C.A.; Magarvey, N.A. Global analysis of prokaryotic tRNA-derived cyclodipeptide biosynthesis. BMC Genom. 2018, 19, 45. [Google Scholar] [CrossRef]
- Brockmeyer, K.; Li, S.-M. Mutations of Residues in Pocket P1 of a Cyclodipeptide Synthase Strongly Increase Product Formation. J. Nat. Prod. 2017, 80, 2917–2922. [Google Scholar] [CrossRef]
- Takahito, M.; Noah, R.; Ana, C.; Dieter, S. Bioinformatic Analysis Reveals Archaeal tRNATyr and tRNATrp Identities in Bacteria. Life 2017, 7, 8. [Google Scholar]
- Jacques, I.B.; Moutiez, M.; Witwinowski, J.; Darbon, E.; Martel, C.; Seguin, J.; Favry, E.; Thai, R.; Lecoq, A.; Dubois, S.; et al. Analysis of 51 cyclodipeptide synthases reveals the basis for substrate specificity. Nat. Chem. Biol. 2015, 11, 721–727. [Google Scholar] [CrossRef]
- Bourgeois, G.; Seguin, J.; Babin, M.; Belin, P.; Moutiez, M.; Mechulam, Y.; Gondry, M.; Schmitt, E. Structural basis for partition of the cyclodipeptide synthases into two subfamilies. J. Struct. Biol. 2018, 203, 17–26. [Google Scholar] [CrossRef]
- Rajendran, V.; Kalita, P.; Shukla, H.; Kumar, A.; Tripathi, T. Aminoacyl-tRNA synthetases: Structure, function, and drug discovery. Int. J. Biol. Macromol. 2018, 111, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Moutiez, M.; Schmitt, E.; Seguin, J.; Thai, R.; Favry, E.; Belin, P.; Mechulam, Y.; Gondry, M. Unravelling the mechanism of non-ribosomal peptide synthesis by cyclodipeptide synthases. Nat. Commun. 2014, 5, 5141. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Bourgeois, G.; Gondry, M.; Aleksandrov, A. Cyclization Reaction Catalyzed by Cyclodipeptide Synthases Relies on a Conserved Tyrosine Residue. Sci. Rep. 2018, 8, 7031. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Gao, J.F. Research Progress of Cyclodipeptides Synthases. Chem. Bioeng. 2013, 30, 17–21. [Google Scholar]
- Poulos, T.L.; Finzel, B.C.; Howard, A.J. High-resolution crystal structure of cytochrome P450cam. J. Mol. Biol. 1987, 195, 687–700. [Google Scholar] [CrossRef]
- Rittle, J.; Green, M.T. Cytochrome P450 Compound I: Capture, Characterization, and C-H Bond Activation Kinetics. Science 2010, 330, 933–937. [Google Scholar] [CrossRef]
- Mclean, K.J.; Leys, D.; Munro, A.W. Microbial Cytochromes P450, 4th ed. In The Cytochrome P450; Ortiz de Montellano, P., Ed.; Springer: Cham, Switzerland, 2015; pp. 261–407. ISBN 978-3-319-12108-6. [Google Scholar]
- Nelson, D.R. Cytochrome P450 Nomenclature, 2004. Methods Mol. Biol. 2006, 320, 1–10. [Google Scholar]
- Nelson, D.R. Progress in tracing the evolutionary paths of cytochrome P450. Biochim. Biophys. Acta 2011, 1814, 14–18. [Google Scholar] [CrossRef]
- Sezutsu, H.; Le Goff, G.; Feyereisen, R. Origins of P450 diversity. Philos. Trans. Biol. Sci. 2013, 368, 20120428. [Google Scholar] [CrossRef]
- Greule, A.; Stok, J.E.; De Voss, J.J.; Cryle, M.J. Unrivalled diversity: The many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat. Prod. Rep. 2018, 35, 757–791. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Qiu, Y.; Nomura, C.T.; Li, J.; Chen, S. Untangling the transcription regulatory network of the bacitracin synthase operon in Bacillus licheniformis DW2. Res. Microbiol. 2017, 168, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. Research of Bacitracin and Pulcherriminc Acid Biosynthesis Regulation in Bacillus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Perego, M.; Spiegelman, G.B.; Hoch, J.A. Structure of the gene for the transition state regulator, abrB: Regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol. Microbiol. 1988, 2, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Zuber, P. Specificity through flexibility. Nat. Struct. Biol. 2000, 7, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Devine, K.M. Expression of AbrB, a transition state regulator from Bacillus subtilis, is growth phase dependent in a manner resembling that of Fis, the nucleoid binding protein from Escherichia coli. J. Bacteriol. 1997, 179, 522–529. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Matoz-Fernandez, D.A.; Porter, M.; Kalamara, M.; Abbott, J.; MacPhee, C.E.; Davidson, F.A.; Stanley-Wall, N.R. Pulcherrimin formation controls growth arrest of the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2019, 116, 13553–13562. [Google Scholar] [CrossRef]
- Lanza, A.M.; Alper, H.S. Global Strain Engineering by Mutant Transcription Factors. Methods Mol. Biol. 2011, 765, 253–274. [Google Scholar]
- Lin, Z.; Zhang, Y.; Wang, J. Engineering of Transcriptional Regulators Enhances Microbial Stress Tolerance. Biotechnol. Adv. 2013, 31, 986–991. [Google Scholar] [CrossRef]
- Alper, H.; Stephanopoulos, G. Global transcription machinery engineering: A new approach for improving cellular phenotype. Metab. Eng. 2007, 9, 258–267. [Google Scholar] [CrossRef]
- Lynch, S.; Eckert, C.; Yu, J.; Gill, R.; Maness, P.C. Overcoming substrate limitations for improved production of ethylene in E. coli. Biotechnol. Biofuels 2016, 9, 3. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, J.; Zhu, S.; Lu, Y.; Zhang, B.; Lu, K.; Li, J.; Ma, X.; Chen, S. Metabolic Engineering of Main Transcription Factors in Carbon, Nitrogen, and Phosphorus Metabolisms for Enhanced Production of Bacitracin in Bacillus licheniformis. ACS Synth. Biol. 2019, 8, 866–875. [Google Scholar] [CrossRef]
- Zhu, C.; Xiao, F.; Qiu, Y.; Wang, Q.; He, Z.; Chen, S. Lichenysin production is improved in codY null Bacillus licheniformis by addition of precursor amino acids. Appl. Microbiol. Biotechnol. 2017, 101, 6375–6383. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Haas, S.; Klaffl, S.; Polen, T.; Eggeling, L.; Van Ooyen, J.; Bott, M. Pushing product formation to its limit: Metabolic engineering of Corynebacterium glutamicum for L-leucine overproduction. Metab. Eng. 2014, 22, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, D.; Xu, H.; Liu, Z.; Zhang, B.; Wu, F.; Li, J.; Chen, S. Enhancement of precursor amino acid supplies for improving bacitracin production by activation of branched chain amino acid transporter BrnQ and deletion of its regulator gene lrp in Bacillus licheniformis. Synth. Syst. Biotechnol. 2018, 3, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.J.; Jiang, W.; Guamán, L.P.; Xiao, Y.; Zhang, F. Engineering Escherichia coli to produce branched-chain fatty acids in high percentages. Metab. Eng. 2016, 38, 148–158. [Google Scholar] [CrossRef]
- Xia, X.X.; Qian, Z.-G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yong, X.; Zhao, T.; Li, Y.; Liu, J. Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus. Molecules 2020, 25, 5611. https://doi.org/10.3390/molecules25235611
Yuan S, Yong X, Zhao T, Li Y, Liu J. Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus. Molecules. 2020; 25(23):5611. https://doi.org/10.3390/molecules25235611
Chicago/Turabian StyleYuan, Siqi, Xihao Yong, Ting Zhao, Yuan Li, and Jun Liu. 2020. "Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus" Molecules 25, no. 23: 5611. https://doi.org/10.3390/molecules25235611
APA StyleYuan, S., Yong, X., Zhao, T., Li, Y., & Liu, J. (2020). Research Progress of the Biosynthesis of Natural Bio-Antibacterial Agent Pulcherriminic Acid in Bacillus. Molecules, 25(23), 5611. https://doi.org/10.3390/molecules25235611





