Maillard Reaction Intermediates and Related Phytochemicals in Black Garlic Determined by EPR and HPLC Analyses
Abstract
:1. Introduction
2. Sample Preparations
2.1. Black Garlic Samples
2.2. EPR Measurements
2.3. Melanoidin Measurements
2.4. Total Polyphenol Measurements
2.5. 5-HMF Quantiication by HPLC and HPLC-ESI-Tof-MS
2.6. Statistical Analysis
3. Results and Discussion
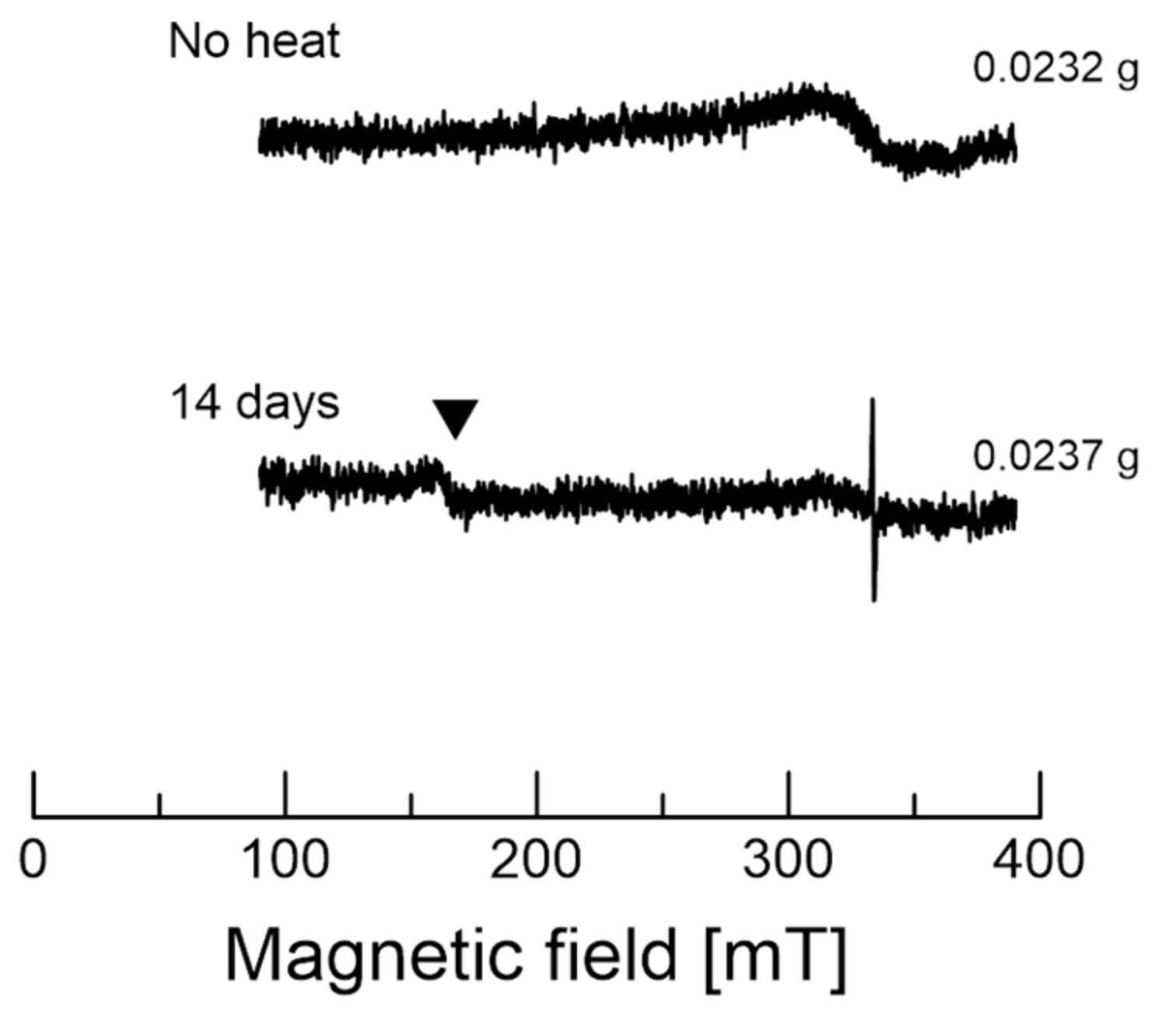
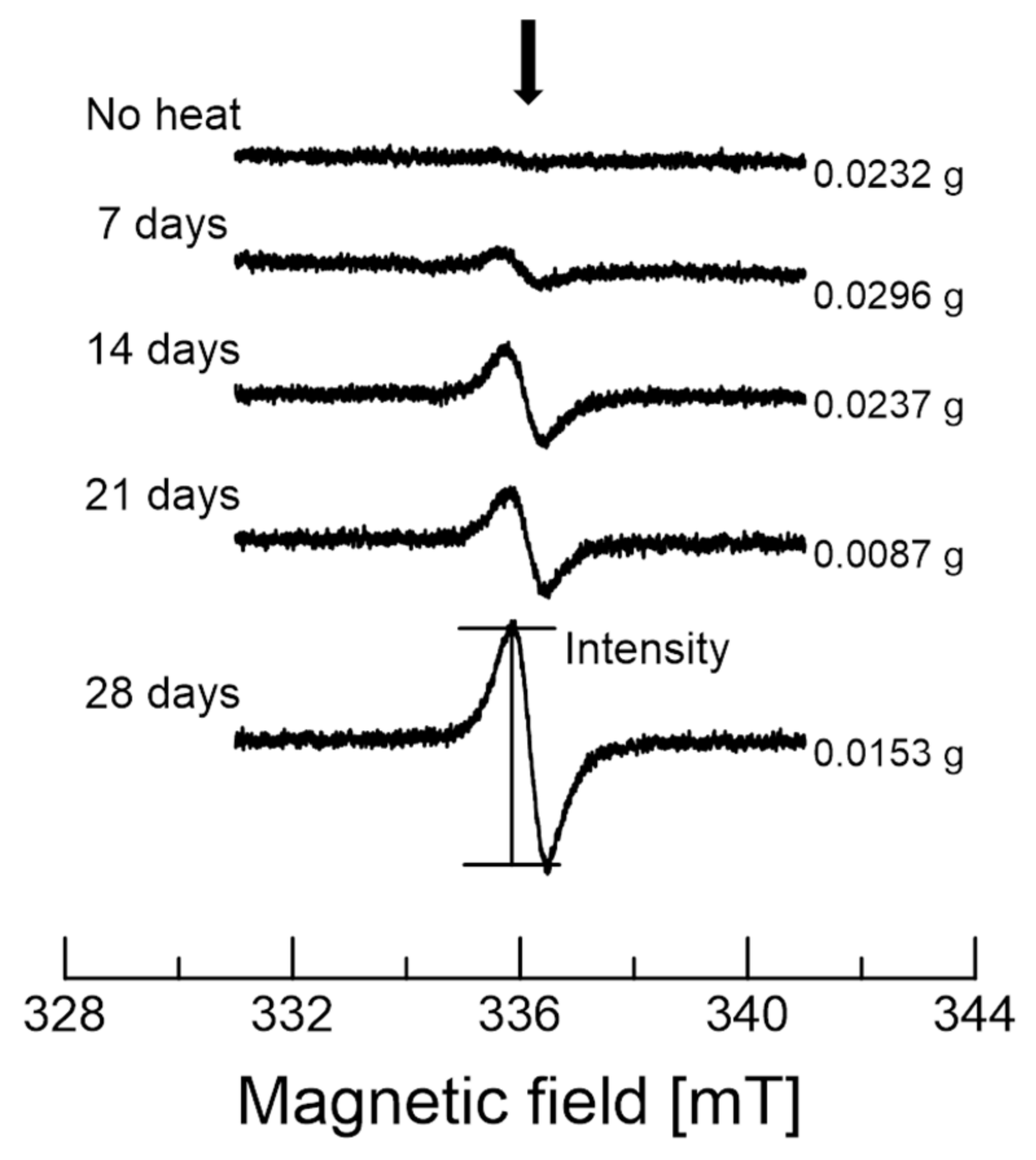
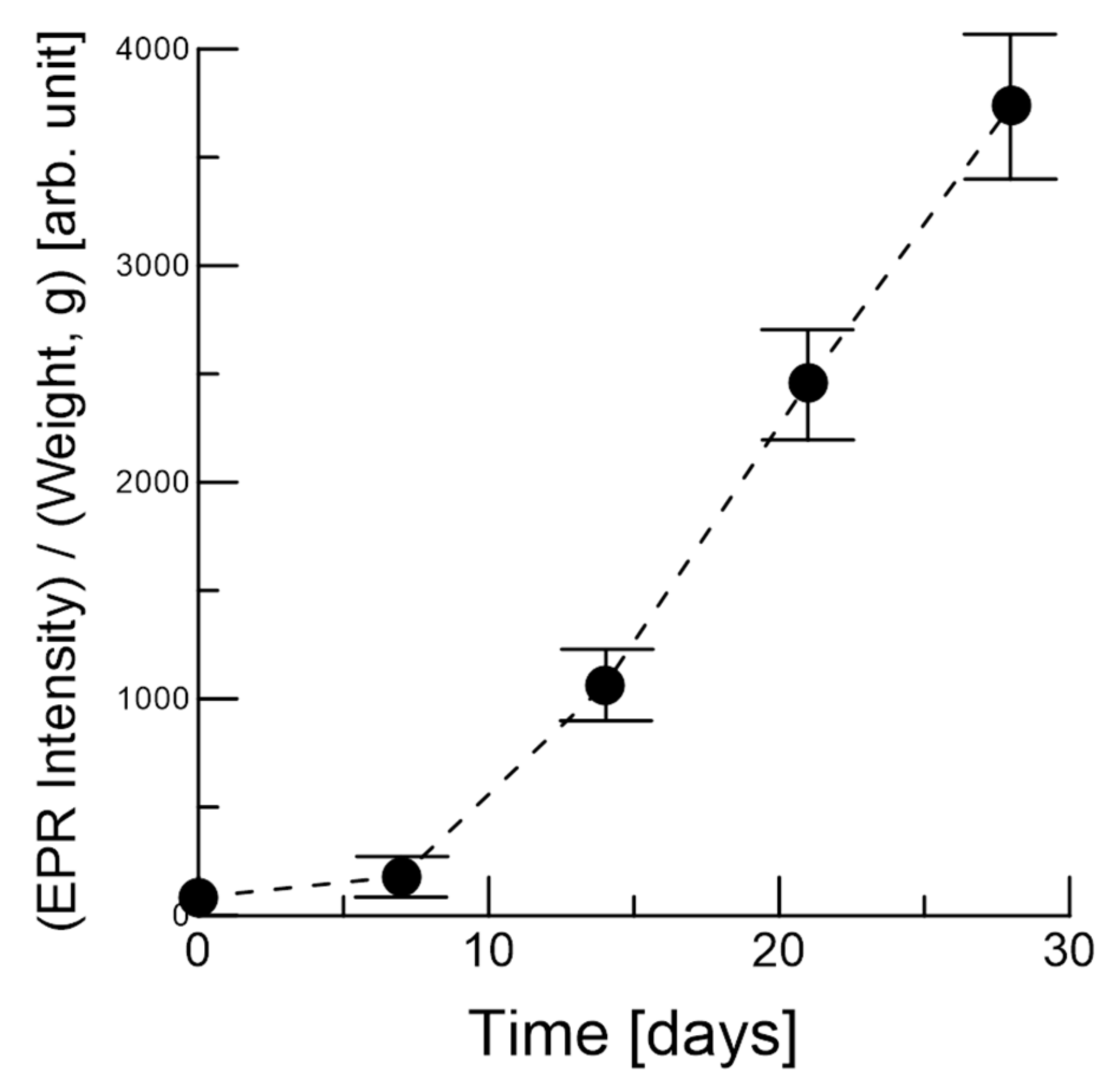
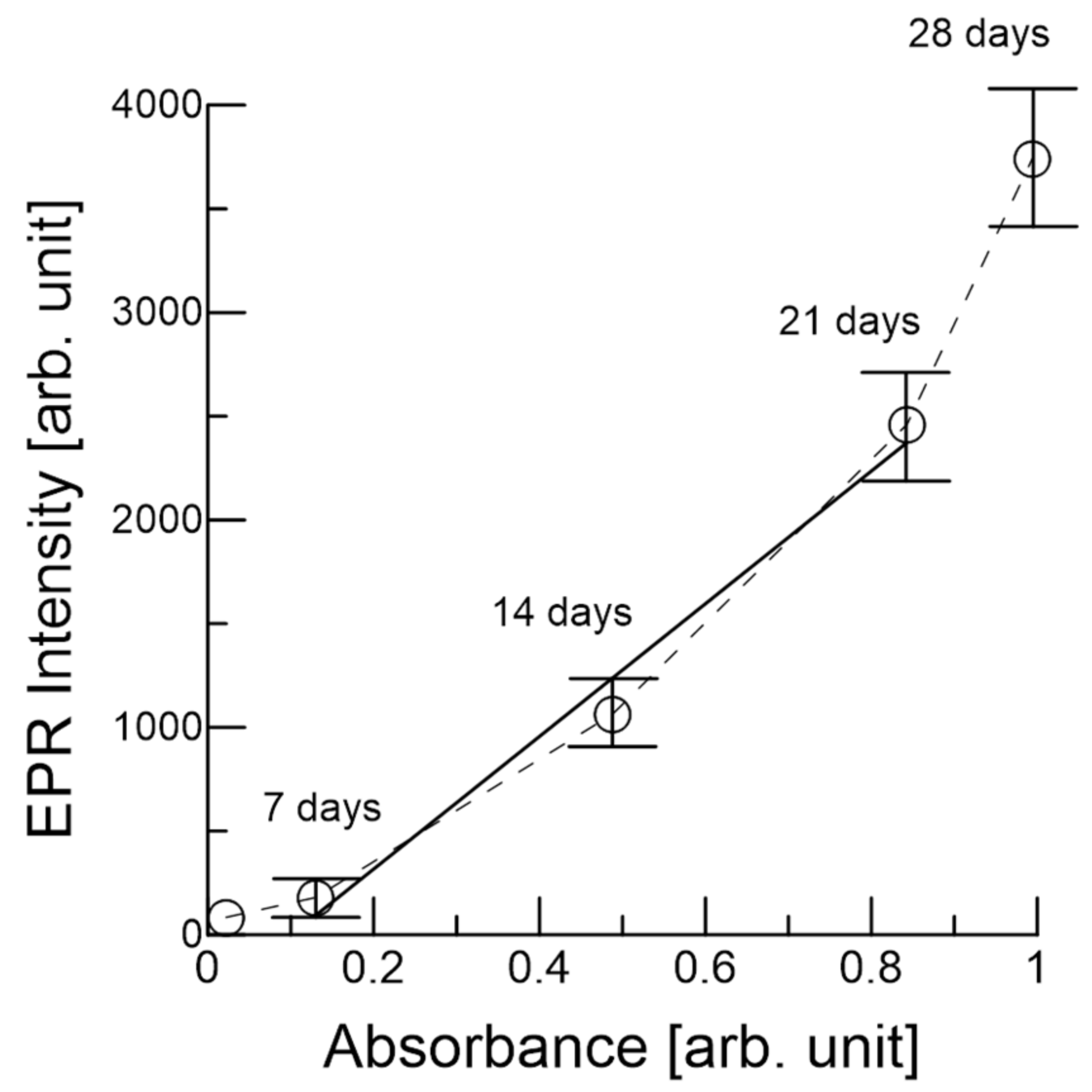
3.1. EPR of the Garlic Samples
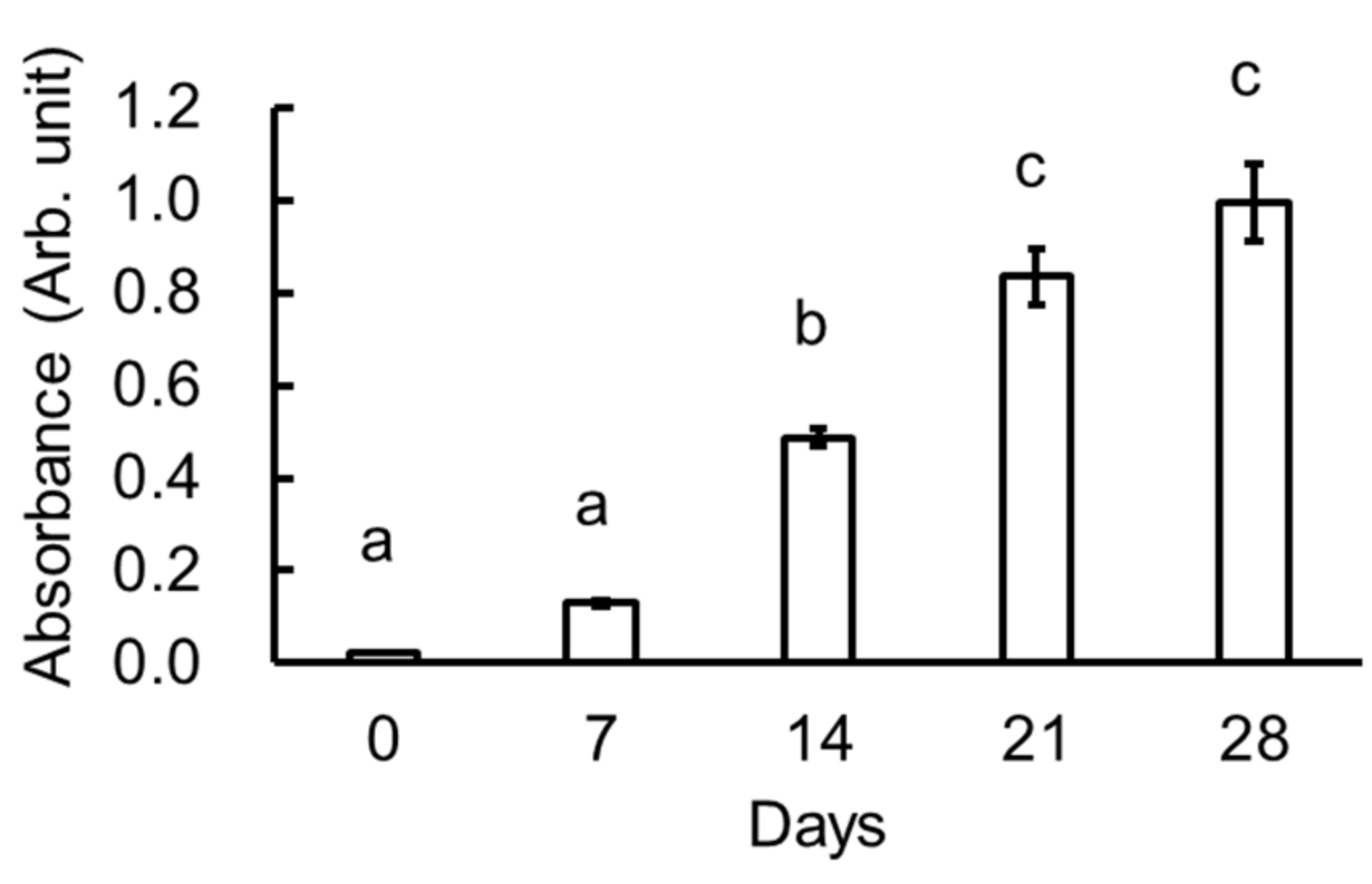
3.2. Melanoidin of the Garlic Samples
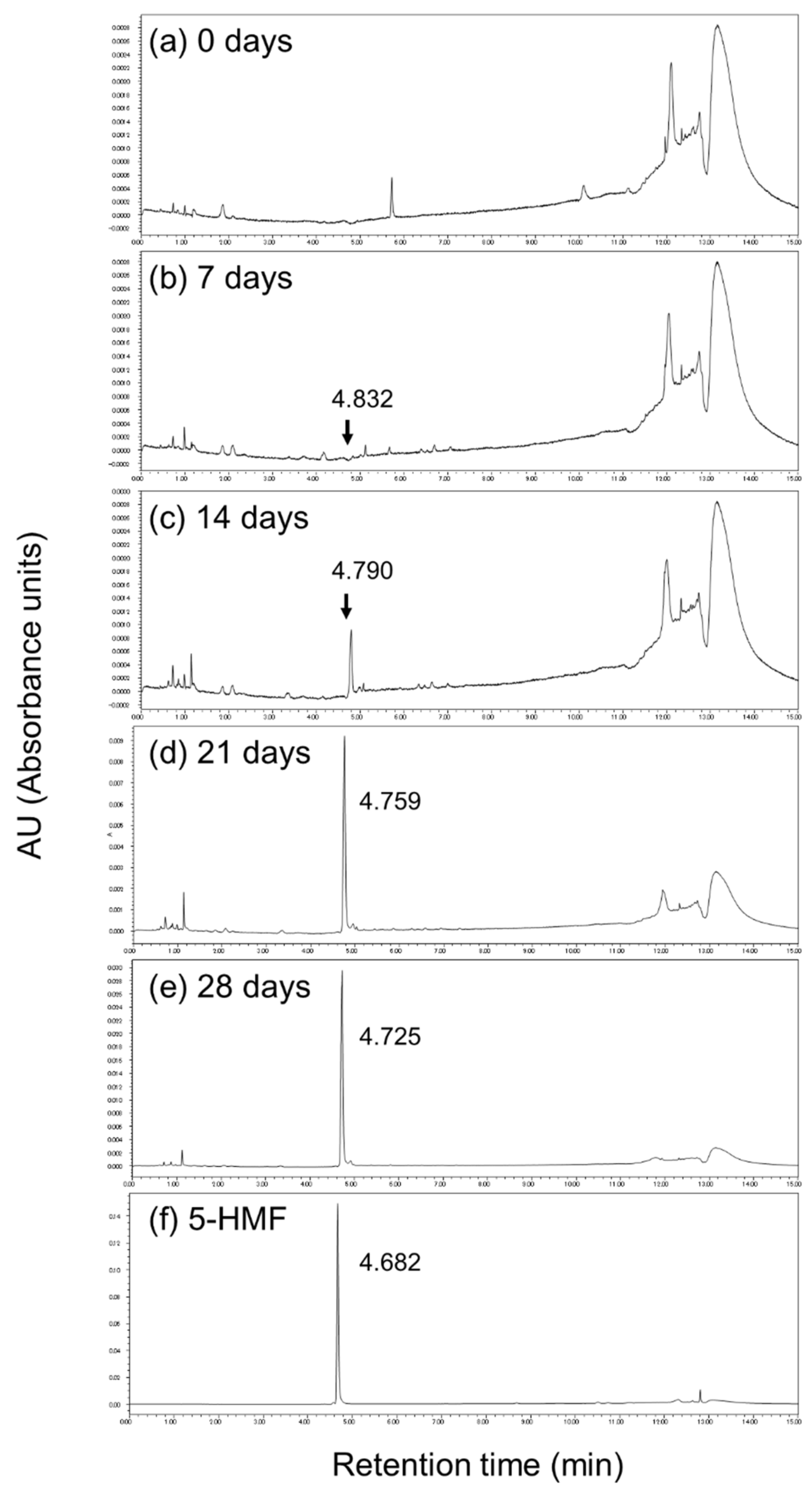
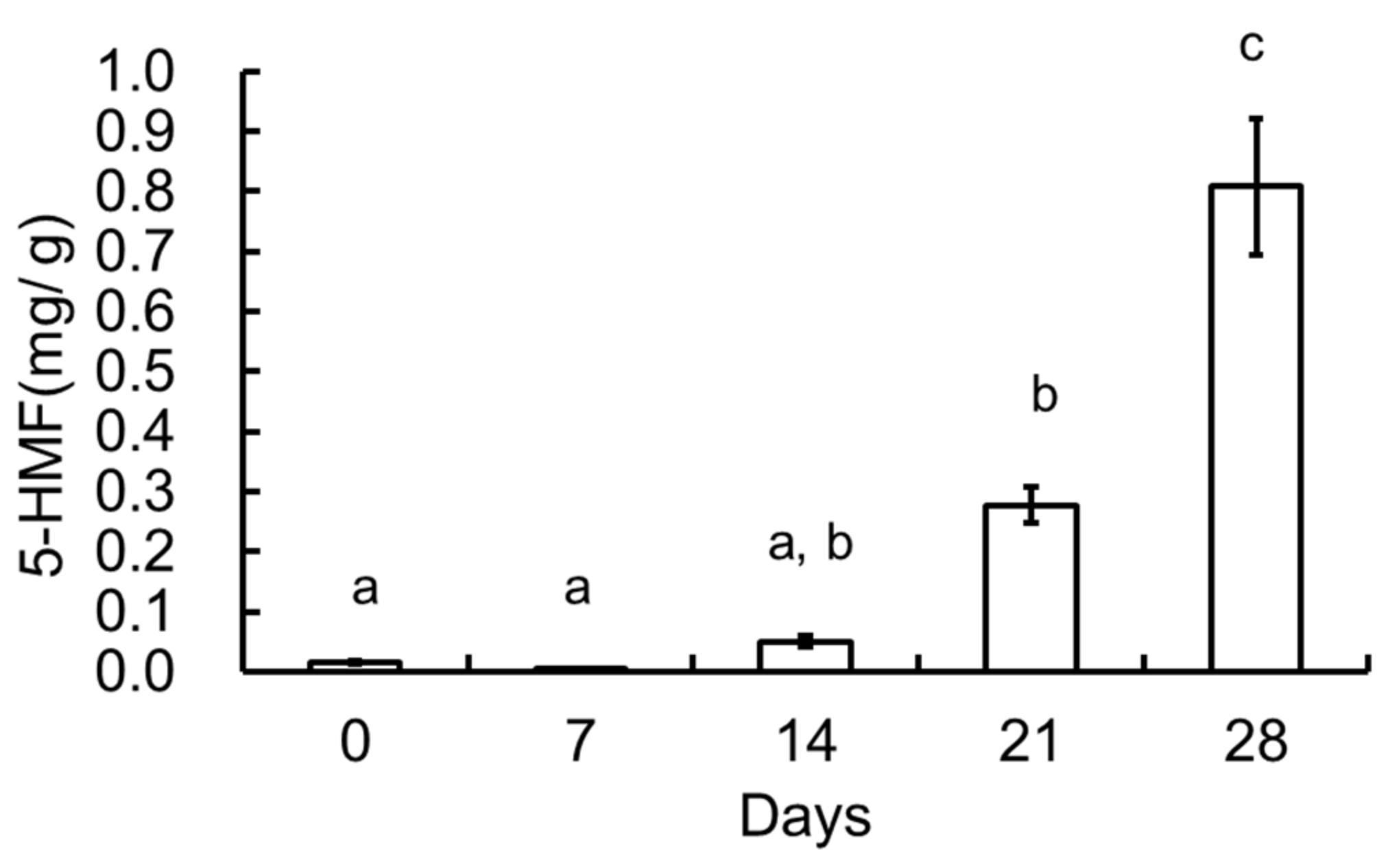
3.3. 5-HMF of the Garlic Samples
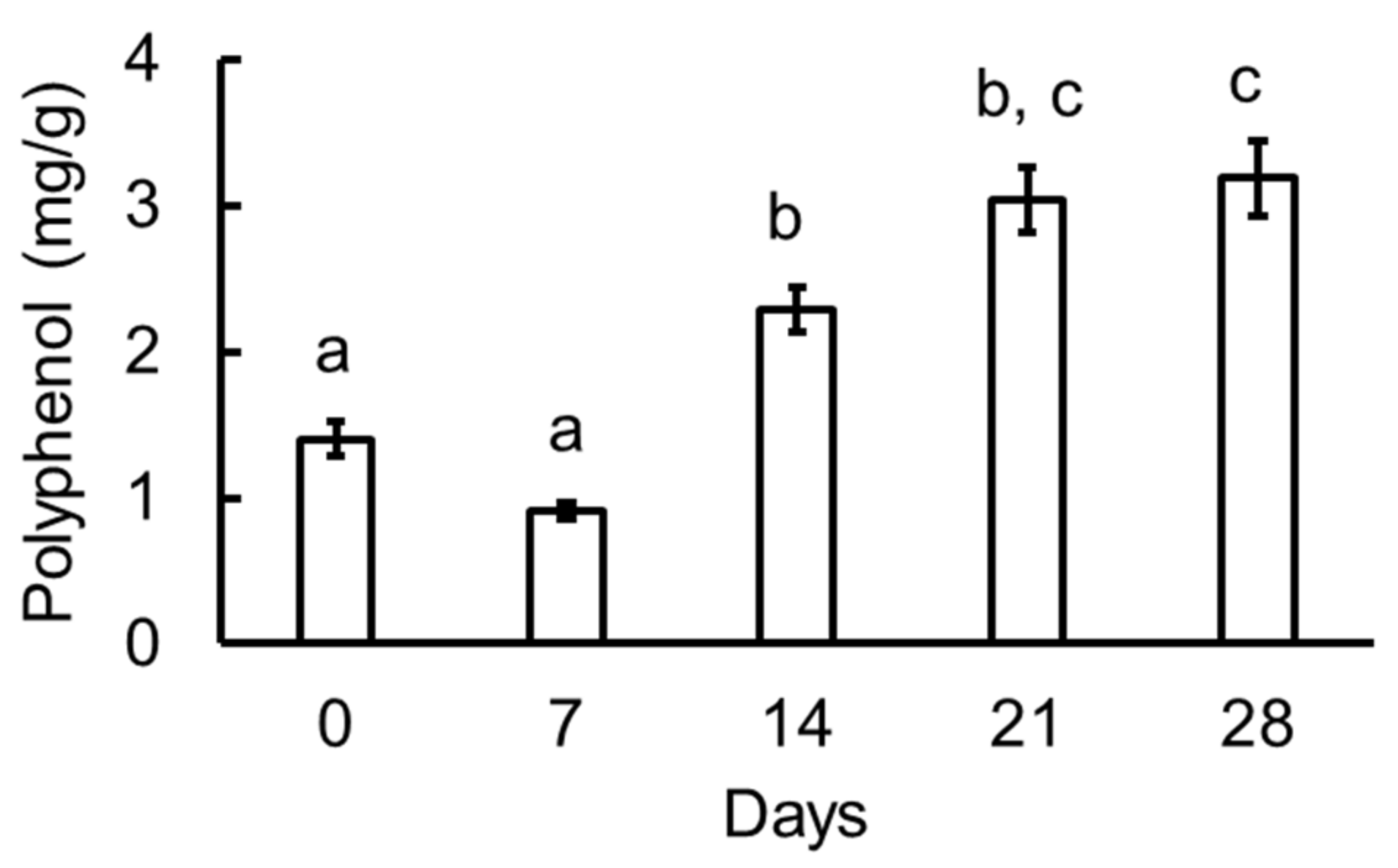
3.4. Polyphenols in the Garlic Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Block, E. The chemistry of garlic and onion. Sci. Am. 1985, 251, 114–119. [Google Scholar]
- Ryu, J.H.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 6–70. [Google Scholar]
- Lee, C.-H.; Chen, Y.-T.; Hsieh, H.-J.; Chen, K.-T.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Lin, J.-A.; Hsieh, C.-W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. LWT-Food Sci. Technol. 2020, 117, 108628. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of nonenzymatic browning and Antioxidant capacity in processed foods. Trends in Food Sci. Technol. 2001, 11, 340–346. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Vázquez-Barrios, M.; Gaytán-Martínez, E.M.; Olano, A.; Montilla, A.; Villamiel, M. 2-Furoylmethyl amino acids as indicators of Maillard reaction during the elaboration of black garlic. Food Chem. 2018, 240, 1106–1112. [Google Scholar] [CrossRef] [Green Version]
- Wakamatsu, J.; Stark, T.D.; Hofmann, T. Taste-Active Maillard Reaction Products in Roasted Garlic (Allium sativum). J. Agric. Food Chem. 2016, 64, 5845–5854. [Google Scholar] [CrossRef]
- Liu, P.; Lu, X.; Li, N.; Zheng, Z.; Zhao, R.; Tanga, X.; Qiao, X. Effects and mechanism of free amino acids on browning in the processing of black garlic. J. Sci. Food Agric. 2019, 99, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Epel, B. Location of radical species in a black pepper seed investigated by CW EPR and 9 GHz EPR imaging. Spectrochim Acta Part A 2014, 131, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Minakawa, S.; Sawamura, D.; Hara, H. Characterization of melanin radicals in paraffin-embedded malignant melanoma and nevus pigmentosus using X-band EPR and EPR imaging. Ana. Sci. 2017, 33, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Saenjum, C.; Pattananandecha, T.; Nakagawa, K. Detection of antioxidant phytochemicals isolated from Camellia japonica seeds using HPLC and EPR imaging. Antioxidants 2020, 9, 493. [Google Scholar]
- Hirano, M.; Miura, M.; Gomyo, T. A Tentative Measurement of Brown Pigments in Various Processed Foods. Biosci. Biotech. Biochem. 1996, 60, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Petra, M.; Marija, S.; Lidija, J. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar]
- Nakagawa, K.; Yooin, W.; Saenjum, C. EPR and HPLC investigation of pigments in Thai purple rice. J. Oleo Sci. 2018, 67, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Matsumoto, K.; Chaiserm, N.; Priprem, A. X-band electron paramagnetic resonance investigation of stable organic radicals present under cold stratification in ‘Fuji’ apple seeds. J. Oleo Sci. 2017, 66, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug. 2017, 25, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Tero-Kubota, S.; Ikegami, Y.; Tsuchihashi, N. EPR and TREPR Spectroscopic Studies of Antioxidant Sesamolyl and Related Phenoxyl Radicals. Photochem. Photobiol. 1994, 60, 199–204. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, K.; Maeda, H.; Yamaya, Y.; Tonosaki, Y. Maillard Reaction Intermediates and Related Phytochemicals in Black Garlic Determined by EPR and HPLC Analyses. Molecules 2020, 25, 4578. https://doi.org/10.3390/molecules25194578
Nakagawa K, Maeda H, Yamaya Y, Tonosaki Y. Maillard Reaction Intermediates and Related Phytochemicals in Black Garlic Determined by EPR and HPLC Analyses. Molecules. 2020; 25(19):4578. https://doi.org/10.3390/molecules25194578
Chicago/Turabian StyleNakagawa, Kouichi, Hayato Maeda, Yoshifumi Yamaya, and Yudai Tonosaki. 2020. "Maillard Reaction Intermediates and Related Phytochemicals in Black Garlic Determined by EPR and HPLC Analyses" Molecules 25, no. 19: 4578. https://doi.org/10.3390/molecules25194578






