An Overview of the Potential Antineoplastic Effects of Casticin
Abstract
1. Introduction
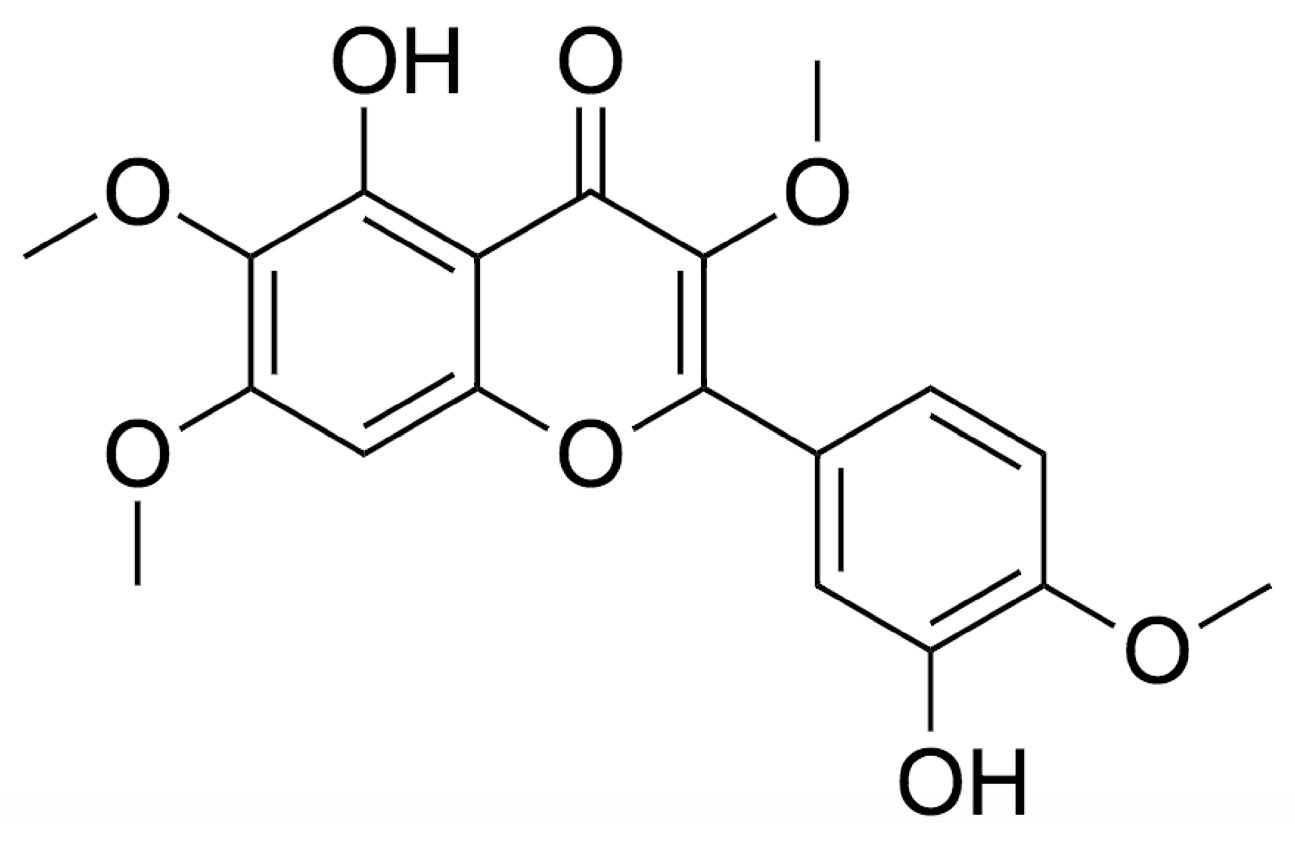
2. Chemistry
3. Antineoplastic Effects of Casticin
3.1. Biological Techniques
3.2. In vitro Cancer Cell lines
3.2.1. Casticin and Breast Cancer
3.2.2. Casticin and Bladder Cancer
3.2.3. Casticin and Cervical Cancer
3.2.4. Casticin and Colon Cancer
3.2.5. Casticin and Esophageal Cancer
3.2.6. Casticin and Gallbladder Cancer
3.2.7. Casticin and Hepatocellular Carcinoma
3.2.8. Casticin and Leukemia
3.2.9. Casticin and Lung Cancer
3.2.10. Casticin and Melanoma
3.2.11. Casticin and Oral Cancer
3.2.12. Casticin and Ovarian Cancer
3.2.13. Casticin and Prostate Cancer
3.3. In vivo Studies: Mouse Models
4. Effect of Casticin on Epithelial-Mesenchymal Transition (EMT)
5. Effect of Casticin on Metastasis
6. Other Important Pharmacological Actions
7. Cytotoxicity Data
8. Limitations and Future Prospects
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| Bcl-2 | B cell lymphoma-2 |
| Bcl-xL | B cell lymphoma-extra large |
| Bax | BCL2-associated X protein |
| CD44 | Cluster of differentiation 44 |
| CDK1 | Cyclin-dependent kinase 1 |
| CDK4 | Cyclin-dependent kinase 4 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A |
| CDKN1B | Cyclin-dependent kinase inhibitor 1B |
| COX-2 | Cyclooxygenase 2 |
| CREB1 | cAMP responsive element binding protein |
| DDIT3 | DNA-damage-inducible transcript 3 |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| EGFR | Epidermal cell growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| FOXO3a | Forkhead box class O 3a |
| FoxM1 | Forkhead box M1 |
| GBC | Gallbladder cancer |
| Gli-1 | Glioma-associated oncogene 1 |
| GSH | Glutathione |
| HCC | Hepatocellular carcinoma |
| IFGR | Insulin-like growth factors |
| IL-6 | Interleukin 6 |
| IL-B | Interleukin B |
| JNK | c-Jun N-terminal kinase |
| LCSC | Liver cancer stem cells |
| MAPK | Mitogen-activated protein kinases |
| MMP | Matrix metalloproteinase |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-2 | Matrix metalloproteinase 2 |
| MMP-9 | Matrix metalloproteinase 9 |
| MUC5AC | Mucin 5AC |
| NAC | Acetylcysteine |
| NDUFS4 | NADH dehydrogenase (ubiquinone) Fe-S protein 4 |
| NF-κB | Nuclear factor kappa light-chain enhancer of activated B cells |
| OSCC | Oral squamous cell carcinoma |
| PARP | Poly ADP-ribose polymerase |
| PBMC | Peripheral blood mononuclear cell |
| PI3K | Phosphatidylinositol 3-kinase |
| PKB | Protein kinase B |
| PLK1 | Polo-like kinase 1 |
| ROS | Reactive oxygen species |
| SCN1B | Cell adhesion molecule 1 |
| TIMP2 | TIMP metallopeptidase inhibitor 2 |
| Twist1 | Twist basic helix-loop-helix transcription factor 1 |
| VEGFA | Vascular endothelial growth factor A |
| XIAP | X-linked inhibitor of apoptosis protein |
| ΔΨm | Mitochondria membrane potential |
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 16 January 2020).
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs. 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide in 2012. Available online: http://globocan.iarc.fr (accessed on 20 January 2020).
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014.
- International Agency for Research on Cancer; Global Initiative for Cancer Registry Development: Lyon, France, 2015.
- Primeau, A.S.B. Cancer Recurrence Statistics. Available online: https://www.cancertherapyadvisor.com/home/tools/fact-sheets/cancer-recurrence-statistics/. (accessed on 17 December 2019).
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Tarver, T.; American Cancer Society. Cancer facts and figures 2014. J. Consum. Health Internet 2012, 16, 366–367. [Google Scholar] [CrossRef]
- Weiderpass, E. Lifestyle and cancer risk. J. Prev. Med. Public Health 2010, 43, 459–471. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F.; Shanmugam, S.W.M.K. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef]
- Naz, I.; Ramchandani, S.; Khan, M.R.; Yang, M.H.; Ahn, K.S. Anticancer Potential of Raddeanin A, a Natural Triterpenoid Isolated from Anemone raddeana Regel. Molecules 2020, 25, 1035. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis 2011, 16, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma. Front. Pharmacol. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Kannaiyan, R.; Goh, J.N.; Wong, K.F.; Wang, W.; Khin, E.; Tergaonkar, V.; Kumar, A.P.; et al. Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev. Res. 2012, 5, 631–643. [Google Scholar] [CrossRef] [PubMed]
- De Padua, L.S.; Bunyapraphatsara, N.; Lemmens, R.H.M.J. Plant resources of SouthEast Asia No. 12: Medicinal and poisonous plants 1; Leiden Backhuys Publisher: Leiden, The Netherlands, 1999; pp. 497–502. [Google Scholar]
- Ganapaty, S.; Vidyadhar, K.N. Phytoconstituents and biological activities of Vitex—a review. J. Nat. Remed. 2005, 5, 75–95. [Google Scholar]
- The Plant List 2013. Vitex. Version 1.1. 20 November 2017. Available online: http://www.theplantlist.org/tpl1.1/search?q=vitex (accessed on 16 January 2020).
- Chan, E.W.C.; Baba, S.; Chan, H.T.; Kainuma, M.; Tangah, J. Medicinal plants of sandy shores: A short review on Vitex trifolia L. and Ipomoea pes-caprae (L.). R Br. Indianj. Nat. Prod. Resour. 2016, 7, 107–115. [Google Scholar]
- Rasul, A.; Zhao, B.J.; Liu, J.; Liu, B.; Sun, J.X.; Li, J.; Li, X.M. Molecular mechanisms of casticin action: An update on its antitumor functions. Asian Pac. J. Cancer Prev. 2014, 15, 9049–9058. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Ko, J.-H.; Jung, Y.Y.; Jung, S.H.; Kim, E.; Kong, M.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Casticin inhibits growth and enhances ionizing radiation–induced apoptosis through the suppression of STAT3 signaling cascade. J. Cell. Biochem. 2018, 120, 9787–9798. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Hsiao, Y.-T.; Huang, Y.-P.; Peng, S.-F.; Huang, W.-W.; Liu, K.-C.; Hsia, T.-C.; Way, T.-D.; Chung, J. Casticin Induces DNA Damage and Affects DNA Repair Associated Protein Expression in Human Lung Cancer A549 Cells (Running Title: Casticin Induces DNA Damage in Lung Cancer Cells). Molecules 2020, 25, 341. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Murad, S.A.; Khan, K.M.; Tareen, R.B.; Ahmed, A.; Atta-ur, R.; Choudhary, M.I. Isolation and immunomodulatory properties of a flavonoid, casticin from Vitex agnus-castus. Phytother. Res. 2009, 23, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Inokuchi, T.; Fujioka, S.; Kimura, Y. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Zeitschrift für Naturforschung C 2005, 59, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Cheng, W.X.; Feng, Y.; Gu, K.; Yang, L.J.; Zhang, Y.P. Studies on flavonoid constituents of Vitex trifolia L. var. simplicifolia Cham. Nat. Prod. Res. Dev. 2008, 20, 582–584. [Google Scholar]
- Kim, H.; Yi, J.M.; Kim, N.S.; Lee, Y.J.; Kim, J.; Oh, D.S.; Oh, S.M.; Bang, O.S.; Lee, J. Cytotoxic compounds from the fruits of Vitex rotundifolia against human cancer cell lines. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 433–437. [Google Scholar] [CrossRef]
- Kondo, Y.; Sugiyama, K.; Nozoe, S. Studies on the constituents of Vitex rotundifolia L. fil. Chem. Pharm. Bull. 1986, 34, 4829–4832. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Fang, Z.; Wu, Y.; Zhang, H.; Wu, Y.; Zhang, H. Chemical constituents of the fruits of Vitex trifolia L. Zhongguo Zhong Yao Za Zhi 1996, 21, 167–168. [Google Scholar] [PubMed]
- Wang, Y.; Hamburger, M.; Gueho, J.; Hostettmann, K. Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochem. 1989, 28, 2323–2327. [Google Scholar] [CrossRef]
- Xie, H.; Liang, Y.; Ito, Y.; Wang, X.; Chen, R.; He, J.; Li, H.; Zhang, T. Preparative isolation and purification of four flavonoids from Daphne genkwa Sieb. et Zucc. by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2360–2372. [Google Scholar] [CrossRef]
- Zani, C.; De Oliveira, A.B.; Murta, S.M.; Ceravolo, I.P.; Romanha, A.J.; Alves, T.M.A. Trypanocidal components ofPluchea quitoc L. Phytotherapy Res. 1994, 8, 375–377. [Google Scholar] [CrossRef]
- Winikoff, S.E.; Lotze, M.T. MTT Assay. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/mtt-assay (accessed on 15 January 2020).
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Methods Mol Biol. 2018, 1709, 107–127. [Google Scholar] [PubMed]
- Bosserman, L.; Rogers, K.; Willis, C.; Davidson, D.; Whitworth, P.; Karimi, M.; Upadhyaya, G.; Rutledge, J.; Hallquist, A.; Perree, M.; et al. Application of a Drug-Induced Apoptosis Assay to Identify Treatment Strategies in Recurrent or Metastatic Breast Cancer. PLoS ONE. 2015, 10, e0122609. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.O.; Ascoli, C.A. Direct Competitive Enzyme-Linked Immunosorbent Assay (ELISA). Cold Spring Harb. Protoc. 2017, 2017. [Google Scholar] [CrossRef]
- Khan-Malek, R.; Wang, Y. Statistical Analysis of Quantitative RT-PCR Results. Methods Mol. Biol. 2017, 1641, 281–296. [Google Scholar]
- Hnasko, T.S.; Hnasko, R.M. The western blot. Methods Mol. Biol. 2015, 1318, 87–96. [Google Scholar]
- Kim, B. Western Blot Techniques. Methods Mol Biol. 2017, 1606, 133–139. [Google Scholar]
- Jiang, L.; Cao, X.-C.; Cao, J.-G.; Liu, F.; Quan, M.-F.; Sheng, X.-F.; Ren, K. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol. Lett. 2013, 5, 1605–1610. [Google Scholar] [CrossRef]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anti-Cancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Hou, Y.; Zhu, Z.; He, J.; Zhao, H.; Ye, X.; Li, D.; Wu, Z.; Huang, Z.; et al. Casticin inhibits nasopharyngeal carcinoma growth by targeting phosphoinositide 3-kinase. Cancer Cell Int. 2019, 19, 348. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Rangappa, K.S.; Dharmarajan, A.; Sethi, G.; Kumar, A.P.; Warrier, S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharmacol. 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Anbalagan, D.; Lee, L.H.; Samy, R.P.; Shanmugam, M.K.; Kumar, A.P.; Sethi, G.; Lobie, P.E.; Lim, L.H. ANXA1 inhibits miRNA-196a in a negative feedback loop through NF-kB and c-Myc to reduce breast cancer proliferation. Oncotarget 2016, 7, 27007–27020. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Y.; Zhou, Q.; Liu, Y.; Gong, B.; Lü, J.; Zhu, H.; Zhu, G.; Xu, Y.; Huang, G. Casticin inhibits breast cancer cell migration and invasion by down-regulation of PI3K/Akt signaling pathway. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Um, J.Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Bladder Cancer - Statistics. Available online: https://www.cancer.net/cancer-types/bladder-cancer/statistics (accessed on 18 December 2019).
- Huang, A.-C.; Cheng, Y.-D.; Huang, L.-H.; Hsiao, Y.-T.; Peng, S.-F.; Lu, K.-W.; Lien, J.-C.; Yang, J.-L.; Lin, T.-S.; Chung, J. Casticin Induces DNA Damage and Impairs DNA Repair in Human Bladder Cancer TSGH-8301 Cells. Anticancer. Res. 2019, 39, 1839–1847. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Devi, N.; Taha, M.M.; Al-zubairi, A.S.; Mohan, S.; Mariod, A.A. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: Involvement of mitochondria-regulated apoptosis. Exp. Toxicol. Pathol. 2010, 62, 461–469. [Google Scholar] [CrossRef]
- Ningegowda, R.; Shivananju, N.S.; Rajendran, P.; Rangappa, K.S.; Chinnathambi, A.; Achar, R.R.; Bist, P.; Alharbi, S.A.; Lim, L.H.K.; Rangappa, K.S. A novel 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFα by abrogating NF-κB activation cascade. Apoptosis 2016, 22, 145–157. [Google Scholar]
- Chen, D.; Cao, J.; Tian, L.; Liu, F.; Sheng, X. Induction of apoptosis by casticin in cervical cancer cells through reactive oxygen species-mediated mitochondrial signaling pathways. Oncol Rep. 2011, 26, 1287–12894. [Google Scholar]
- Zeng, F.; Tian, L.; Liu, F.; Cao, J.; Quan, M.; Sheng, X. Induction of apoptosis by casticin in cervical cancer cells: Reactive oxygen species-dependent sustained activation of Jun N-terminal kinase. Acta Biochim Biophys Sin. 2012, 44, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and Treatment of Colorectal Cancer by Natural Agents From Mother Nature. Curr. Colorectal Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.S.; Liu, J.Y.; Lu, H.F.; Chiang, H.S.; Lin, C.H.; Chen, A.; Lin, Y.F.; Chung, J.G. Casticin induced apoptotic cell death and altered associated gene expression in human colon cancer colo 205 cells. Environ. Toxicol. 2017, 32, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Cheng, Y.; Liu, S.; Ma, Z.; Li, S.; Zhang, W. Casticin inhibits esophageal cancer cell proliferation and promotes apoptosis by regulating mitochondrial apoptotic and JNK signaling pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gallbladder Cancer Incidence and Death Rates. Available online: https://www.cdc.gov/cancer/dcpc/research/articles/gallbladder.htm (accessed on 16 January 2020).
- Song, X.L.; Zhang, Y.J.; Wang, X.F.; Zhang, W.J.; Wang, Z.; Zhang, F.; Zhang, Y.J.; Lu, J.H.; Mei, J.W.; Hu, Y.P.; et al. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 9. [Google Scholar] [CrossRef]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Swamy, S.G.; Kameshwar, V.H.; Shubha, P.B.; Looi, C.Y.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Sethi, G.; Shivananju, N.S.; Bishayee, A. Targeting multiple oncogenic pathways for the treatment of hepatocellular carcinoma. Target. Oncol. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anti-Cancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of β-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharm. Exp Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- He, G.; Cao, X.; He, M.; Sheng, X.; Wu, Y.; Ai, X. Casticin inhibits self-renewal of liver cancer stem cells from the MHCC97 cell line. Oncol. Lett. 2014, 7, 2023–2028. [Google Scholar] [CrossRef]
- He, L.; Yang, X.; Cao, X.; Liu, F.; Quan, M.; Cao, J. Casticin induces growth suppression and cell cycle arrest through activation of FOXO3a in hepatocellular carcinoma. Oncol. Rep. 2013, 29, 103–108. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Tian, L.; Sheng, X.F.; Liu, F.; Cao, J.G. Casticin-induced apoptosis involves death receptor 5 upregulation in hepatocellular carcinoma cells. World J. Gastroenterol. 2011, 17, 4298–4307. [Google Scholar] [CrossRef]
- He, M.; Cao, X.C.; He, G.C.; Sheng, X.F.; Ai, X.H.; Wu, Y.H. Casticin inhibits epithelial-mesenchymal transition of liver cancer stem cells of the SMMC-7721 cell line through downregulating Twist. Oncol. Lett. 2014, 7, 1625–1631. [Google Scholar] [CrossRef][Green Version]
- Shishodia, S.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007, 74, 118–130. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yuan, B.; Yuhara, E.; Takagi, N.; Toyoda, H. Involvement of histone H3 phosphorylation through p38 MAPK pathway activation in casticin-induced cytocidal effects against the human promyelocytic cell line HL-60. Int. J. Oncol. 2013, 43, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.K.; Du, H.P.; Yang, M.; Wang, Y.G.; Jin, J. Casticin induces leukemic cell death through apoptosis and mitotic catastrophe. Ann. Hematol. 2009, 88, 743–752. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Wang, L.; Chia, D.M.; Seah, J.Y.; Kong, L.R.; Thuya, W.L.; Chinnathambi, A.; Lau, J.Y.; Wong, A.L.; Yong, W.P.; et al. A novel combinatorial strategy using Seliciclib((R)) and Belinostat((R)) for eradication of non-small cell lung cancer via apoptosis induction and BID activation. Cancer Lett. 2016, 381, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Nam, D.; Um, J.Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Cao, X.; Cao, J.; Yang, X.; Zeng, W. Casticin suppresses the carcinogenesis of small cell lung cancer H446 cells through activation of AMPK/FoxO3a signaling. Oncol. Rep. 2018, 40, 1401–1410. [Google Scholar] [CrossRef]
- Liu, F.; Cao, X.; Liu, Z.; Guo, H.; Ren, K.; Quan, M.; Zhou, Y.; Xiang, H.; Cao, J. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from A549 cells through down-regulation of pAkt. Acta Biochim Biophys Sin. (Shanghai) 2014, 46, 15–21. [Google Scholar] [CrossRef]
- Liou, C.-J.; Huang, W.-C.H. Casticin inhibits interleukin-1β–induced ICAM-1 and MUC5AC expression by blocking NF-κB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget 2017, 8, 101175–101188. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Y.; Mao, Q.Q.; Li, X.; Chen, M.W.; Su, J.; Tian, L.; Mao, N.Q.; Long, L.Z.; Quan, M.F.; et al. Casticin induces caspase-mediated apoptosis via activation of mitochondrial pathway and upregulation of DR5 in human lung cancer cells. Asian Pac. J. Trop Med. 2013, 6, 372–378. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Morrison, C.C.; Blasi, P.R.; Nguyen, M.; Shibuya, K.C.; Patnode, C.D. Behavioral Counseling for Skin Cancer Prevention: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Chou, H.M.; Chou, H.C.; Lu, H.F.; Chu, Y.L.; Shang, H.S.; Chung, J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-κB signaling pathways. Environ. Toxicol. 2017, 32, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicine 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Shiue, Y.W.; Lu, C.C.; Hsiao, Y.P.; Liao, C.L.; Lin, J.P.; Lai, K.C.; Chung, J.G. Casticin induced apoptosis in a375.s2 human melanoma cells through the inhibition of nf-kb and mitochondria-dependent pathways in vitro and inhibited human melanoma xenografts in a mouse model in vivo. Am. J. Chin. Med. 2016, 44, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer. 2007, 120, 2545–2556. [Google Scholar] [CrossRef]
- Behera, A.K.; Kumar, M.; Shanmugam, M.K.; Bhattacharya, A.; Rao, V.J.; Bhat, A.; Vasudevan, M.; Gopinath, K.S.; Mohiyuddin, A.; Chatterjee, A.; et al. Functional interplay between YY1 and CARM1 promotes oral carcinogenesis. Oncotarget 2019, 10, 3709–3724. [Google Scholar] [CrossRef]
- Xie, Y.; Zhong, L.; Duan, D.; Li, T. Casticin inhibits invasion and proliferation via downregulation of β-catenin and reversion of EMT in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 897–905. [Google Scholar] [CrossRef]
- Chou, G.L.; Peng, S.F.; Liao, C.L.; Ho, H.C.; Lu, K.W.; Lien, J.C.; Fan, M.J.; La, K.C.; Chung, J.G. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ. Toxicol. 2018, 33, 127–141. [Google Scholar] [CrossRef]
- Ovarian Cancer Statistics: How Common is Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 15 January 2020).
- Zhang, J.; Cui, Y.; Sun, S.; Cao, J.; Fang, X. Casticin inhibits the epithelial-mesenchymal transition in ovarian carcinoma via the hedgehog signaling pathway. Oncol Lett. 2018, 15, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lo, U.G.; Hsieh, J.T. The regulatory pathways leading to stem-like cells underlie prostate cancer progression. Asianj. Androl. 2019, 21, 233–240. [Google Scholar]
- Zainfeld, D.; Goldkorn, A. Liquid Biopsy in Prostate Cancer: Circulating Tumor Cells and Beyond. Cancer Treat. Res. 2018, 175, 87–104. [Google Scholar] [PubMed]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.-H.; Lee, S.G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2016, 8, 17700–17711. [Google Scholar] [CrossRef]
- Meng, F.; Yang, J.; Yang, C.; Jiang, Y.; Zhou, Y.; Yu, B.; Yang, H. Vitexicarpin Induces Apoptosis in Human Prostate Carcinoma PC-3 Cells through G2/M Phase Arrest. Asian Pac. J. Cancer Prev. 2012, 13, 6369–6374. [Google Scholar] [CrossRef]
- Lai, K.-C.; Lu, H.-F.; Chen, K.-B.; Hsueh, S.-C.; Chung, J.; Huang, W.-W.; Chen, C.-C.; Shang, H.-S. Casticin Promotes Immune Responses, Enhances Macrophage and NK Cell Activities, and Increases Survival Rates of Leukemia BALB/c Mice. Am. J. Chin. Med. 2019, 47, 223–236. [Google Scholar] [CrossRef]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of tumor-derived exosomes in cancer metastasis. Biochim Biophys. Acta Rev. Cancer. 2019, 1871, 12–19. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Chang, Y.-C.; Kumar, D.; et al. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef]
- Baek, S.-H.; Ko, J.-H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2016, 232, 346–354. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharm. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.; Sethi, G.; Xin, H.-W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, J.H.; Ko, J.-H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Mol. 2019, 24, 1584. [Google Scholar] [CrossRef]
- Chen, X.; Tang, F.R.; Arfuso, F.; Cai, W.; Ma, Z.; Yang, J.; Sethi, G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomol. 2019, 10, 66. [Google Scholar] [CrossRef]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox Signal 2016, 24, 575–589. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Scapozza, L.; I Altaba, A.R. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta Bioenerg. 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ong, T.H.; Subramaniam, A.; Siveen, K.S.; Perumal, E.; Samy, R.P.; Bist, P.; Lim, L.H.K.; Kumar, A.P.; et al. Emodin Suppresses Migration and Invasion through the Modulation of CXCR4 Expression in an Orthotopic Model of Human Hepatocellular Carcinoma. PLoS ONE 2013, 8, e57015. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li., F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef]
- Chua, A.W.; Hay, H.S.; Rajendran, P.; Shanmugam, M.K.; Li, F.; Bist, P.; Koay, E.S.; Lim, L.H.; Kumar, A.P.; Sethi, G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-κB activation in breast and pancreatic tumor cells. Biochem Pharmacol. 2010, 80, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Lien, J.C.; Huang, Y.P.; Liao, C.L.; Lin, J.J.; Fan, M.J.; Ko, Y.C.; Hsiao, Y.P.; Lu, H.F.; Chung, J.G. Casticin Inhibits A375.S2 Human Melanoma Cell Migration/Invasion through Downregulating NF-κB and Matrix Metalloproteinase-2 and -1. Molecules 2016, 21, 384. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, K.B.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Peng, S.F.; Chung, J.G. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-κB signaling pathways. J. Food Biochem. 2019, 43, e12902. [Google Scholar] [CrossRef]
- Webster, D.E.; He, Y.; Chen, S.N.; Pauli, G.F.; Farnsworth, N.R.; Wang, Z.J. Opioidergic mechanisms underlying the actions of Vitex agnus-castus L. Biochem. Pharmacol. 2011, 81, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Cheng, C.Y.; Yeh, K.W.; Wu, Y.H.; Huang, W.C. Protective Effects of Casticin From Vitex trifolia Alleviate Eosinophilic Airway Inflammation and Oxidative Stress in a Murine Asthma Model. Front. Pharmacol. 2018, 9, 635. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.; Zhao, L.; Wang, Y.; Xue, L.; Han, T.; Zheng, C.; Qin, L. Quantitative determination and pharmacokinetic study of casticin in rat plasma by liquid chromatography-mass spectrometry. J. Pharm Biomed. Anal. 2012, 61, 242–246. [Google Scholar] [CrossRef]
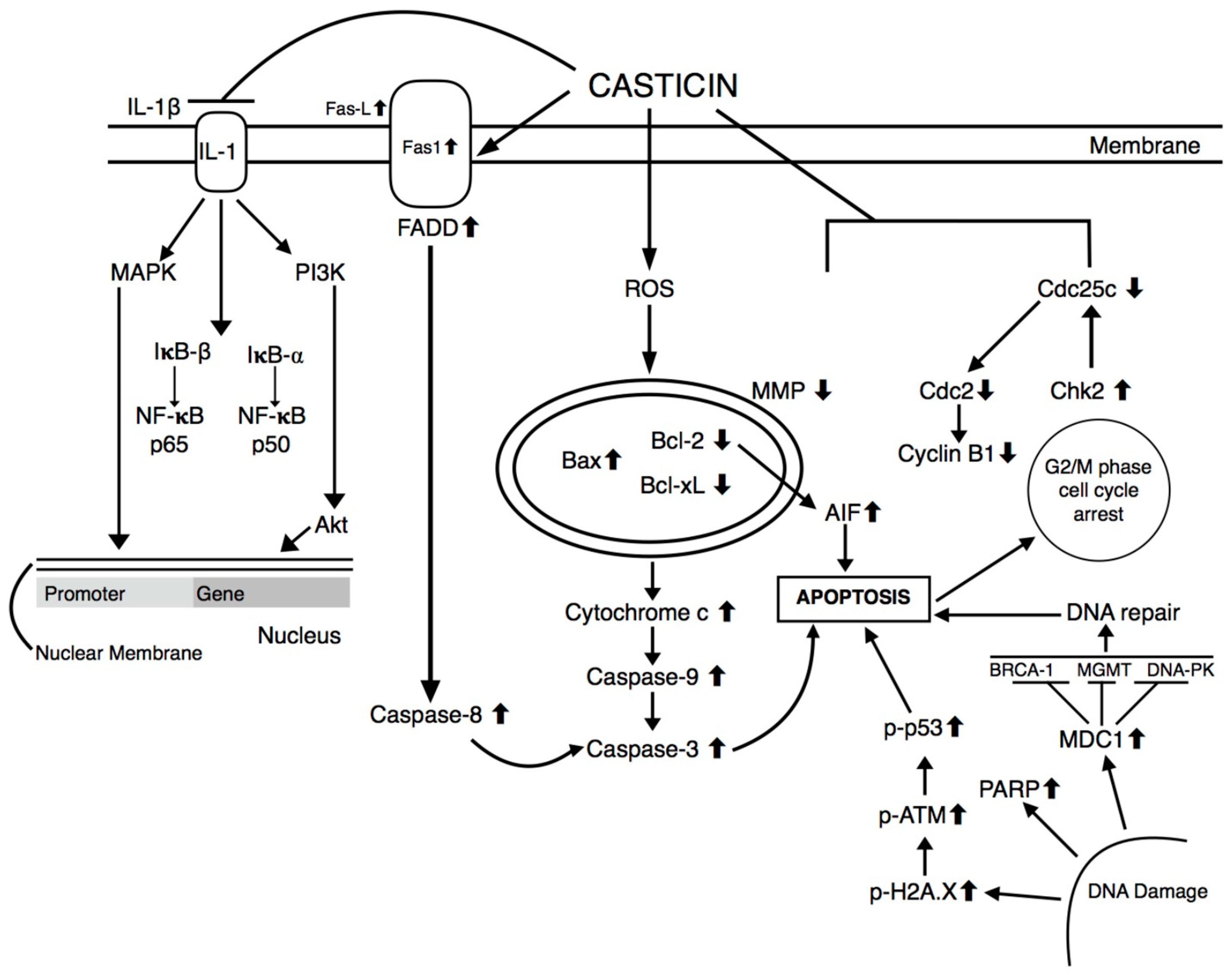
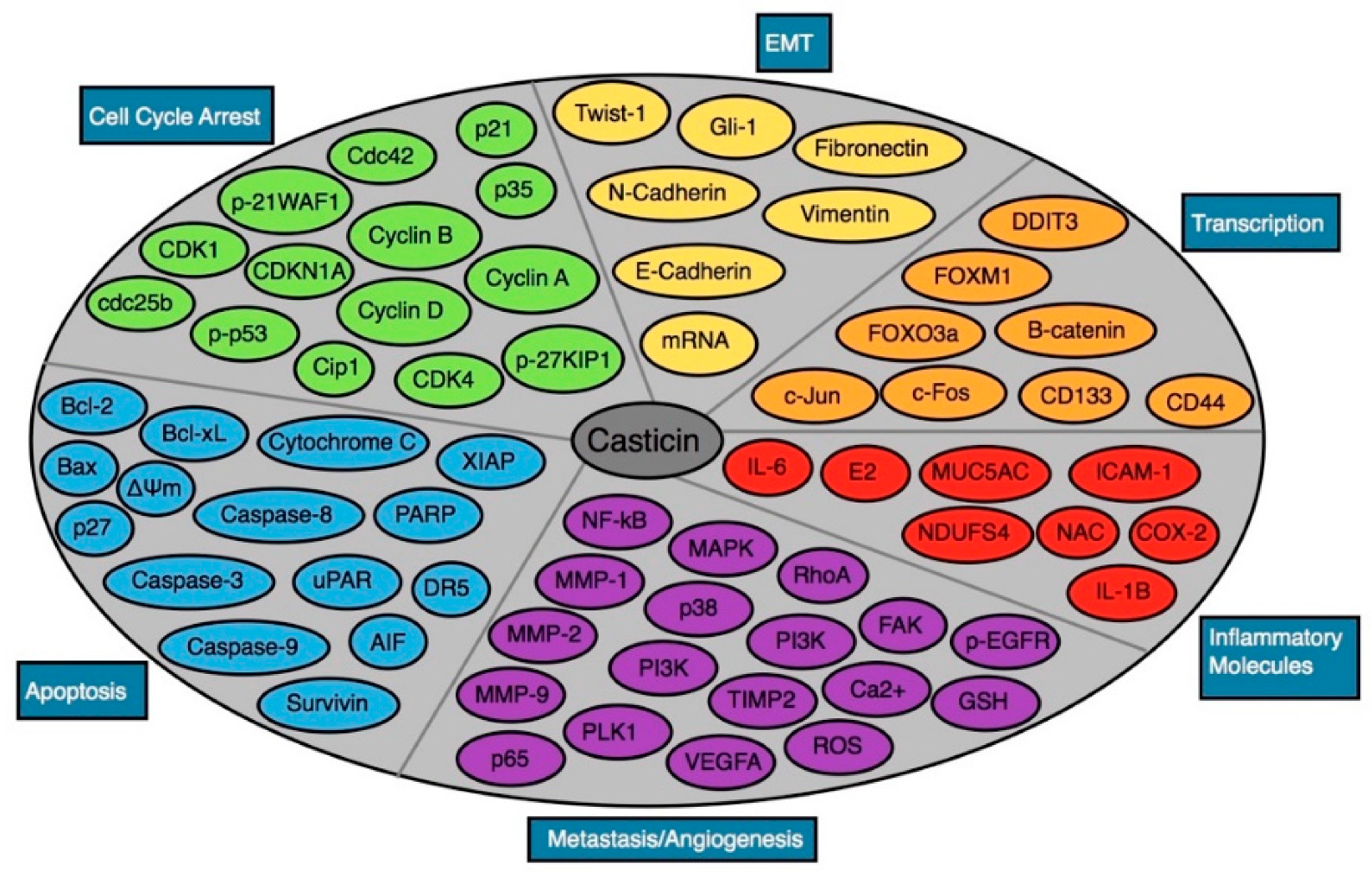

| Cancer | Cell Line | IC50 Values | Phenotypic Effects | Mechanisms of Action | Ref |
|---|---|---|---|---|---|
| Breast Cancer | MDA-MB-231, 4T1 cells | Inhibited migratory activity and invasion | ↓ MMP-9, ↓ c-Jun, ↓ c-Fos, ↓ Skt, ↑ MMP-2 | [51] | |
| Bladder Cancer | TSGH-8301 | Induces DNA damage and impairs DNA repair | ↓ p-ATM, ↓ p-ATR, ↓ MDC1, ↓ MGMT, ↑ p-p53, ↑ p-H2A.X, ↑ PARP | [56] | |
| Cervical Cancer | HeLa, CasKi, SiHa, PBMC’s | 1.268 µM | Accumulation of sub-G1 cells and induced mitochondrial apoptosis | ↑ Cytochrome c, ↓ MMP, ↑ caspase-3, ↑ caspase-9, ↑ ROS, ↑ Bax, ↓ Bcl-xL, ↓ XIAP | [59] |
| HeLa, CasKi, SiHa | Induced apoptosis | ↑ ROS, ↑ JNK, ↑ c-Jun | [60] | ||
| Colon Cancer | Colo 205 cells | Induced apoptosis and altered associated gene expression | ↑ ROS, ↑ caspase-3, ↑ caspase-8, ↑ caspase-9, ↓ ΔΨm, ↓ Ca2+, ↓ CDKN1A, ↓ p21, ↓ Cip1, Cdc42 | [62] | |
| Esophageal cancer | TE-1, ECA-109 | Sub-G1/G2 cell cycle arrest, Induced EC apoptosis and inhibited proliferation and clonogenogenicity | ↓ Bcl-2, ↑ Bax, ↑ caspase-3, ↑ caspase-9, ↑ PARP | [63] | |
| Gallbladder Cancer | SGC996 | 2 µM | Induced mitochondrial-related apoptosis and G0/G1 cell cycle arrest | ↑ Bax, ↓ Bcl-2, ↑ p27, ↓ cyclinD/CDK4, ↓ ΔΨm | [65] |
| Hepatocellular Carcinoma | LCSC’s HCC MHCC97 | Inhibited proliferation | ↓ B-catenin | [69] | |
| HepG2, PLC/PRF/5 | 17.9 µM for parental cells and 0.5 µM for LCSCs | Inhibited cell viability and colony formation and induced apoptosis G2/M cell cycle arrest | ↓ FOXO3a, ↓ FoxM1, ↓ CDK1, ↓ cdc25B, ↓ cyclin B, ↑ p27/KIP1 | [76] | |
| HepG2, PLC/PRF/5 | Induced apoptosis and increased sub-G1 population | ↑ Histone/DNA, ↑ caspase-3, ↑ caspase-8, ↑ caspase-9, ↓ GSH, ↓ DR5, ↑ NAC | [77] | ||
| CD133+ | Overexpression of Twist and cadherin switching | ↑ E-cadherin, ↓ N-cadherin, ↓ EMT associated Twist-1 | [78] | ||
| Leukemia | HL-60 | 0.29 µM for 24 h and 1.15 µM for 48 h | Induced apoptosis and G2/M cell cycle arrest | ↑ caspase-3, ↑ caspase-8, ↑ caspase-9, ↑ H3 phosphorylation, ↑ intracellular ATP, ↑ ROS, ↓ MAPK | [79] |
| HL-60, Kasumi-2, K562 cells | Induced apoptosis and mitotic catastrophe via PI3K/Akt pathway | ↑ P21waf1, ↑ P27kip1, ↑ AV-positive PI-negative cells, ↑ PARP, ↑ caspase-3 | [82] | ||
| Lung cancer | SCLC H446 | Induced apoptosis | ↓ uPAR, ↓ CD33, ↑ AMPK, ↑ ACC | [83] | |
| A549 | Suppressed self-renewal and invasion of lung cancer stem-like cells (LCSLCs) | ↓ CD133, ↓ CD44, ↓ ALDH1, ↓ MMP-9 | [88] | ||
| A549 | 14.3 µM for A549 cells and 0.4 µM for parental cells | Blocked proinflammatory cytokine and reduced inflammation | ↓ IL-6, ↓ COX-2, ↓ E2, ↓ MU5AC, ↓ ICAM-1, ↓ proinflammatory cytokine, ↓ chemokine gene expression, ↓ MAPK, ↓ NF-κB p65 | [89] | |
| H460, A548, H157 | Induced caspase-mediated apoptosis | ↑ MMP, ↑ Cytochrome c, ↑ caspase-9, ↑ caspase-3, ↑ Bax, ↓ XIAP, ↓ Bcl-XL | [90] | ||
| Melanoma | B16F10 | Inhibits gene expression of cell migration and invasion | ↓ MMP-9, ↓ MMP-2, ↓ MMP-1, ↓ FAK, ↓ 14–3-3, ↓ GRB2, ↓ Akt, ↓ NF-κB p65, ↓ SOS-1, ↓ p-EGFR, ↓ p-JNK 1.2, ↓ Rho A, ↑ SCN1B, ↑ TIMP2, ↓ NDUFS4, ↓ VEGFA, ↓ DDIT3 | [91] | |
| A365.S2 | Induced apoptosis and morphological changes, condensed and damaged DNA, decreased cell viability and induced G2/M cell cycle arrest | ↑ ROS, ↑ caspase-3, ↓ ΔΨm, ↑ p35, ↑ p21, ↑ CHK-1, ↓ Cdc25c, CDK1, ↓ Cyclin A, ↓ Cyclin B, ↓ NF-κB p65 | [94] | ||
| Oral cancer | UM1 and HSC-3 | Inhibited cell viability, invasion and migration | ↓ B-catenin | [95] | |
| SCC-4 | Induced apoptosis and G2/M cell cycle arrest, decreased cell viability and condensed and damaged DNA | ↑ ROS and Ca2+, ↓ ΔΨm, ↑ caspase-3, ↑ caspase-8, ↑ caspase-9, ↑ AIF, ↑ Cytochrome C | [100] | ||
| Ovarian cancer | SKOV3, A2780 | Induced apoptosis through the loss of FoxM1 | ↓ PLK1, ↓ survivin, ↑ p27 | [47] | |
| SKOV3 | Reduced SKOV3 cell viability, migration and invasion | ↓ Gli-1, ↓ Twist-1, ↓ N-cadherin, ↑ E-cadherin | [102] | ||
| Prostate Cancer | PC-3 | 28.7 µM | Induced apoptosis and G2/M cell cycle arrest | ↑ Bax, ↓ Bcl-2, ↑ Cytochrome c, ↓ ΔΨm, ↑ ROS | [106] |
| Cancer | Cell Line | Phenotypic Effects | Mechanisms of Action | Ref |
|---|---|---|---|---|
| Esophageal cancer | Mouse xenograft model | Inhibited proliferation and induced apoptosis | ↓ Bcl-2, ↑ Bax, ↑ caspase-3, ↑ caspase-9, ↑ PARP | [63] |
| Hepatocellularcarcinoma | Mouse CD133+ | Overexpression of Twist and cadherin switching | ↑ E-cadherin, ↓ N-cadherin, ↓ EMT associated Twist-1 | [79] |
| Leukemia | Mouse WEHI-3 | Increased macrophage phagocytosis from peripheral blood mononuclear cells (PBMCs) | ↑ NK cells cytotoxic activity, ↑ T-cells | [107] |
| Melanoma | Mouse A375.S2 xenograft model | Early-G2/M cell cycle arrest and induced mitochondrial apoptosis | ↓ Tumor volume | [95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramchandani, S.; Naz, I.; Lee, J.H.; Khan, M.R.; Ahn, K.S. An Overview of the Potential Antineoplastic Effects of Casticin. Molecules 2020, 25, 1287. https://doi.org/10.3390/molecules25061287
Ramchandani S, Naz I, Lee JH, Khan MR, Ahn KS. An Overview of the Potential Antineoplastic Effects of Casticin. Molecules. 2020; 25(6):1287. https://doi.org/10.3390/molecules25061287
Chicago/Turabian StyleRamchandani, Shanaya, Irum Naz, Jong Hyun Lee, Muhammad Rashid Khan, and Kwang Seok Ahn. 2020. "An Overview of the Potential Antineoplastic Effects of Casticin" Molecules 25, no. 6: 1287. https://doi.org/10.3390/molecules25061287
APA StyleRamchandani, S., Naz, I., Lee, J. H., Khan, M. R., & Ahn, K. S. (2020). An Overview of the Potential Antineoplastic Effects of Casticin. Molecules, 25(6), 1287. https://doi.org/10.3390/molecules25061287






