Abstract
As important cancer therapeutic agents, macrocyclic peptides have recently drawn great attention, mainly because they are synthetically accessible and have lower toxicity towards normal cells. In the present work, we synthesized newly macrocyclic pyridoheptapeptide derivatives. The synthesized derivatives were characterized using standard chemical and spectroscopic analytical techniques, and their anticancer activities against human breast and hepatocellular cancer cells were investigated. Results showed that compounds 1a and 1b were the most effective against hepatocellular (HepG2) and breast (MCF-7) cancer cell lines, respectively.
1. Introduction
Cancer is the second most common causative disease threatening human life. Recently, researchers have focused their works on developing successful therapeutic drugs capable of treating different cancer cells. Most research has been focused on developing early-stage cancer-treating drugs, which have received better attention in comparison to drugs used to treat late cancer phases [1]. Besides naturally obtained preparations; i.e., plant-derived extracts and microbially produced antibiotics, chemical synthesis is still used as a traditional method for obtaining potential anticancer drugs.
Among chemically synthesized pharmaceutical drugs, macrocyclic compounds with a ring of 12 or more atoms [2] are generally favored for synthesizing potential anticancer derivatives, mainly in the chemical, biological, and medical sectors [3,4,5]. Macrocyclic derivatives include peptide- and non-peptide-derived compounds, synthesized peptides, and macrocyclic derivatives [6].
Additionally, peptides comprise a major group of pharmaceutical drugs with potential anticancer effects [7]. Previous reports showed that chemical synthesis of peptides proved successful in obtaining new derivatives with potent antimicrobial, anti-inflammatory [8,9,10,11,12,13,14,15,16], and anticancer properties [17,18,19,20,21]. In our previous works, we were able to synthesize, chemically characterize, and biologically evaluate different bis-amino acid and peptide conjugates of dipicolinic acid [22]. Our previous work with compound (A) (Figure 1) showed potential anti-proliferative effects, mainly due to DNA intercalation, and metal sensor properties, particularly for pollutant lead (Pb2+) cations [23].
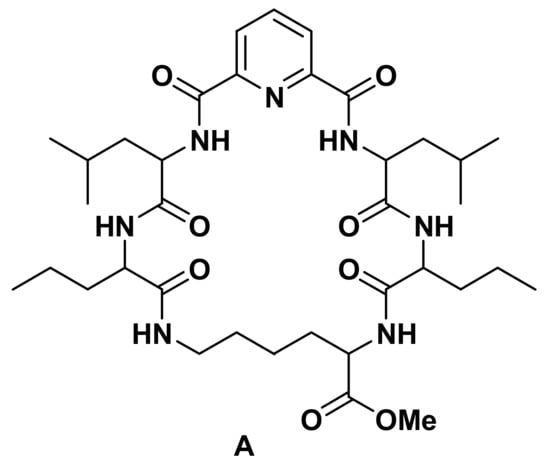
Figure 1.
Structure of the anticancer agent.
Moreover, human cells react towards toxicants; i.e., injury and infection, by developing a natural inflammatory response, which finally results in damage to the concerned tissues [24]. Inflammatory reactions include the initiation of various biological pathways such as production and secretion of pro-inflammatory mediators [25]. There has been a well-established connection between inflammatory response and cancer development. Tumor growth and development is dramatically increased by the presence of inflammatory cells; inflammatory mediators are considered a tumor microenvironment [26,27]. Recently, cancer treatments based on the application of synthesized peptides as anti-inflammatory agents have been used [28].
Based on previous investigations and our continuous work in the field of peptide synthesis [17,18,19,20,21,22,23], we have prepared different new macrocyclic heptapeptidopyridine candidates, and evaluated their anticancer potential in relation to standard used anticancer drugs.
2. Results and Discussion
2.1. Chemistry
In the present work we report the synthesis of newly macrocyclic pyridoheptapeptide derivatives 2–6 using Nα-dipicolinoyl-bis[dipeptide-carboxylic acid] (1a–c) [29] as starting material, and they were screened as anticancer agents. Treatment of 1a-c with L-amino acid methyl ester in the presence of ethyl chloroformate in dichloromethane afforded the corresponding Nα-dipicolinoyl-bis[tripeptide methyl ester] derivatives 2a–c, respectively. The latter bis-methyl ester derivatives 2a–c were hydrolyzed with sodium hydroxide in methanol to afford the corresponding Nα-dipicolinoyl-bis[tripeptide carboxylic acid] derivatives 3a–c, which were cyclized with L-lysine methyl ester by different methods to afford the corresponding cyclo-(Nα- dipicolinoyl)-bis-[(tripeptide)-L-Lys- methyl ester] derivatives 4a–c, respectively. The heptapeptide esters 3a–c were hydrolyzed or underwent hydrazonolysis with sodium hydroxide or hydrazine hydrate in methanol to give the cyclo-(Nα-dipicolinoyl)-bis-[(tripeptide)-L-Lys-carboxylic acid] derivatives 5a–c and cyclo-(Nα-dipicolinoyl)-bis-[(tripeptide)-L-Lys-acid hydrazide] derivatives 6a–c, respectively (Scheme 1).
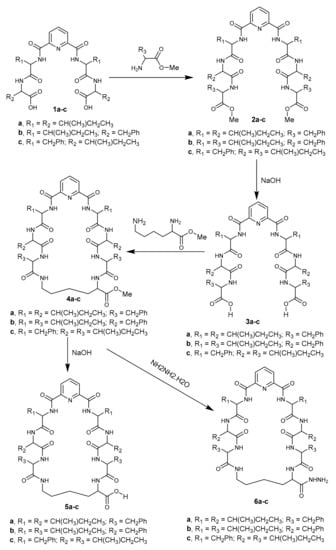
Scheme 1.
Synthetic pathway for compounds 2–6.
2.2. Anticancer Activity
The newly synthesized derivatives were evaluated for their anticancer potential towards breast (MCF-7) and hepatocellular (HepG2) cancer cell lines cell lines. Compounds 6a–c did not show any cytotoxic activity against both tested cell lines. Generally, all other derivatives, with the exception of compound 5c, showed concentration-dependent effects on both cell lines (Figure 2). Moreover, potential positive anticancer compounds showed varying effects ranging from potent to moderate effects. Increasing the applied concentration gradually increased the cytotoxic effects and correspondingly decreased cell viability. On the other hand, compound 5c did not show any activity against MCF-7 cells, and was only active against HepG2 cells. Additionally, compounds 2a and 5a–c were considered practically inactive against MCF-7 cells, since they exhibited higher compound concentrations required to inhibit cell viability by 50% (IC50; > 100 μM) at the investigated concentration ranges. Furthermore, all compounds which exhibited anticancer activities were found to be more effective (lower IC50 values, Table 1) on HepG2 cells than MCF-7 cells. This can be attributed to the fact that different biological systems react differently toward same affecting compounds due to inherent morphological and membrane-structural differences among different cell lines [30,31,32]. For HepG2 cells, compounds 1a,b, 3a,b, 4a–c, and 5a–c were more potent than compounds 1c, 2a–c, and 3c, which were the least effective against HepG2 cells. According to the IC50 values, the order of activity of the most potent compounds can be arranged as 1a > 5b > 5a > 4b > 4a > 3a > 1b > 5c > 4c > 3b (IC50: 6.62 ± 0.35, 7.09 ± 0.78, 7.12 ± 0.79, 7.17 ± 0.89, 7.31 ± 0.59, 8.06 ± 0.86, 8.73 ± 0.47, 9.57 ± 0.1.14, 9.63 ± 0.93, and 9.66 ± 0.79 µM, respectively). On the other hand, the least effective compounds showed a decreasing order of 3c > 1c > 2a > 2b > 2c (IC50: 16.96 ± 1.16, 17.01 ± 0.97, 17.51 ± 0.87, 18.68 ± 0.99, and 19.32 ± 1.06 µM, respectively). On the other hand, for MCF-7 cells, compounds 1b, 3a,b, and 4a,b were the most potent derivatives, where their decreasing order can be arranged as 4b > 4a > 3b > 3a > 1b (IC50: 9.19 ± 0.55, 9.30 ± 0.54, 9.91 ± 0.49, 10.32 ± 0.55, and 10.90 ± 0.53 µM, respectively). Furthermore, compound 1b showed moderate activity with an IC50 value of 15.33 ± 0.67 µM. Moreover, the order of the least effective compounds can be arranged as 4c > 2b > 3c > 2c > 1c (IC50: 19.55 ± 1.03, 20.70 ± 0.97, 22.86 ± 0.99, 24.94 ± 1.08, and 34.89 ± 1.27 µM, respectively). On the other hand, compounds 2a and 5a–c were ineffective against MCF-7 cells in terms of IC50 values. The IC50 values obtained for positive controls (tamoxifen and cisplatin) were 29.34 ± 1.15 and 10.93 ± 0.96 µM for HepG2 cells, respectively, and 22.37 ± 2.41 and 8.89 ± 0.37 µM, for MCF-7 cells, respectively.
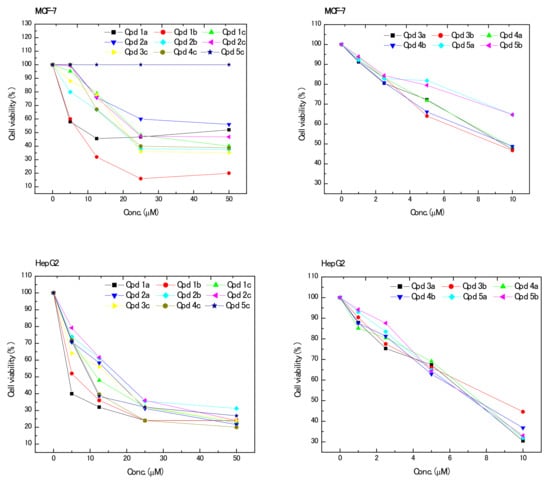
Figure 2.
Effect of different concentrations of the prepared compounds on the viability of hepatocellular (HepG-2) and breast (MCF-7) cancer cell lines.

Table 1.
IC50 of the tested compounds 1–5 against the MCF-7 and HepG-2 cell lines.
From the aforementioned results, it can be concluded that most of the prepared compounds with potent promising anticancer activities against HepG2 cells showed better activities than the positive control tamoxifen, whereas the most potent compounds against MCF-7 cells showed cytotoxic effects comparable to those of tamoxifen. Finally, it can be concluded that compounds 1a and 1b were the most potent synthesized derivatives against HepG2 and MCF-7 cells, respectively.
The structure activity relationship (SAR) was outlined in order to explain the activity of the prepared derivatives. The order of activity against cancer cell lines can be explained due to presence of free carboxylic groups, which increase the acidity, and thus increase the potential anticancer activity. Moreover, the difference in cytotoxic effects can be correlated with the substituted amino acid residues.
3. Materials and Methods
3.1. Chemistry
Melting points were determined in open glass capillary tubes with an “Electro Thermal” Digital melting point apparatus, (model: IA9100) and are uncorrected. Elemental micro-analyses results for carbon, hydrogen, and nitrogen (Microanalytical Unit, NRC) were found within the acceptable limits of the calculated values. Infrared spectra (KBr) were recorded on a Nexus 670 FTIR Nicolet, Fourier Transform infrared spectrometer. Proton nuclear magnetic resonance (1H NMR) spectra were run in [D6] DMSO on Jeol 270 MHz or 500 MHz instruments. Chemical shifts d are given in ppm. Mass spectra were run on a MAT Finnigan SSQ 7000 spectrometer, using the electron impact technique (EI). Analytical thin layer chromatography (TLC) was performed on silica gel aluminum sheets, 60 F254 (E. Merck). Specific optical rotations were measured with a A. Krawss, Optronic, P8000a polarimeter, in a 1-dm length observation tube at the indicated conditions, and according to the equation: [a]T D = 100. a = (c l), where: a = observed rotation angle, D = sodium line (l = 589 nm), c = concentration (g = 100 mL), l = path length in dm, and T = temperature (°C). The following solvent systems (by volume) were used as eluents for the development of the plates: S: chloroform-methanol-acetic acid (85: 10: 5); S1: S-petroleum ether (40–60 °C) (1:1); S2: S-petroleum ether (40–60 °C) (3: 2); S3: S-petroleum ether (40–60 °C) (1:2), and S4: butanol-water-acetic acid-pyridine (120:48:12:40). It is generally known that basic reaction media enhance racemization. However, under the reaction conditions employed in this work, especially with short reaction times and temperatures below 0 °C, only negligible racemization was observed.
3.1.1. Synthesis of Nα-dipicolinoyl-bis[tripeptide methyl ester] derivatives (2a–c)
To a cold (−15 °C) suspension of Nα-dipicolinoyl-bis[dipeptide-carboxylic acid] (1a–c) [29] (1 mmol) and N-methylmorpholine (0.2 mL, 2 mmol) in dichloromethane (25 mL), ethyl chloroformate (0.2 mL, 2 mmol) was added with stirring at the same temperature (−15 °C). The reaction mixture was stirred for 20 min. Then, a cold dichloromethane solution (20 mL) of the free amino acid methyl ester of L-Phe and/or L-ILeu (2 mmol) was added, with stirring for 3 h at −15 °C and then for 12 h at room temperature. The reaction mixture was washed with water, 1 N sodium bicarbonate, and 1 N hydrochloric acid and water, and dried over anhydrous calcium chloride. The solvent was evaporated under reduced pressure; the obtained residue was triturated with dry ether/n-hexane mixture. The obtained solid was filtered off and crystallized from ethanol/n-hexane to give the corresponding bis-esters (2a–c), respectively.
Nα-Dipicolinoyl-bis[L-ILe-L-ILe-L-Phe-methyl ester] (2a). Yield: 66%; m.p. 96–98 °C, [α]: -103 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3399 (NH str.), 2964 (C-H, arom.), 2872 (C-H, aliph.), 1660 (C=O, ester), 1528, 1449, 1379 (C=O amide I, II and III, respectively). 8.56–8.50 (m, 3H, Pyr-H), 8.60, 8.40, 8.36 (3s, 6H, 6NH, D2O exchangeable), 7.25–7.14 (m, 10H, Ar-H, L-Phe-ala), 4.68 (t, 2H, NHCH, L-Phe-ala), 4.45-4.33 (m, 4H, 4NHCH, L-Ile), 4.12 (d, 4H, 2CH2, L-Phe-ala), 3.60 (s, 6H, 2OCH3), 3.22-3.18 (m, 4H, 4NHCHCH, L-Ile), 1.20–1.12 (m, 8H, 4CH2, L-Ile), 0.95–0.86 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 172.65 (2C, CO, ester), 172.00, 171.78 (4C, 4CO, L-Phe-ala, L-Ile), 163.74 (2C, 2CO, dicarbonyl pyridine), 147.80, 144.55, 125.80 (5C, pyr-C), 137.82, 127.90, 127.56, 125.62 (12C, Ph-C), 61.80, 58.32, 57.34 (6C, 6CHNH), 52.45 (2C, 2OCH3), 46.00 (2C, 2CH2, L-Phe-ala), 34.42, 34.10 (4C, 4CH, L-Ile), 34.12, 33.90 (4C, 4CH2), 14.20, 11.30 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 943 (M++1, 16), 642 (M+, 26), 704 (64), 636 (93), 605 (85), 603 (100), 577 (80), 461 (35), 302 (45), 86 (32), 57 (39), 50 (17). Analysis for C51H71N7O10 (942.15): Calcd. C, 65.02; H, 7.60; N, 10.41. Found: C, 64.96, H, 7.54, N, 10.36.
Nα-Dipicolinoyl-bis[L-ILe-L-L-Phe-ILe-methyl ester] (2b). Yield: 73%; m.p. 150–152 °C, [α]: -50 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3440 (NH, str.), 3099 (C-H, arom.), 2020 (C-H, aliph.), 1710 (C=O, ester), 1600, 1580, 1496 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 8.65–8.58 (m, 3H, Pyr-H), 8.46, 8.35, 8.32 (3s, 6H, 6NH, D2O exchangeable), 7.35–7.20 (m, 10H, Ar-H, L-Phe-ala), 4.72 (t, 2H, NHCH, L-Phe-ala), 4.50–4.34 (m, 4H, 4NHCH, L-Ile), 4.26 (d, 4H, 2CH2, L-Phe-ala), 3.62 (s, 6H, 2OCH3), 3.25–3.16 (m, 4H, 4NHCHCH, L-Ile), 1.25–1.15 (m, 8H, 4CH2, L-Ile), 0.94–0.82 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.65 (2C, CO, ester), 171.00, 169.98 (4C, 4CO, L-Phe-ala, L-Ile), 162.90 (2C, 2CO, dicarbonyl pyridine), 147.72, 144.68, 125.72 (5C, pyr-C), 137.80, 127.72, 127.50, 125.60 (12C, Ph-C), 62.00, 58.40, 57.56 (6C, 6CHNH), 52.52 (2C, 2OCH3), 45.68 (2C, 2CH2, L-Phe-ala), 34.40, 34.28 (4C, 4CH, L-Ile), 34.10, 33.92 (4C, 4CH2), 13.82, 11.30 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 942 (M+, 11), 777 (80), 593 (34), 428 (58), 340 (33), 293 (32), 207 (70), 125 (100), 90 (12), 70 (33), 57 (45), 50 (85). Analysis for C51H71N7O10 (942.15): Calcd. C, 65.02; H, 7.60; N, 10.41. Found: C, 64.94, H, 7.56, N, 10.38.
Nα-Dipicolinoyl-bis[L-Phe-L-ILe-L-ILe-methyl ester] (2c). Yield: 70%; m.p. 94–96 °C, [α]: -70 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3320 (NH str.), 3066 (C-H, arom.), 2966 (C-H, aliph.), 1740 (C=O, ester), 1656, 1530, 1447 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 8.70–8.64 (m, 3H, Pyr-H), 8.50, 8.40, 8.30 (3s, 6H, 6NH, D2O exchangeable), 7.44–7.13 (m, 10H, Ar-H, L-Phe-ala), 4.80 (t, 2H, NHCH, L-Phe-ala), 4.52–4.35 (m, 4H, 4NHCH, L-Ile), 4.15 (d, 4H, J = 8.1 Hz, 2CH2, L-Phe-ala), 3.64 (s, 6H, 2OCH3), 3.28-3.20 (m, 4H, 4NHCHCH, L-Ile), 1.23–1.12 (m, 8H, 4CH2, L-Ile), 0.83–0.79 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 172.80 (2C, CO, ester), 171.05, 169.95 (4C, 4CO, L-Phe-ala, L-Ile), 161.75 (2C, 2CO, dicarbonyl pyridine), 147.70, 144.82, 125.70 (5C, pyr-C), 137.75, 127.84, 127.60, 125.52 (12C, Ph-C), 61.85, 58.48, 57.55 (6C, 6CHNH), 52.40 (2C, 2OCH3), 45.60 (2C, 2CH2, L-Phe-ala), 34.53, 34.32 (4C, 4CH, L-Ile), 33.90, 33.80 (4C, 4CH2), 13.74, 11.46 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 943 (M++1, 32), 942 (M+, 38), 829 (33), 769 (29), 685 (66), 684 (100), 656 (53), 624 (34), 511 (34), 409 (11), 370 (89), 332 (35), 268 (15), 57 (29), 51 (72). Analysis for C51H71N7O10 (942.15): Calcd. C, 65.02; H, 7.60; N, 10.41. Found: C, 64.92, H, 7.52, N, 10.34.
3.1.2. Synthesis of Nα-dipicolinoyl-bis[tripeptide carboxylic acid]derivatives (3a–c)
To a stirred and cold methanolic solution (−15 °C, 20 mL) of the corresponding tripeptide ester (2a–c) (1 mmol), sodium hydroxide (1N, 25 mL) was gradually added. The reaction mixture was stirred for 2 h at the same temperature and then for 3 h at room temperature. The solvent was distilled off under reduced pressure, and the remaining aqueous solution was cooled and acidified with 1 N hydrochloric acid to pH = 3. The obtained solid was filtered off, washed with water, dried, and crystallized from ethanol–water to give the corresponding acids (3a–c).
Nα-Dipicolinoyl-bis[L-ILe-L-ILe-L-Phe-carboxylic acid] (3a). Yield: 90 %; m.p. 137–139 °C, [α]: -65 (C, 0.02, MeOH). IR (KBr, cm−1): ν = 3287 (NH str), 3070 (C-H, arom), 2966 (C-H, aliph), 1646 (C=O, acid), 1531, 1452, 1386 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.55 (s, 2H, 2OH, D2O exchangeable), 8.52–8.48 (m, 3H, Pyr-H), 8.20–8.11 (3s, 6H, 6NH, D2O exchangeable), 7.36-7.25 (m, 10H, Ar-H), 4.50-4.46 (t, 2H, NHCH, L-Phe-ala), 4.24 (d, 4H, 4NHCH, L-Ile), 3.26 (d, 4H, 2CH2, L-Phe-ala), 3.16-2.98 (m, 4H, 4NHCHCH, L-Ile), 1.28–1.08 (m, 8H, 4CH2, L-Ile), 0.87–0.75 (m, 24H, 8CH3, L-Ile). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.85 (2C, CO, acid), 171.65, 171.60 (4C, 4CO, L-Phe-ala, L-Ile), 167.34 (2C, 2CO, Dicarbonyl pyridine), 148.70, 140.45, 124.24 (5C, pyr-C), 137.00, 128.04, 127.30, 125.90 (12C, Ph-C), 60.90, 58.86, 58.70 (6C, 6CHNH), 42.65 (2C, 2CH2, L-Phe-ala), 35.50, 35.00 (4C, 4CH, L-Ile), 25.04, 24.92 (4C, 4CH2), 13.40, 10.90 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 915 (M++1, 6), 914 (M+, 11), 913 (7), 833 (76), 804 (42), 776 (64), 702 (44), 641 (100), 539 (44), 461 (69), 401(27), 302 (70), 86 (39), 57 (20), 50 (5). Analysis for C49H67N7O10 (914.1): Calcd. C, 64.38; H, 7.39; N, 10.73. Found: C, 64.37, H, 7.37, N, 10.70.
Nα-Dipicolinoyl-bis[L-ILe-L-Phe-L-ILe-carboxylic acid] (3b). Yield: 82%; m.p. 222–224 °C, [α]: -77 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3402 (NH str), 3073 (C-H, arom), 2966 (C-H, aliph), 1652 (C=O, acid), 1530, 1453, 1387 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.60 (s, 2H, 2OH, D2O exchangeable), 8.50-8.42 (m, 3H, Pyr-H), 8.24-8.15 (3s, 6H, 6NH, D2O exchangeable), 7.22–7.12 (m, 10H, Ar-H), 4.70–4.62 (t, 2H, NHCH, L-Phe-ala), 4.36 (d, 4H, 4NHCH, L-Ile), 3.30 (d, 4H, 2CH2, L-Phe-ala), 3.06-3.00 (m, 4H, 4NHCHCH, L-Ile), 1.42–1.24 (m, 8H, 4CH2, L-Ile), 0.86–0.78 (m, 24H, 8CH3, L-Ile). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 174.00 (2C, CO, acid), 171.650, 171.58 (4C, 4CO, L-Phe-ala, L-Ile), 166.92 (2C, 2CO, dicarbonyl pyridine), 148.85, 140.64, 124.60 (5C, pyr-C), 137.15, 128.16, 127.24, 125.70 (12C, Ph-C), 60.82, 58.84, 58.75 (6C, 6CHNH), 42.68 (2C, 2CH2, L-Phe-ala), 35.34, 35.06 (4C, 4CH, L-Ile), 25.10, 24.95 (4C, 4CH2), 13.35, 10.76 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 915 (M++1, 8), 914 (M+, 22), 833 (M+, 52), 760 (31), 637 (69), 590 (100), 477 (66), 430 (44), 400 (20), 302 (70), 69 (64), 57 (25), 51 (12). Analysis for C49H67N7O10 (914.10): Calcd. C, 64.38; H, 7.39; N, 10.73. Found: C, 64.37, H, 7.37, N, 10.69.
Nα-Dipicolinoyl-bis[L-Phe-L-ILe-L-ILe-carboxylic acid] (3c). Yield: 66%; m.p. 138–140 °C, [α]: -96 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3326 (NH str), 3072 (C-H, arom), 2966 (C-H, aliph), 1721 (C=O, ester), 1655, 1529, 1452 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.54 (s, 2H, 2OH, D2O exchangeable), 8.62–8.52 (m, 3H, Pyr-H), 8.10–8.09 (3s, 6H, 6NH, D2O exchangeable), 7.32–7.22 (m, 10H, Ar-H), 4.86-4.80 (t, 2H, NHCH, L-Phe-ala), 4.40–4.18 (d, 4H, 4NHCH, L-Ile), 3.31-3.20 (d, 4H, 2CH2, L-Phe-ala), 3.12–3.05 (m, 4H, 4NHCHCH, L-Ile), 1.34-1.02 (m, 8H, 4CH2, L-Ile), 0.92–0.77 (m, 24H, 8CH3, L-Ile). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.58 (2C, CO, acid), 171.72, 171.60 (4C, 4CO, L-Phe-ala, L-Ile), 167.05 (2C, 2CO, dicarbonyl pyridine), 148.68, 140.60, 124.45 (5C, pyr-C), 137.25, 128.10, 127.36, 125.75 (12C, Ph-C), 60.68, 58.80, 58.65 (6C, 6CHNH), 42.65 (2C, 2CH2, L-Phe-ala), 35.45, 35.36 (4C, 4CH, L-Ile), 25.16, 24.90 (4C, 4CH2), 13.32, 10.92 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 914 (M+, 5), 913 (2), 782 (6), 641 (2), 551 (8), 396 (9), 305 (8), 234 (9), 87 (10), 59 (100), 57 (12), 50 (4). Analysis for C49H67N7O10 (914.10): Calcd. C, 64.38; H, 7.39; N, 10.73. Found: C, 64.30, H, 7.30, N, 10.70.
3.1.3. Synthesis of cyclo-(Nα-dipicolinoyl)-bis-[tripeptide]-L-Lys-OMe (cyclic heptapeptide methyl esters) (4a–c)
Ethyl chloroformate (0:2 mL, 2 mmol) was added to a stirred and cold (−15 °C) dichloromethane solution (20 mL) of the corresponding Nα-dipicolinoyl-bis[tripeptide] (3a–c) (1 mmol), containing N-methylmorpholine (0:2 mL, 2 mmol). The reaction mixture was stirred for additional 20 min, and then a cold (−15 °C) dichloromethane solution (20 mL) of the free L-lysine methyl ester (1 mmol) was added. Stirring was maintained for 3 h at (−15 °C), and then for 12 h at room temperature. The reaction mixture was washed with water, 1 N sodium bicarbonate, and 1 N potassium hydrogen sulfate and water, and then dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to dryness, and the obtained oily residue was solidified by trituration with dry ether-n-hexane mixture. The crude product was purified by preparative thin layer chromatography using S3 as eluent to give the corresponding cyclic heptapeptide methyl esters (4a–c).
Cyclo-(Nα-dipicolinoyl)-bis-[(L-ILe-L-ILe-L-Phe)-L-Lys methyl ester] (4a). Yield: 82%; m.p. 86–88 °C, [α]: -15 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3306 (NH str), 3066 (C-H, arom), 2876 (C-H, aliph), 1741 (C=O, ester), 1651, 1529, 1450, 1381 (C=O amide I, II, III and V, respectively). 8.74–8.60 (m, 3H, Pyr-H), 8.42–8.15 (4s, 8H, 8NH, D2O exchangeable), 7.28–7.12 (m, 10H, Ar-H), 4.65 (t, 2H, NHCH, L-Phe-ala), 4.50-4.35 (m, 4H, 4CH, L-Ile), 4.25 (t, 1H, CH, L-Lys), 4.16 (d, 4H, 2CH2Ph), 3.68-3.60 (m, 2H, CH2, NHCH2, L-Lys), 3.48 (s, 3H, OCH3), 2,80–2.70 (m, 4H, 4CH, L-Ile), 2.18-1.80 (m, 6H, 3CH2, Lys-CH2), 1.25-1.12 (m, 8H, 4CH2, L-Ile), 0.95-0.76 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.02 (1C, CO, ester), 172.60, 172.52, 171.98 (6C, 6CO, L-Phe-ala, L-Ile), 163.82 (2C, 2CO, dicarbonyl pyridine), 147.72, 144.68, 125.84 (5C, pyr-C), 137.75, 127.94, 127.60, 125.70 (12C, Ph-C), 59.98, 58.36, 58.24, 58.00 (7C, 7CHNH), 52.12 (1C, OCH3, ester), 48.68 (1C, NHCH2, L-Lys), 46.34, 45.98 (2C, 2CH2, L-Phe-ala), 37.56, 37.26 (4C, 4CH, L-Ile), 35.24, 34.70 (4C, 4CH2), 32.28, 29.12, 21.32 (3C, 3CH2, Lys), 14.30, 11.30 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1039 (M++1, 8), 1038 (M+, 10), 857 (10), 782 (10), 565 (24), 250 (13), 69 (40), 59 (100), 57 (78), 55 (80), 53 (9). Analysis for C56H79N9O10 (1038.28): Calcd. C, 64.78; H, 7.67; N, 12.14. Found: C, 64.77, H, 7.65, N, 12.11.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-ILe-L-Phe-L-Ile)-L-Lys methyl ester] (4b). Yield: 70%; m.p. 90–92 °C, [α]: -44 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3305 (NH str), 3065 (C-H, arom), 2965 (C-H, aliph), 1740 (C=O, ester), 1655, 1528, 1450 (C=O amide I, II and III respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 8.60–8.52 (m, 3H, Pyr-H), 8.35-8.18 (4s, 8H, 8NH, D2O exchangeable), 7.26-7.18 (m, 10H, Ar-H), 4.62 (t, 2H, NHCH, L-Phe-ala), 4.48-4.37 (m, 4H, 4NHCH, L-Ile), 4.22 (t, 1H, CH, L-Lys), 4.12 (d, 4H, 2CH2Ph), 3.61-3.57 (m, 2H, CH2, NHCH2, L-Lys), 3.42 (s, 3H, OCH3), 2.86-2.78 (m, 4H, 4CH, L-Ile), 2.00–1.76 (m, 6H, 3CH2, Lys-CH2), 1.27–1.10 (m, 8H, 4CH2, L-Ile), 0.99–0.71 (m, 24H, 8CH3, L-Ile). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.14 (1C, CO, ester), 172.42, 172.38, 171.84 (6C, 6CO, L-Phe-ala, L-Ile), 164.80 (2C, 2CO, dicarbonyl pyridine), 147.70, 144.64, 125.80 (5C, pyr-C), 137.70, 127.90, 127.64, 125.65 (12C, Ph-C), 60.65, 58.62, 58.34, 58.06 (7C, 7CHNH), 52.18 (1C, OCH3, ester), 48.79 (1C, NHCH2, L-Lys), 46.34 (2C, 2CH2, L-Phe-ala), 37.55, 37.20 (4C, 4CH, L-Ile), 35.08, 34.87 (4C, 4CH2), 32.45, 29.18, 21.46 (3C, 3CH2, Lys), 14.18, 10.96 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1039 (M++1, 3), 1038 (M+, 6), 924 (26), 868 (17), 650 (70), 622 (50), 590 (33), 505 (40), 477 (68), 347 (36), 330 (69), 302 (100), 293 (38), 86 (59), 69 (59), 57 (25), 50 (3). Analysis for C56H79N9O10 (1038.28): Calcd. C, 64.78; H, 7.67; N, 12.14. Found: C, 64.71, H, 7.65, N, 12.12.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-Phe-L-ILe-L-Ile)-L-Lys methyl ester] (4c). Yield: 85%; m.p. 110–112 oC, [α]: -18 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3315 (NH str), 3065 (C-H, arom), 2964 (C-H, aliph), 1658 (C=O, ester), 1528, 1447, 1379, 1241 (C=O amide I, II, III and IV, respectively). 8.72–8.60 (m, 3H, Pyr-H), 8.45–8.14 (4s, 8H, 8NH, D2O exchangeable), 7.30-7.16 (m, 10H, Ar-H), 4.62 (t, 2H, NHCH, L-Phe-ala), 4.54-4.38 (m, 4H, 4CH, L-Ile), 4.256 (t, 1H, CH, L-Lys), 4.18 (d, 4H, 2CH2Ph), 3.66-3.58 (m, 2H, CH2, NHCH2, L-Lys), 3.46 (s, 3H, OCH3), 2.76–2.65 (m, 4H, 4CH, L-Ile), 2.24–1.86 (m, 6H, 3CH2, Lys-CH2), 1.24–1.18 (m, 8H, 4CH2, L-Ile), 0.96–0.82 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.32 (1C, CO, ester), 172.60, 172.30, 171.72 (6C, 6CO, L-Phe-ala, L-Ile), 164.75 (2C, 2CO, dicarbonyl pyridine), 147.75, 144.65, 125.85 (5C, pyr-C), 137.72, 127.93, 127.64, 125.62 (12C, Ph-C), 61.14, 58.60, 58.30, 58.00 (7C, 7CHNH), 52.44 (1C, OCH3, ester), 48.85 (1C, NHCH2, L-Lys), 46.60 (2C, 2CH2, L-Phe-ala), 37.58, 37.24 (4C, 4CH, L-Ile), 35.00, 34.92 (4C, 4CH2), 32.44, 29.22, 21.45 (3C, 3CH2, Lys), 14.12, 10.95 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1039 (M++1, 4), 10.38 (M+, 5), 925 (9), 867 (10), 812 (19), 726 (26), 684 (84), 656 (48), 571 (56), 511 (32), 409 (20), 370 (100), 86 (23), 57 (10), 51 (2). Analysis for C56H79N9O10 (1038.28): Calcd. C, 64.78; H, 7.67; N, 12.14. Found: C, 64.73, H, 7.65, N, 12.14.
3.1.4. Synthesis of cyclo-(Nα-dipicolinoyl)-bis[tripeptide]-L-Lys-carboxylic acid (5a–c)
To a stirred and cold methanolic solution (−5 °C, 20 mL) of cyclic heptapeptide methyl ester (4a–c) (1 mmol), sodium hydroxide (1 N, 25 mL) was gradually added. The reaction mixture was stirred for 2 h at the same temperature, and then for 3 h at room temperature. The solvent was distilled off under reduced pressure, and the remaining aqueous solution was cooled and acidified with 1 N hydrochloric acid to pH = 3. The obtained solid was filtered off, washed with water, dried, and crystallized from ethanol/water to give the corresponding cyclic heptapeptide methyl acids (5a–c).
Cyclo-(Nα-dipicolinoyl)-bis-[(L-ILe-L-Ile-L-Phe)-L-Lys carboxylic acid] (5a). Yield: 70 %; m.p. 132–134 °C, [α]: -20 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3310 (NH str), 3066 (C-H, arom), 2965 (-CH, aliph), 1652 (C=O, acid), 1529, 1452, 1383, 1227 (C=O amide I, II, III, and IV, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.50 (s, 1H, OH, D2O exchangeable), 8.75-8.65 (m, 3H, Pyr-H), 8.50-8.18 (m, 8H, 8NH, D2O exchangeable), 7.50–7.21 (m, 10H, Ar-H), 4.60 (t, 2H, 2 CHCH2, L-Phe-ala), 4.55-4.45 (m, 4H, 4CH, L-Ile), 4.35 (t, 1H, CH, L-Lys), 4.18 (t, 4H, 2CH2Ph), 3.75–3.70 (m, 2H, NHCH2, L-Lys), 2.72–2.60 (m, 4H, 4CH, L-Ile), 2.00-1.80 (m, 6H, 3CH2, Lys-CH2), 1.27–1.14 (m, 8H, 4CH2, L-Ile), 0.93–0.74 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 174.00 (1C, CO, Acid), 172.90, 172.80, 171.16 (6C, 6CO, L-Phe-ala, L-Ile), 168.10 (2C, 2CO, dicarbonyl pyridine), 147.60, 144.45, 125.80 (5C, pyr-C), 137.70, 128.55, 127.60, 125.78 (12C, Ph-C), 59.95, 58.32, 58.22, 58.04 (7C, 7CHNH), 48.56 (1C, NHCH2, L-Lys), 46.30, 45.95 (2C, 2CH2, L-Phe-ala), 37.55, 37.22 (4C, 4CH, L-Ile), 35.16, 34.80 (4C, 4CH2), 32.20, 29.12, 21.30 (3C, 3CH2, Lys), 14.24, 11.36 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1025 (M+ + 1, 22), 1024 (M+, 6), 816 (26), 645 (26), 562 (36), 388 (32), 221 (31), 157 (30), 91 (83), 69 (53), 59 (58), 57 (100), 55 (98), 51 (32), 50 (15). Analysis for C55H77N9O10 (1024.25): Calcd. C, 64.49; H, 7.58; N, 12.31. Found: C, 64.40, H, 7.50, N, 12.28.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-ILe-L-Phe-L-Ile)-L-Lys carboxylic acid] (5b). Yield: 65 %; m.p. 138–140 °C, [α]: -45 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3312 (NH str.), 3065 (C-H, arom), 2965 (C-H, aliph), 1654 (C=O, acid), 1529, 1451, 1384 (C=O amide I, II and III respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.45 (s, 1H, OH, D2O exchangeable), 8.95-8.85 (m, 3H, Pyr-H), 8.42–8.12 (s, 8H, 8NH, D2O exchangeable), 7.45–7.30 (m, 10H, Ar-H), 4.70 (t, 2H, 2CHCH2, L-Phe-ala), 4.40–4.36 (m, 4H, 4CH, L-Ile), 4.25 (t, 1H, CH, L-Lys), 4.05 (t, 4H, 2CH2Phe), 3.70–3.62 (m, 2H, CH2, NHCH2, L-Lys), 2.80–2.70 (m, 4H, 4CH, L-Ile), 2.00–1.45 (m, 6H, 3CH2, Lys-CH2), 1.35–1.15 (m, 8H, 4CH2, L-Ile), 0.90–0.71 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.85 (1C, CO, Acid), 173.65, 173.33, 170.35 (6C, 6CO, L-Phe-ala, L-Ile), 167.60 (2C, 2CO, dicarbonyl pyridine), 147.66, 144.18, 125.80 (5C, pyr-C), 137.90, 128.57, 127.75, 125.60 (12C, Ph-C), 60.16, 58.28, 58.18, 58.08 (7C, 7CHNH), 48.28 (1C, NHCH2, L-Lys), 46.26, 45.95 (2C, 2CH2, L-Phe-ala), 37.56, 37.28 (4C, 4CH, L-Ile), 35.00, 34.78 (4C, 4CH2), 32.18, 29.05, 21.08 (3C, 3CH2, Lys), 14.75, 11.26 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1025 (M++1, 26), 789 (19), 654 (25), 514 (22), 330 (35), 69 (52, 59 (54), 57 (100), 54 (22). Analysis for C55H77N9O10 (1024.25). Calcd. C, 64.49; H, 7.58; N, 12.31. Found: C, 64.44, H, 7.56, N, 12.30.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-Phe-L-ILe-L-Ile)-L-Lys carboxylic acid] (5c). Yield: 90%; m.p. 147–149 °C, [α]: -41 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3322 (NH str.), 3065 (C-H, arom), 2965 (C-H, aliph), 1600 (C=O, acid), 1527, 1449, 1383, 1231 (C=O amide I, II, III and IV, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 12.65 (s, 1H, OH, D2O exchangeable), 8.86–8.70 (m, 3H, Pyr-H), 8.56–8.22 (m, 8H, 8NH, D2O exchangeable), 7.41–7.35 (m, 10H, Ar-H), 4.75 (t, 2H, 2CHCH2, L-Phe-ala), 4.60-4.42 (m, 4H, 4CH, L-Ile), 4.35 (t, 1H, CH, L-Lys), 3.96 (t, 4H, 2CH2Phe), 3.65–3.55 (m, 2H, CH2, NHCH2, L-Lys), 2.90–2.75 (m, 4H, 4CH, L-Ile), 2.00–1.65 (m, 6H, 3CH2, Lys-CH2), 1.30–1.10 (m, 8H, 4CH2, L-Ile), 0.90–0.76 (m, 24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 174.02 (1C, CO, Acid), 173.18, 173.06, 170.15 (6C, 6CO, L-Phe-ala, L-Ile), 167.60 (2C, 2CO, Dicarbonyl pyridine), 147.95, 144.12, 126.15 (5C, pyr-C), 137.60, 128.40, 127.80, 125.72 (12C, Ph-C), 59.85, 58.60, 58.45, 58.28 (7C, 7CHNH), 48.32 (1C, NHCH2, L-Lys), 46.42, 45.94 (2C, 2CH2, L-Phe-ala), 37.62, 37.54 (4C, 4CH, L-Ile), 35.12, 34.88 (4C, 4CH2), 32.25, 29.16, 21.18 (3C, 3CH2, Lys), 14.52, 11.26 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1025 (M++1, 2), 10.24 (M+, 4), 689 (2), 621 (26), 565 (12), 406 (5), 113 (4), 59 (100), 57 (21), 50 (8). Analysis for C55H77N9O10 (1024.25): Calcd. C, 64.49; H, 7.58; N, 12.31. Found: C, 64.43, H, 7.55, N, 12.29.
3.1.5. Synthesis of cyclo-[(Nα-dipicolinoyl)-bis-(tripeptide)-L-Lys-NHNH2] (cyclic heptapeptide hydrazides) (6a–c).
To a stirred methanolic solution (20 mL) of the corresponding cyclic pentapeptide methyl ester (4a–c) (1 mmol), anhydrous hydrazine hydrate (0:35 mL, 10 mmol) was added with refluxing for 3 h. The solvent was evaporated and the obtained residue was triturated with ether, filtered off, and crystallized from methanol/ether to afford the hydrazides (6a–c).
Cyclo-(Nα-dipicolinoyl)-bis-[(L-Ile-L-Ile-L-Phe)-L-Lys acid hydrazide] (6a). Yield: 75%; m.p. 168–170 °C, [α]: -35 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3300 (NH str.), 3066 (C-H, arom.), 2965 (CH, aliph.), 1652, 1530, 1452 and 1385 (C=O amide I, II, III and IV, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.40 (s, 1H, NH, D2O exchangeable), 8.80–8.60 (m, 3H, Pyr-H), 8.45–8.21 (m, 8H, 8NH, D2O exchangeable), 7.60–7.50 (m, 10H, Ar-H), 4.90 (t, 2H, 2 CHCH2, L-Phe-ala), 4.70–4.55 (m, 4H, 4CH, L-Ile), 4.35 (t, 1H, CH, L-Lys), 4.25 (s, 2H, NH2, D2O exchangeable), 4.15 (t, 4H, 2CH2Ph), 3.85–3.80 (m, 2H, CH2, L-Lys), 2.35–2.20 (m, 4H, 4CH, L-Ile), 1.60–1.40 (m, 6H, 3CH2, Lys-CH2), 1.25–1.00 (m, 8H, 4CH2, L-Ile), 0.90–0.75 (24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.16, 173.02, 170.16 (6C, 6CO, L-Phe-ala, L-Ile), 171.55 (1C, CO, Hydrazide), 167.72 (2C, 2CO, dicarbonyl pyridine), 147.65, 144.14, 125.82 (5C, pyr-C), 137.90, 128.30, 127.65, 125.70 (12C, Ph-C), 60.12, 58.25, 58.13, 58.00 (7C, 7CHNH), 48.10 (1C, NHCH2, L-Lys), 46.32, 45.92 (2C, 2CH2, L-Phe-ala), 37.56, 37.24 (4C, 4CH, L-Ile), 35.12, 34.68 (4C, 4CH2), 32.18, 29.05, 21.04 (3C, 3CH2, Lys), 14.70, 11.30 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1038 (M+, 17), 1027 (30), 965 (33), 878 (30), 754 (42), 597 (34), 375 (36), 167 (36), 71 (58), 60 (58), 57 (100), 55 (84), 51 (14). Analysis for C55H79N11O9 (1038.28): Calcd. C, 63.62; H, 7.67; N, 14.84. Found: C 63.55, H 7.60, N 14.76.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-Ile-L-Phe-L-Ile)-L-Lys acid hydrazide] (6b). Yield: 70%; m.p. 182–184 °C, [α]: -55 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3296 (NH str.), 3064 (C-H, arom.), 2931 (C-H, aliph.), 1752, 1529, 1451, 1383 (C=O amide I, II, III, IV and V, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.35 (s, 1H, NH, D2O exchangeable), 8.90–8.75 (m, 3H, Pyr-H), 8.35–8.20 (m, 8H, 8NH, D2O exchangeable), 7.65–7.45 (m, 10H, Ar-H), 4.85 (t, 2H, 2 CHCH2, L-Phe-ala), 4.72–4.58 (m, 4H, 4CH, L-Ile), 4.44 (t, 1H, CH, L-Lys), 4.30 (s, 2H, NH2, D2O exchangeable), 4.12 (t, 4H, 2CH2Ph), 3.80–3.70 (m, 2H, CH2, L-Lys), 2.50–2.40 (m, 4H, 4CH, L-Ile), 1.70–1.50 (m, 6H, 3CH2, Lys-CH2), 1.30–1.05 (m, 8H, 4CH2, L-Ile), 0.95-0.85 (24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.30, 173.15, 170.25 (6C, 6CO, L-Phe-ala, L-Ile), 171.60 (1C, CO, Hydrazide), 167.80 (2C, 2CO, dicarbonyl pyridine), 147.7, 144.12, 125.9 (5C, pyr-C), 137.95, 128.35, 127.70, 125.75 (12C, Ph-C), 60.10, 58.25, 58.15, 58.04 (7C, 7CHNH), 48.12 (1C, NHCH2, L-Lys), 46.20, 45.98 (2C, 2CH2, L-Phe-ala), 37.5, 37.2 (4C, 4CH, L-Ile), 35.10, 34.32 (4C, 4CH2), 32.15, 29.00, 21.00 (3C, 3CH2, Lys), 14.7, 11.25 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1039 (M++1, 14), 1038 (M+, 19), 918 (20), 767 (21), 737 (20), 661 (60), 590 (49), 522 (84), 429 (43), 375 (63), 347 (100), 302 (68), 69 (64), 57 (85), 55 (66), 51 (16). Analysis for C55H79N11O9 (1038.28): Calcd. C, 63.62; H, 7.67; N, 14.84. Found: C 63.50, H 7.60, N 14.74.
Cyclo-(Nα-dipicolinoyl)-bis-[(L-Phe-L-ILe-L-Ile)-L-Lys acid hydrazide] (6c). Yield: 74 %; m.p. 175–177 °C, [α]: -66 (c, 0.02, MeOH). IR (KBr, cm−1): ν = 3298 (NH str.), 3064 (C-H, arom.), 2964 (C-H, aliph.), 1653, 1527, 1448, 1383, 1241 (C=O amide I, II, III, IV and V, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.35 (s, 1H, NH, D2O exchangeable), 9.05–8.60 (m, 3H, Pyr-H), 8.10–8.00 (m, 8H, 8NH, D2O exchangeable), 7.50–7.15 (m, 10H, Ar-H), 4.86 (t, 2H, 2 CHCH2, L-Phe-ala), 4.50-4.40 (m, 4H, 4CH, L-Ile), 4.25 (t, 1H, CH, L-Lys), 4.05 (s, 2H, NH2, D2O exchangeable), 3.98 (t, 4H, 2CH2Ph), 3.70 (t, 2H, CH2, L-Lys), 2.80–2.65 (m, 4H, 4CH, L-Ile), 1.90–1.70 (m, 6H, 3CH2, Lys-CH2), 1.35–1.12 (m, 8H, 4CH2, L-Ile), 1.00–0.82 (24H, 8CH3). 13C-NMR (125 MHz, ppm, DMSO-d6): δ = 173.36, 173.16, 170.10 (6C, 6CO, L-Phe-ala, L-Ile), 171.580 (1C, CO, Hydrazide), 167.56 (2C, 2CO, dicarbonyl pyridine), 148.5, 144.0, 126.1 (5C, pyr-C), 137.78, 128.42, 127.76, 125.80 (12C, Ph-C), 60.00, 58.34, 58.18, 58.06 (7C, 7CHNH), 48.18 (1C, NHCH2, L-Lys), 46.35, 45.95 (2C, 2CH2, L-Phe-ala), 37.6, 37.3 (4C, 4CH, L-Ile), 35.16, 34.38 (4C, 4CH2), 32.22, 29.04, 21.08 (3C, 3CH2, Lys), 14.5, 11.20 (8C, 8CH3, L-Ile). MS (EI, 70 eV): m/z (%) = 1038 (M+, 8), 10.37 (12), 926 (24), 778 (22), 664 (25), 624 (43), 522 (45), 471 (26), 409 (73), 370 (100), 324 (21), 165 (12), 86 (53), 57 (69), 55 (51), 50 (10). Analysis for C55H79N11O9 (1038.28): Calcd. C 63.62; H 7.67; N 14.84. Found: C 63.54, H 7.63, N 14.75.
3.2. Anticancer Activity
The cytotoxic effects of the prepared compounds on MCF-7 and HepG2 cells were investigated with the help of standard MTT assay [33,34]. Cells were plated in RPMI 1640 medium in 96-well culture plates 2 × 104/mL, incubated for 24 h for adherence, and then different concentrations of the prepared derivatives (0–1 μM/DMSO) were added to well plates. Plates were then further incubated for 72 h. Afterwards, 20 μL of MTT (5 mg/mL in PBS) were pipetted to the wells and incubated for another 4 h. The medium was then aspirated and 100 μL DMSO were added/well. Absorbance was read using a microplate reader at 570 nm [35]. IC50 values, used to compare compound toxicity with control cells, were obtained from linear regression of the dose–response curve using Origin® 6.1 software (OriginLab Corporation, Northampton, MA, USA). Experiments were performed in triplicates, and tamoxifen and cisplatin were used as positive control. Data were represented as mean ± SD.
4. Conclusions
In summary, a series of macrocyclic derivatives bearing a pyrido-heptapeptide moiety were designed and synthesized from Nα-dipicolinoyl-bis[dipeptide-carboxylic acid]. Two human cancer cell lines (MCF-7 and HepG-2) were used to evaluate the anticancer potency of all synthesized compounds. All compounds which exhibited anticancer activities were found to be more effective (lower IC50 values, Table 1) on HepG2 cells than MCF-7 cells. For HepG2 cells, compounds 1a,b, 3a,b, 4a–c, and 5a–c were the most effective. On the other hand, for MCF-7 cells, compounds 2a and 5a–c were not effective against MCF-7 cancer cells. Furthermore, compounds 1b, 3a,b, and 4a,b were the most potent derivatives.
Author Contributions
The listed authors contributed to this work as described in the following. M. H. A., A.G. E. A.; G.O. M., A.M.N. provided the concepts of the work, interpreted the results, developed the experimental section, and prepared the manuscript. M. A. A. cooperated in the preparation of the manuscript. E. A. E. and A.H.B. performed the anticancer analysis of the new compounds. All authors read and approved the final manuscript.
Funding
This research was funded by Authors are grateful to King Saud University, Riyadh, Saudi Arabia through Researchers Supporting Project (No. RSP-2019/66).
Acknowledgments
Authors are grateful to King Saud University, Riyadh, Saudi Arabia for funding the work through Researchers Supporting Project (No. RSP-2019/66).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Controll. Release 2017, 250, 62–76. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Krahn, D.; Ottmann, C.; Kaiser, M. Macrocyclic proteasome inhibitors. Curr. Med. Chem. 2011, 18, 5052–5060. [Google Scholar] [CrossRef]
- Marsault, E.; Peterson, M.L. Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef] [PubMed]
- Erb, W.; Zhu, J. From natural product to marketed drug: The tiacumicin odyssey. Nat. Prod. Rep. 2013, 30, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.; Collins, I. Macrocycles in new drug discovery. Future Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Amr, A.E.; Abo-Ghaliaa, M.H.; Abdalah, M.M. Synthesis of novel macrocyclic peptido-calix [4]arenes and peptidopyridines as precursors for potential molecular metallacages, chemosensors and biologically active candidates. Z. Naturforsch. 2006, 61b, 1335–1345. [Google Scholar]
- Amr, A.E.; Abdel-Salam, O.I.; Attia, A.; Stibor, I. Synthesis of new potential bis-intercallators based on chiral pyridine-2,6-dicarbox-amides. Collect. Czech. Chem. Commun. 1999, 64, 288–298. [Google Scholar] [CrossRef]
- Attia, A.; Abdel-Salam, O.I.; Amr, A.E.; Stibor, I.; Budesinsky, M. Synthesis and antimicrobial activity of some new chiral bridged macrocyclic pyridines. Egypt. J. Chem. 2000, 43, 187–201. [Google Scholar] [CrossRef]
- Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A.; Al-Salem, H.S.A.; Hozzein, W.N. Synthesis, characterization and in vitro antimicrobial investigation of novel amino acids and dipeptides based on dibenzofuran-2-sulfonyl-chloride. J. Comput. Theor. Nanosci. 2017, 14, 3183–3190. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Amr, A.E. Synthesis of some new pyridine-2,6-carboxamide-derived Schiff Bases as potential antimicrobial agents. Molecules 2010, 15, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, O.I.; Al-Omar, M.A.; Fayed, A.A.; Flefel, E.M.; Amr, A.E. Synthesis of new macrocyclic polyamides as antimicrobial agent candidates. Molecules 2012, 17, 14510–14521. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, H.S.A.; Naglah, A.M.; Moustafa, G.O.; Mahmoud, A.Z.; Al-Omar, M.A. Synthesis of novel tripeptides based on dibenzofuran-2-sulfonyl-[aromatic and hydroxy aromatic residues]: Towards antimicrobial and antifungal agents. J. Comput. Theor. Nanosci. 2017, 14, 3958–3966. [Google Scholar] [CrossRef]
- Moustafa, G.; Khalaf, H.; Naglah, A.; Al-Wasidi, A.; Al-Jafshar, N.; Awad, H. Synthesis, molecular docking studies, in vitro antimicrobialand antifungal activities of novel dipeptide derivatives based on n-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide. Molecules 2018, 23, 761. [Google Scholar] [CrossRef]
- Khayyat, S.; Amr, A.E. Synthesis and biological activities of some new (Nα-dinicotinoyl)-bis- L-leucyl lnear and macrocyclic peptides. Molecules 2014, 19, 10698–10716. [Google Scholar] [CrossRef]
- Amr, A.E.; Abo-Ghalia, M.H.; Abdalah, M.M. Synthesis of new (Nα-dipicolinoyl)-bis-L-valyl- L-phenyl linear and macrocyclic bridged peptides as anti-inflammatory agents. Arch. Pharm. Chem. Life Sci. 2007, 340, 304–309. [Google Scholar] [CrossRef]
- Abo-Ghalia, M.H.; Amr, A.E. Synthesis and investigation of a new cyclo (Nα-pentapeptide of a breast and CNS cytotoxic activity and an ionophoric specificity. Amino Acids 2004, 26, 283–289. [Google Scholar] [CrossRef]
- Masereel, B.; Dupont, L.; Laeckmann, D.; Liégeois, J.F.; Pirotte, B.; de Tullio, P.; Delarge, J. Synthesis and pharmacology of pyrid-3-ylsulfonylcyanoguanidines as diuretic. Eur. J. Med. Chem. 1995, 30, 235–240. [Google Scholar] [CrossRef]
- Abo-Ghalia, M.H.; Moustafa, G.O.; Alwasidi, A.S.; Naglah, A.M. Cytotoxic investigation of isophthaloyl cyclopentapeptides. Lat. Am. J. Pharm. 2017, 36, 1957–1962. [Google Scholar] [CrossRef]
- Moustafa, G.O.; El-Sawy, A.A.; Abo-Ghalia, M.H. Synthesis of novel cyclopeptide candidates: I-cyclo-[Nα-isophthaloyl-bis-(Glycine-amino acid)-L-lysine] derivatives with expected anticancer activity. Egypt. J. Chem. 2013, 5, 473–494. [Google Scholar] [CrossRef]
- Amr, A.E.; Mohamed, A.M.; Ibrahim, A.A. Synthesis of some new chiral tricyclic and macrocyclic pyridine derivatives as antimicrobial agents. Z. Naturforsch. 2003, 58b, 861–868. [Google Scholar] [CrossRef]
- Abo-Ghaliaa, M.H.; Amr, A.E.; Abdalah, M.M. Synthesis of some new (Nα-dipicolinoyl)-bis- L-leucyl-DL-norvalyl linear tetra and cyclic octa bridged peptides as new antiinflammatory agents. Z. Naturforsch. 2003, 58b, 903–910. [Google Scholar] [CrossRef]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammationassociated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Irish J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef]
- La Manna, S.; Scognamiglio, P.L.; Di Natale, C.; Leone, M.; Mercurio, F.A.; Malfitano, A.M.; Cianfarani, F.; Madonna, S.; Caravella, S.; Albanesi, C.; et al. Characterization of linear mimetic peptides of Interleukin-22 from dissection of protein interfaces. Biochimie 2017, 138, 106–115. [Google Scholar] [CrossRef]
- Amr, A.E.; Abo-Ghalia, M.H.; Moustafa, G.O.; Al-Omar, M.A.; Nossir, E.; Elsayed, E.A. Synthesis and characterization of some newly macrocyclic pentapeptide derivatives as anticancer activity. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef]
- El-Faham, A.; Elzatahry, A.; Al-Othman, Z.; Elsayed, E.A. Facile method for the synthesis of silver nanoparticles using 3-hydrazino-isatin derivatives in aqueous methanol and their antibacterial activity. Int. J. Nanomed. 2014, 9, 1167–1174. [Google Scholar] [CrossRef]
- Amr, A.E.; Abo-Ghalia, M.; Moustafa, G.; Al-Omar, M.A.; Nossier, E.; Elsayed, E.A. Design, synthesis and docking studies of novel macrocyclic pentapeptides as anticancer multi-targeted kinase inhibitors. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.; El-Naggar, M.; Al-Omar, M.A.; Elsayed, E.A. In vitro and in vivo anti-breast cancer activities of some synthesized pyrazolinyl-estran-17-one candidates. Molecules 2018, 23, 1572. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; El-Enshasy, H.A.; Wadaan, M.A. In Vitro assessment of anticancer properties of moringa peregrine essential seed oil on different cell lines. Pak. J. Zool. 2016, 48, 853–859. [Google Scholar]
- Dailin, D.; Elsayed, E.A.; Othman, N.Z.; Malek, R.A.; Ramli, S.; Sarmidi, M.; Aziz, R.; Wadaan, M.; El-Enshasy, H.A. Development of cultivation medium for high yield kefiran production by Lactobacillus kefiranofaciens. Int. J. Pharm. Pharm. Sci. 2015, 7, 159–163. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).