Linking Genes to Molecules in Eukaryotic Sources: An Endeavor to Expand Our Biosynthetic Repertoire
Abstract
:1. Introduction
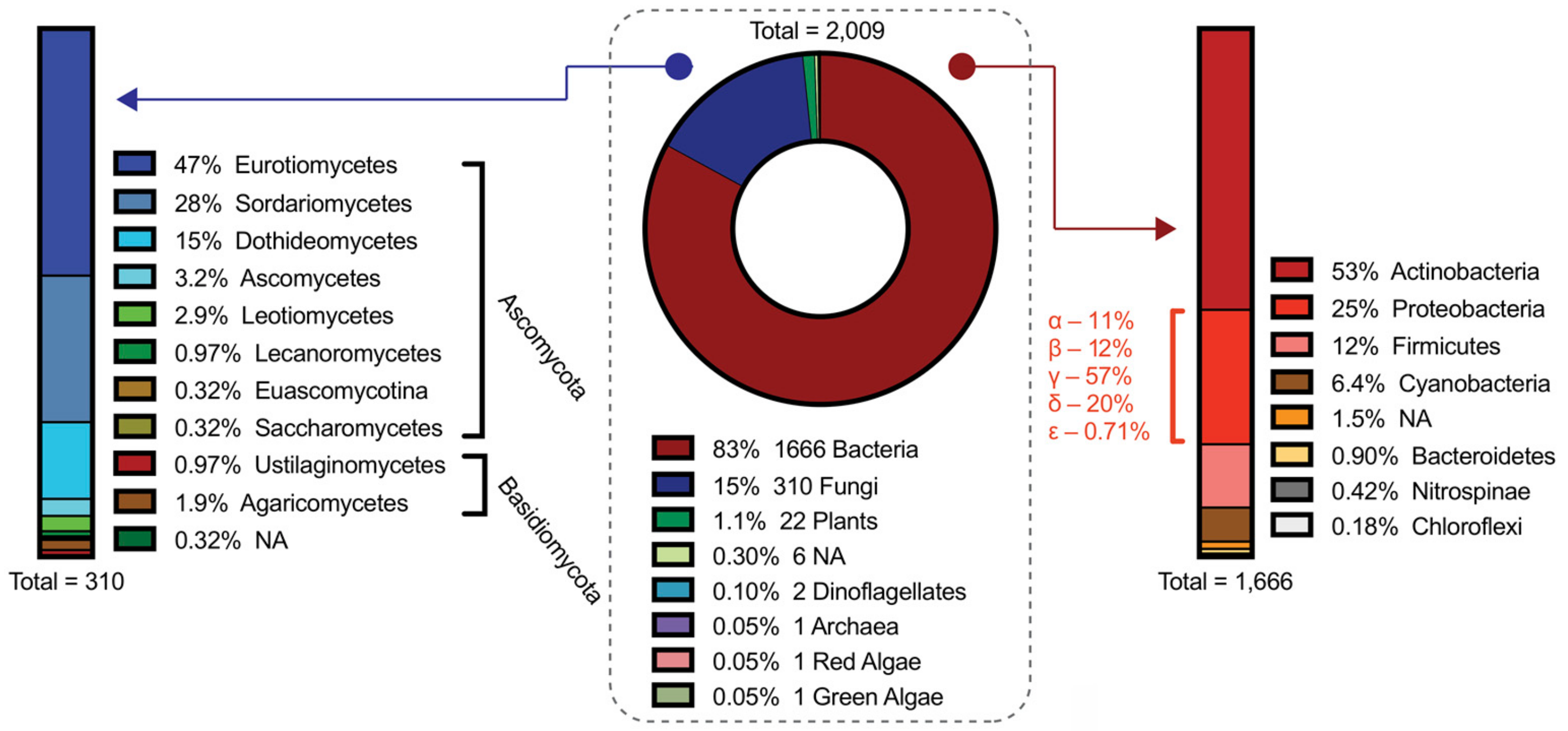
2. Current State of Connecting Genes to Molecules and Evaluation of the MiBIG Repository
3. From Molecules to Gene Clusters in Dinoflagellates and Algae
4. From Molecules to Gene Clusters in Animals
5. Genetics-Guided Discovery of Natural Products in Eukaryotes
6. Outlook and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J. Natural Products as Leads to Potential Drugs: An Old Process or the New Hope for Drug Discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef]
- Cantley, A.M.; Clardy, J. Animals in a bacterial world: Opportunities for chemical ecology. Nat. Prod. Rep. 2015, 32, 888–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baquero, F.; Alvarez-Ortega, C.; Martinez, J.L. Ecology and evolution of antibiotic resistance. Environ. Microbiol. Rep. 2009, 1, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Maplestone, R.A.; Stone, M.J.; Williams, D.H. The evolutionary role of secondary metabolites—A review. Gene 1992, 115, 151–157. [Google Scholar] [CrossRef]
- Fischbach, M.A. Microbiome: Focus on Causation and Mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Ritzman, J.; Brodbeck, A.; Brostrom, S.; McGrew, S.; Dreyer, S.; Klinger, T.; Moore, S.K. Economic and sociocultural impacts of fisheries closures in two fishing-dependent communities following the massive 2015 U.S. West Coast harmful algal bloom. Harmful Algae 2018, 80, 35–45. [Google Scholar] [CrossRef]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [Green Version]
- Leeds, J.A.; Schmitt, E.K.; Krastel, P. Recent developments in antibacterial drug discovery: Microbe-derived natural products – from collection to the clinic. Expert Opin. Investig. Drugs 2006, 15, 211–226. [Google Scholar] [CrossRef]
- Zerikly, M.; Challis, G.L. Strategies for the Discovery of New Natural Products by Genome Mining. ChemBioChem 2009, 10, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kersten, R.D.; Yang, Y.-L.; Xu, Y.; Cimermancic, P.; Nam, S.-J.; Fenical, W.; Fischbach, M.A.; Moore, B.S.; Dorrestein, P.C. A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nat. Chem. Biol. 2011, 7, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.; Philmus, B.; Ziemert, N. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucleic Acids Res. 2017, 45, W42–W48. [Google Scholar] [CrossRef]
- Lewin, H.A.; Robinson, G.E.; Kress, W.J.; Baker, W.J.; Coddington, J.; Crandall, K.A.; Durbin, R.; Edwards, S.V.; Forest, F.; Gilbert, M.T.P.; et al. Earth BioGenome Project: Sequencing life for the future of life. Proc. Natl. Acad. Sci. USA 2018, 115, 4325–4333. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res. 2017, 45, W49–W54. [Google Scholar] [CrossRef] [Green Version]
- Kautsar, S.A.; Suarez Duran, H.G.; Blin, K.; Osbourn, A.; Medema, M.H. plantiSMASH: Automated identification, annotation and expression analysis of plant biosynthetic gene clusters. Nucleic Acids Res. 2017, 45, W55–W63. [Google Scholar] [CrossRef] [Green Version]
- Van Heel, A.J.; de Jong, A.; Montalbán-López, M.; Kok, J.; Kuipers, O.P. BAGEL3: Automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef]
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic mapping of fungal secondary metabolite clusters. Fungal Genet. Biol. 2010, 47, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef] [Green Version]
- Almabruk, K.H.; Dinh, L.K.; Philmus, B. Self-Resistance of Natural Product Producers: Past, Present, and Future Focusing on Self-Resistant Protein Variants. ACS Chem. Biol. 2018, 13, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Ziemert, N.; Lechner, A.; Wietz, M.; Millan-Aguinaga, N.; Chavarria, K.L.; Jensen, P.R. Diversity and evolution of secondary metabolism in the marine actinomycete genus Salinispora. Proc. Natl. Acad. Sci. 2014, 111, E1130–E1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes—A review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Enghiad, B.; Zhao, H. New tools for reconstruction and heterologous expression of natural product biosynthetic gene clusters. Nat. Prod. Rep. 2016, 33, 174. [Google Scholar] [CrossRef] [Green Version]
- Kunjapur, A.M.; Pfingstag, P.; Thompson, N.C. Gene synthesis allows biologists to source genes from farther away in the tree of life. Nat. Commun. 2018, 9, 4425. [Google Scholar] [CrossRef] [Green Version]
- Carlson, R. The changing economics of DNA synthesis. Nat. Biotechnol. 2009, 27, 1091–1094. [Google Scholar] [CrossRef]
- Yoder, R.A.; Johnston, J.N. A Case Study in Biomimetic Total Synthesis: Polyolefin Carbocyclizations to Terpenes and Steroids. Chem. Rev. 2005, 105, 4730–4756. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, X.; Renata, H. Enzymatic CH functionalizations for natural product synthesis. Curr. Opin. Chem. Biol. 2019, 49, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Soisson, S.M.; Young, K.; Shoop, W.; Kodali, S.; Galgoci, A.; Painter, R.; Parthasarathy, G.; Tang, Y.S.; Cummings, R.; et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 2006, 441, 358–361. [Google Scholar] [CrossRef]
- Smanski, M.J.; Peterson, R.M.; Rajski, S.R.; Shen, B. Engineered Streptomyces platensis Strains That Overproduce Antibiotics Platensimycin and Platencin. Antimicrob. Agents Chemother. 2009, 53, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
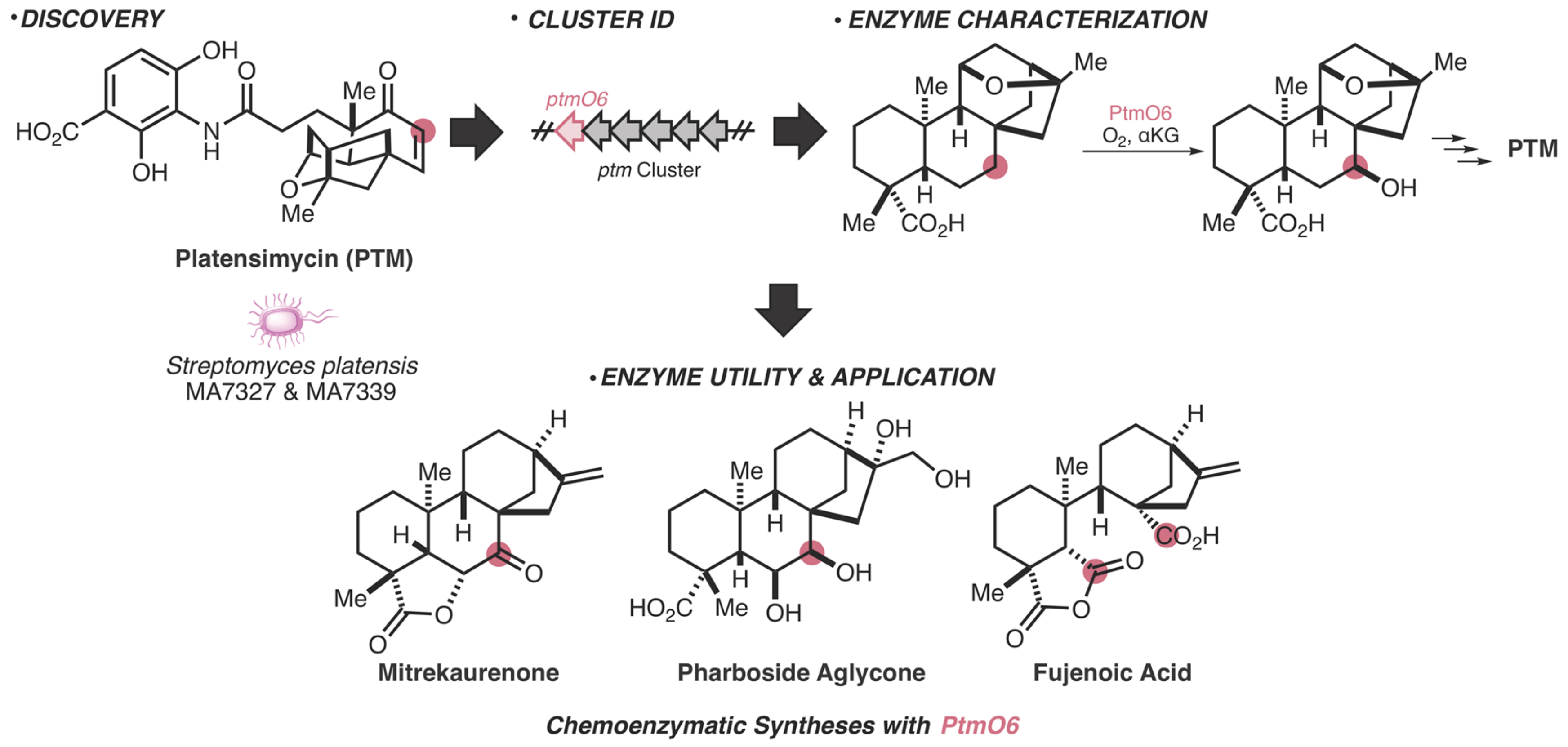
- Dong, L.-B.; Zhang, X.; Rudolf, J.D.; Deng, M.-R.; Kalkreuter, E.; Cepeda, A.J.; Renata, H.; Shen, B. Cryptic and Stereospecific Hydroxylation, Oxidation, and Reduction in Platensimycin and Platencin Biosynthesis. J. Am. Chem. Soc. 2019, 141, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, L.-B.; Yang, L.-C.; Rudolf, J.D.; Shen, B.; Renata, H. Harnessing the Biocatalytic Potential of PtmO6, an α-Ketoglutarate-Dependent Dioxygenase from Platensimycin Biosynthesis, for the Chemoenzymatic Synthesis of Highly Oxidized ent-Kaurane Diterpenes. Chem. Rxiv. 2019. Preprint. [Google Scholar] [CrossRef]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; de Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Amaral-Zettler, L.; Cochrane, G.; Cole, J.R.; Dawyndt, P.; Garrity, G.M.; Gilbert, J.; Glöckner, F.O.; Hirschman, L.; Karsch-Mizrachi, I.; et al. The Genomic Standards Consortium. PLoS Biol. 2011, 9, e1001088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, D.; Okada, B.K.; Wu, Y.; Xu, F.; Seyedsayamdost, M.R. Recent advances in activating silent biosynthetic gene clusters in bacteria. Curr. Opin. Microbiol. 2018, 45, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Huang, A.; Osbourn, A. Plant metabolic clusters—From genetics to genomics. New Phytol. 2016, 211, 771–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nützmann, H.-W.; Scazzocchio, C.; Osbourn, A. Metabolic Gene Clusters in Eukaryotes. Annu. Rev. Genet. 2018, 52, 159–183. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep. 2001, 18, 674–688. [Google Scholar]
- Agrawal, D.C.; Tsay, H.-S.; Shyur, L.-F.; Wu, Y.-C.; Wang, S.-Y. (Eds.) Medicinal Plants and Fungi: Recent Advances in Research and Development; Medicinal and Aromatic Plants of the World; Springer Singapore: Singapore, 2017; Volume 4, ISBN 978-981-10-5977-3. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peláez, F. Handbook of Industrial Mycology; Zhiqiang, A., Ed.; Marcel Dekker: New York, NY, USA, 2005. [Google Scholar]
- Lyu, H.-N.; Liu, H.-W.; Keller, N.P.; Yin, W.-B. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat. Prod. Rep. 2020. In print. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Osbourn, A. Computational genomic identification and functional reconstitution of plant natural product biosynthetic pathways. Nat. Prod. Rep. 2016, 33, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine Natural Products from Microalgae: An -Omics Overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, Y. Dinoflagellates as Sources of Bioactive Molecules. In Pharmaceutical and Bioactive Natural Products; Springer US: Boston, MA, USA, 1993; pp. 391–410. [Google Scholar]
- Torres, J.P.; Schmidt, E.W. The biosynthetic diversity of the animal world. J. Biol. Chem. 2019, 294, 17684–17692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Schmidt, E.W. Parallel lives of symbionts and hosts: Chemical mutualism in marine animals. Nat. Prod. Rep. 2018, 35, 357–378. [Google Scholar] [CrossRef]
- Ireland, C.; Scheuer, P.J. Photosynthetic Marine Mollusks: In vivo 14C Incorporation into Metabolites of the Sacoglossan Placobranchus ocellatus. Science 1979, 205, 922–923. [Google Scholar] [CrossRef]
- Kubanek, J.; Andersen, R.J. Evidence for de Novo Biosynthesis of the Polyketide Fragment of Diaulusterol A by the Northeastern Pacific Dorid Nudibranch Diaulula s andiegensis. J. Nat. Prod. 1999, 62, 777–779. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz, B.D.S.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. Biomed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rey, J.; Wright, L.C.; Van Wagoner, R.M.; Satake, M.; Wright, J.L.C. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1077–1232. [Google Scholar]
- Ahmed, I.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M.N. Microalgae as a source of high-value bioactive compounds. Front. Biosci. 2018, 10, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
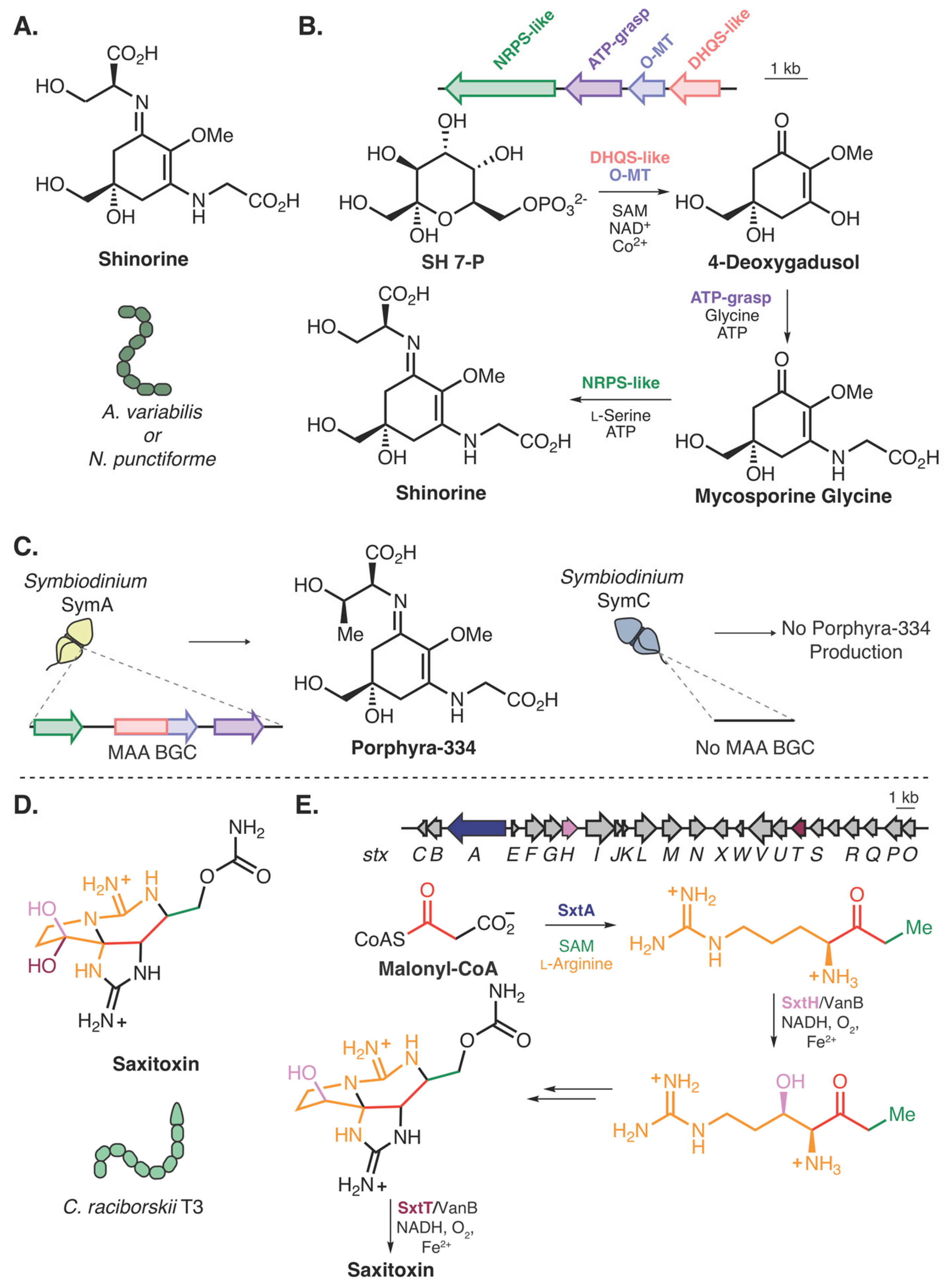
- Shoguchi, E.; Beedessee, G.; Tada, I.; Hisata, K.; Kawashima, T.; Takeuchi, T.; Arakaki, N.; Fujie, M.; Koyanagi, R.; Roy, M.C.; et al. Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genomics 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Dunlap, W.C.; Masaki, K.; Yamamoto, Y.; Larsen, R.M.; Karube, I. A Novel Antioxidant Derived from Seaweed. In New Developments in Marine Biotechnology; Springer US: Boston, MA, USA, 1998; pp. 33–35. [Google Scholar]
- Trione, E.J.; Leach, C.M.; Mutch, J.T. Sporogenic Substances Isolated from Fungi. Nature 1966, 212, 163–164. [Google Scholar] [CrossRef]
- Shinzato, C.; Shoguchi, E.; Kawashima, T.; Hamada, M.; Hisata, K.; Tanaka, M.; Fujie, M.; Fujiwara, M.; Koyanagi, R.; Ikuta, T.; et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 2011, 476, 320–323. [Google Scholar] [CrossRef]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. Elife 2015, 4, e05919. [Google Scholar] [CrossRef] [Green Version]
- Lukowski, A.L.; Narayan, A.R.H. Natural Voltage-Gated Sodium Channel Ligands: Biosynthesis and Biology. ChemBioChem 2019, 20, 1231–1241. [Google Scholar] [CrossRef]
- Hackett, J.D.; Wisecaver, J.H.; Brosnahan, M.L.; Kulis, D.M.; Anderson, D.M.; Bhattacharya, D.; Plumley, F.G.; Erdner, D.L. Evolution of Saxitoxin Synthesis in Cyanobacteria and Dinoflagellates. Mol. Biol. Evol. 2013, 30, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-Z.; Zhang, S.-F.; Zhang, Y.; Lin, L. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. J. Proteomics 2016, 135, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, R.; Mihali, T.K.; Jeon, Y.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.W.; Hinze, M.E.; Skiba, M.A.; Narayan, A.R.H. Chemistry of a Unique Polyketide-like Synthase. J. Am. Chem. Soc. 2018, 140, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, A.L.; Ellinwood, D.C.; Hinze, M.E.; DeLuca, R.J.; Du Bois, J.; Hall, S.; Narayan, A.R.H. C–H Hydroxylation in Paralytic Shellfish Toxin Biosynthesis. J. Am. Chem. Soc. 2018, 140, 11863–11869. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, A.L.; Denomme, N.; Hinze, M.E.; Hall, S.; Isom, L.L.; Narayan, A.R.H. Biocatalytic Detoxification of Paralytic Shellfish Toxins. ACS Chem. Biol. 2019, 14, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Sako, Y.; Yoshida, T.; Uchida, A.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and characterization of a sulfotransferase specific to N-21 of saxitoxin and gonyautoxin 2+3 from the toxic dinoflagellate Gymodinium catenatum (dinophyceae). J. Phycol. 2001, 37, 1044–1051. [Google Scholar] [CrossRef]
- Yoshida, T.; Sako, Y.; Uchida, A.; Kakutani, T.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and characterization of sulfotransferase specific to O-22 of 11-hydroxy saxitoxin from the toxic dinoflagellate Gymnodinium catenatum (dinophyceae). Fish. Sci. 2002, 68, 634–642. [Google Scholar] [CrossRef]
- Granéli, E.; Hansen, P.J. Allelopathy in Harmful Algae: A Mechanism to Compete for Resources? In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–201. [Google Scholar]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; De Vogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef]
- Rao, D.V.S.; Quilliam, M.A.; Pocklington, R. Domoic Acid—A Neurotoxic Amino Acid Produced by the Marine Diatom Nitzschia pungens in Culture. Can. J. Fish. Aquat. Sci. 1988, 45, 2076–2079. [Google Scholar] [CrossRef]
- Takemoto, T.; Daigo, K.; Kondo, Y.; Kondo, K. Studies on the Constituents of Chondria armata. VIII: On the Structure of Domoic Acid. (1). Yakugaku Zasshi 1966, 86, 874–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larm, J.A.; Beart, P.M.; Cheung, N.S. Neurotoxin domoic acid produces cytotoxicity via kainate- and ampa-sensitive receptors in cultured cortical neurones. Neurochem. Int. 1997, 31, 677–682. [Google Scholar] [CrossRef]
- Stewart, G.R.; Zorumski, C.F.; Price, M.T.; Olney, J.W. Domoic acid: A dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp. Neurol. 1990, 110, 127–138. [Google Scholar] [CrossRef]
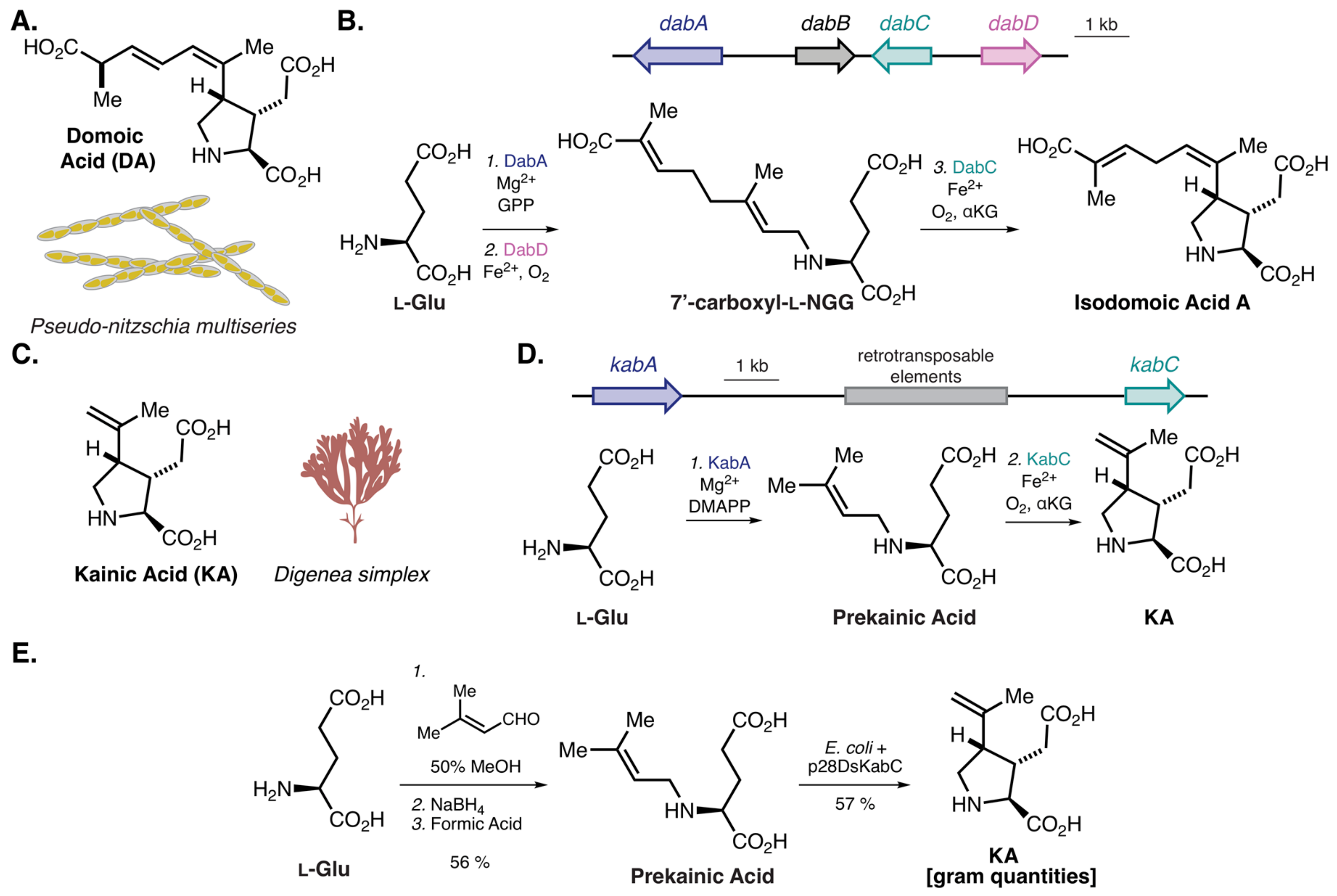
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekan, J.R.; McKinnie, S.M.K.; Moore, M.L.; Poplawski, S.G.; Michael, T.P.; Moore, B.S. Scalable Biosynthesis of the Seaweed Neurochemical, Kainic Acid. Angew. Chemie Int. Ed. 2019, 58, 8454–8457. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Takemoto, T.; Shimizu, Z. Studies on the Effective Principles of Digenea simplex Aq. I. Yakugaku Zasshi 1953, 73, 1026–1028. [Google Scholar] [CrossRef] [Green Version]
- Michael, T.P.; Jupe, F.; Bemm, F.; Motley, S.T.; Sandoval, J.P.; Lanz, C.; Loudet, O.; Weigel, D.; Ecker, J.R. High contiguity Arabidopsis thaliana genome assembly with a single nanopore flow cell. Nat. Commun. 2018, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Stathakis, C.I.; Yioti, E.G.; Gallos, J.K. Total Syntheses of (-)-α-Kainic Acid. Eur. J. Org. Chem. 2012, 2012, 4661–4673. [Google Scholar] [CrossRef]
- Zhang, M.; Watanabe, K.; Tsukamoto, M.; Shibuya, R.; Morimoto, H.; Ohshima, T. A short scalable route to (-)-α-kainic acid using Pt-catalyzed direct allylic amination. Chemistry 2015, 21, 3937–3941. [Google Scholar] [CrossRef]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Hanukoglu, I. Steroidogenic enzymes: Structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1992, 43, 779–804. [Google Scholar] [CrossRef] [Green Version]
- Oetting, W.S. The Tyrosinase Gene and Oculocutaneous Albinism Type 1 (OCA1): A Model for Understanding the Molecular Biology of Melanin Formation. Pigment Cell Res. 2000, 13, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Samuelsson, B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc. Natl. Acad. Sci. USA 1974, 71, 3400–3404. [Google Scholar] [CrossRef] [Green Version]
- Bellés, X.; Martín, D.; Piulachs, M.-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005, 50, 181–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Schooley, D.A.; Hall, M.S.; Judy, K.J. Juvenile hormone biosynthesis: Homomevalonate and mevalonate synthesis by insect corpus allatum enzymes. J. Chem. Soc. Chem. Commun. 1978, 7, 290–292. [Google Scholar] [CrossRef]
- Calestani, C.; Rast, J.P.; Davidson, E.H. Isolation of pigment cell specific genes in the sea urchin embryo by differential macroarray screening. Development 2003, 130, 4587–4596. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.S. Biosynthesis of marine natural products: Macroorganisms (Part B). Nat. Prod. Rep. 2006, 23, 615. [Google Scholar] [CrossRef]
- Salomon, C.; Ellisman, M.; Faulkner, D.; Deerinck, T. The cellular localization of dercitamide in the Palauan sponge Oceanapia sagittaria. Mar. Biol. 2001, 139, 313–319. [Google Scholar]
- Koljak, R.; Järving, I.; Kurg, R.; Boeglin, W.E.; Varvas, K.; Valmsen, K.; Ustav, M.; Brash, A.R.; Samel, N. The Basis of Prostaglandin Synthesis in Coral. J. Biol. Chem. 2001, 276, 7033–7040. [Google Scholar] [CrossRef] [Green Version]
- Kerr, R.G.; Vicchiarelli, R.; Kerr, S.S. Identification and Biosynthetic Origins of Sterols in the Marine Bryozoan Bugula neritina. J. Nat. Prod. 1999, 62, 468–470. [Google Scholar] [CrossRef]
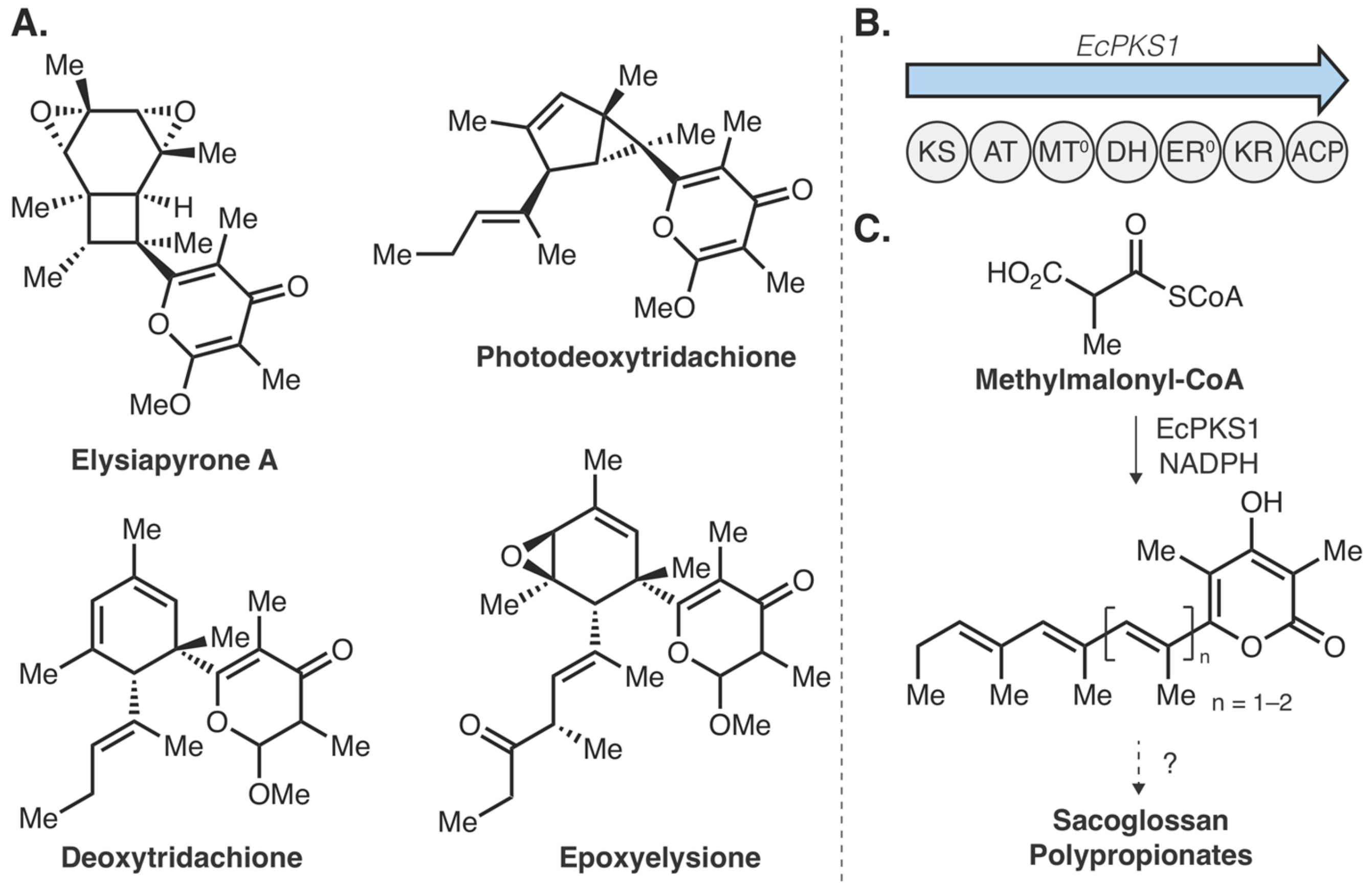
- Torres, J.P.; Lin, Z.; Winter, J.M.; Krug, P.J.; Schmidt, E.W. Animal biosynthesis of complex polyketides in a photosynthetic partnership. bioRxiv 2019, 764225. [Google Scholar]
- Pelletreau, K.N.; Bhattacharya, D.; Price, D.C.; Worful, J.M.; Moustafa, A.; Rumpho, M.E. Sea slug kleptoplasty and plastid maintenance in a metazoan. Plant Physiol. 2011, 155, 1561–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zan, J.; Li, Z.; Tianero, M.D.; Davis, J.; Hill, R.T.; Donia, M.S. A microbial factory for defensive kahalalides in a tripartite marine symbiosis. Science 2019, 364, eaaw6732. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Cimino, G.; Villani, G.; Fontana, A. Shaping the Polypropionate Biosynthesis in the Solar-Powered Mollusc Elysia viridis. ChemBioChem 2009, 10, 315–322. [Google Scholar] [CrossRef]
- de Vries, J.; Woehle, C.; Christa, G.; Wägele, H.; Tielens, A.G.M.; Jahns, P.; Gould, S.B. Comparison of sister species identifies factors underpinning plastid compatibility in green sea slugs. Proc. Biol. Sci. 2015, 282, 20142519. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.X.; Vaysberg, P.; Price, D.C.; Pelletreau, K.N.; Rumpho, M.E.; Bhattacharya, D. Active Host Response to Algal Symbionts in the Sea Slug Elysia chlorotica. Mol. Biol. Evol. 2018, 35, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Rauch, C.; Christa, G.; de Vries, J.; Woehle, C.; Gould, S.B. Mitochondrial Genome Assemblies of Elysia timida and Elysia cornigera and the Response of Mitochondrion-Associated Metabolism during Starvation. Genome Biol. Evol. 2017, 9, 1873–1879. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Li, Q.; Fang, X.; Li, J.; Curtis, N.E.; Altenburger, A.; Shibata, T.; Feng, M.; Maeda, T.; Schwartz, J.A.; et al. A draft genome assembly of the solar-powered sea slug Elysia chlorotica. Sci. Data 2019, 6, 190022. [Google Scholar] [CrossRef]
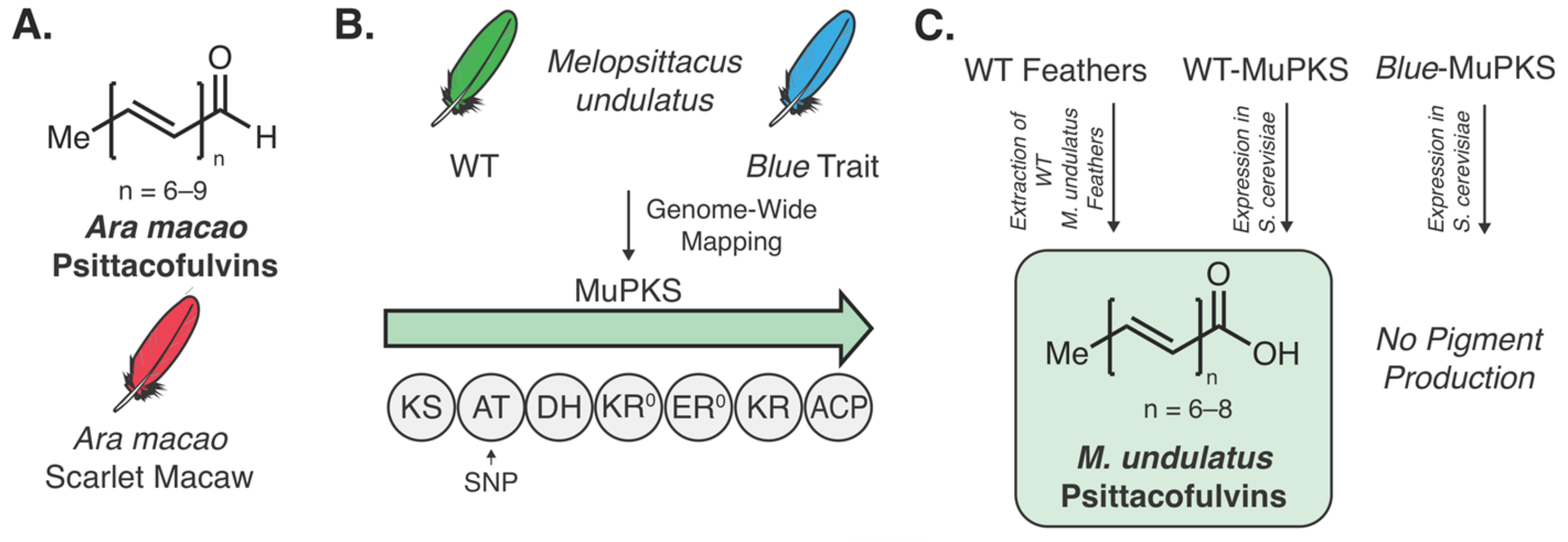
- Cooke, T.F.; Fischer, C.R.; Wu, P.; Jiang, T.-X.; Xie, K.T.; Kuo, J.; Doctorov, E.; Zehnder, A.; Khosla, C.; Chuong, C.-M.; et al. Genetic Mapping and Biochemical Basis of Yellow Feather Pigmentation in Budgerigars. Cell 2017, 171, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Gizzi, A.S.; Grove, T.L.; Arnold, J.J.; Jose, J.; Jangra, R.K.; Garforth, S.J.; Du, Q.; Cahill, S.M.; Dulyaninova, N.G.; Love, J.D.; et al. A naturally occurring antiviral ribonucleotide encoded by the human genome. Nature 2018, 558, 610–614. [Google Scholar] [CrossRef]
- Hill, G.E.; McGraw, K.J. Bird Coloration; Harvard University Press: Cambridge, MA, USA, 2006; ISBN 9780674018938. [Google Scholar]
- Stradi, R.; Pini, E.; Celentano, G. The chemical structure of the pigments in Ara macao plumage. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2001, 130, 57–63. [Google Scholar] [CrossRef]
- McGraw, K.J.; Nogare, M.C. Distribution of unique red feather pigments in parrots. Biol. Lett. 2005, 1, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Auber, L. Colours of feathers and their structural causes in varieties of the budgerigar, Melopsittacus undulatus (Shaw). Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1941. [Google Scholar]
- D’Alba, L.; Kieffer, L.; Shawkey, M.D.; Williamson, S.; Goodman, S.M. Relative contributions of pigments and biophotonic nanostructures to natural color production: A case study in budgerigar (Melopsittacus undulatus) feathers. J. Exp. Biol. 2012, 215, 1272–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, H. Vererbungsstudien am Wellensittich Melopsittacus undulatus (SHAW): Ein kasuistischer Beitrag zum Domestikationsproblem. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 1932. [Google Scholar]
- Taylor, T.G.; Warner, C. Genetics for Budgerigar Breedersrs; Iliffe Books: London, UK, 1961; ISBN 9780592080314. [Google Scholar]
- Stork, N.E.; McBroom, J.; Gely, C.; Hamilton, A.J. New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. Proc. Natl. Acad. Sci. USA 2015, 112, 7519–7523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, E.D. Royal Society of Chemistry (Great Britain) Biosynthesis in insects; Royal Society of Chemistry: London, UK, 2010; ISBN 9781847558084. [Google Scholar]
- Beran, F.; Rahfeld, P.; Luck, K.; Nagel, R.; Vogel, H.; Wielsch, N.; Irmisch, S.; Ramasamy, S.; Gershenzon, J.; Heckel, D.G.; et al. Novel family of terpene synthases evolved from trans-isoprenyl diphosphate synthases in a flea beetle. Proc. Natl. Acad. Sci. USA 2016, 113, 2922–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, J.; Lehner, B.; Khrimian, A.; Muchlinski, A.; Luck, K.; Köllner, T.G.; Weber, D.C.; Gundersen-Rindal, D.E.; Tholl, D. An IDS-Type Sesquiterpene Synthase Produces the Pheromone Precursor (Z)-α-Bisabolene in Nezara viridula. J. Chem. Ecol. 2019, 45, 187–197. [Google Scholar] [CrossRef]
- Hartwig, S.; Dovengerds, C.; Herrmann, C.; Hovemann, B.T. Drosophila Ebony: A novel type of nonribosomal peptide synthetase related enzyme with unusually fast peptide bond formation kinetics. FEBS J. 2014, 281, 5147–5158. [Google Scholar] [CrossRef]
- Richardt, A.; Kemme, T.; Wagner, S.; Schwarzer, D.; Marahiel, M.A.; Hovemann, B.T. Ebony, a novel nonribosomal peptide synthetase for beta-alanine conjugation with biogenic amines in Drosophila. J. Biol. Chem. 2003, 278, 41160–41166. [Google Scholar] [CrossRef] [Green Version]
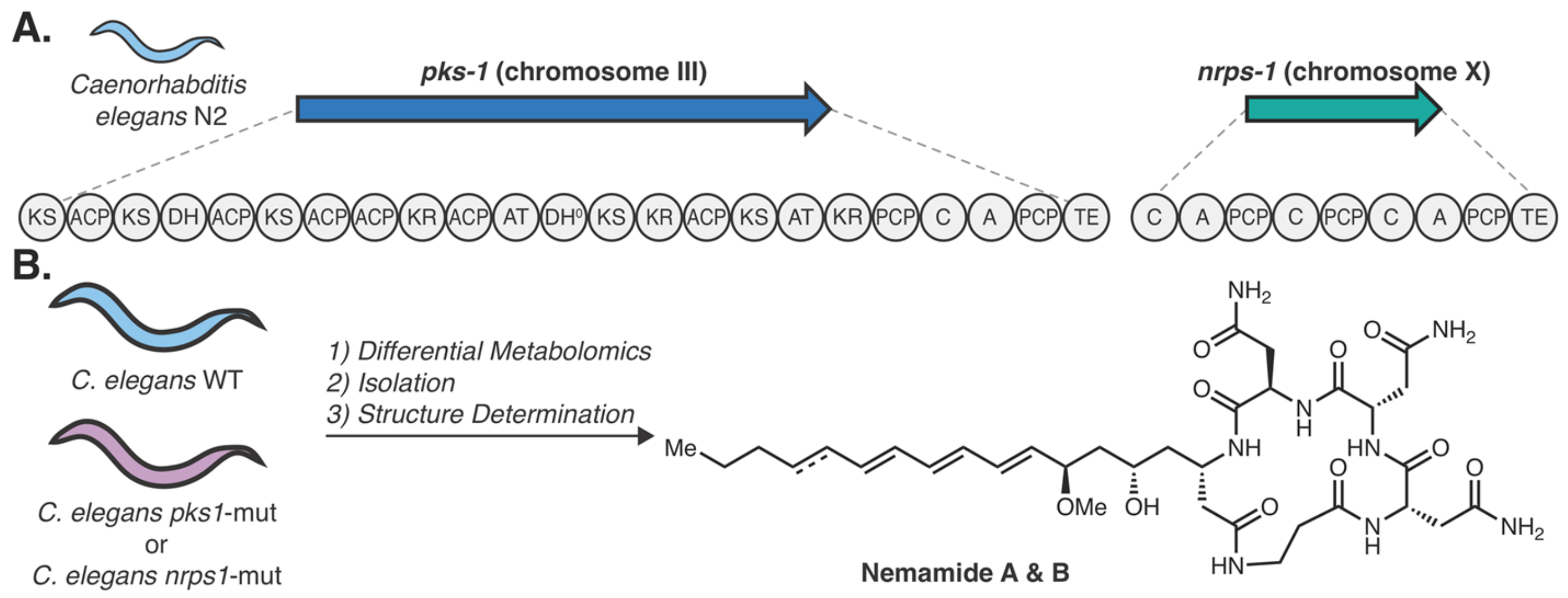
- Shou, Q.; Feng, L.; Long, Y.; Han, J.; Nunnery, J.K.; Powell, D.H.; Butcher, R.A. A hybrid polyketide–nonribosomal peptide in nematodes that promotes larval survival. Nat. Chem. Biol. 2016, 12, 770–772. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.-C.; Lin, H.-C.; Li, D.; Zou, Y.; Li, J.; Xu, W.; Cacho, R.A.; Hillenmeyer, M.E.; Garg, N.K.; Tang, Y. Discovery of Unclustered Fungal Indole Diterpene Biosynthetic Pathways through Combinatorial Pathway Reassembly in Engineered Yeast. J. Am. Chem. Soc. 2015, 137, 13724–13727. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, R.V.; Davis, R.W.; Khosla, C.; Hillenmeyer, M.E. Computational identification and analysis of orphan assembly-line polyketide synthases. J. Antibiot. (Tokyo) 2014, 67, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, R.A. Natural products as chemical tools to dissect complex biology in C. elegans. Curr. Opin. Chem. Biol. 2019, 50, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, K.D.; Grzegorski, S.J.; Chin, Y.; Higuchi, L.N.; Wilkinson, C.J.; Shavit, J.A.; Kramer, K.L. Zebrafish otolith biomineralization requires polyketide synthase. Mech. Dev. 2019, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Omi, A.; Hamanaka, G.; Shindo, K.; Shimada, A.; Kondo, M.; Narita, T.; Kiyomoto, M.; Katsuyama, Y.; Ohnishi, Y.; et al. Unexpected link between polyketide synthase and calcium carbonate biomineralization. Zool. Lett. 2015, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Sibley, L.D. Intracellular Parasite Invasion Strategies. Science 2004, 304, 248–253. [Google Scholar] [CrossRef]
- Fast, N.M.; Kissinger, J.C.; Roos, D.S.; Keeling, P.J. Nuclear-Encoded, Plastid-Targeted Genes Suggest a Single Common Origin for Apicomplexan and Dinoflagellate Plastids. Mol. Biol. Evol. 2001, 18, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.T.; Keeling, P.J. Nucleus-Encoded, Plastid-Targeted Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Indicates a Single Origin for Chromalveolate Plastids. Mol. Biol. Evol. 2003, 20, 1730–1735. [Google Scholar] [CrossRef] [Green Version]
- Ganley, J.G.; Toro-Moreno, M.; Derbyshire, E.R. Exploring the Untapped Biosynthetic Potential of Apicomplexan Parasites. Biochemistry 2018, 57, 365–375. [Google Scholar] [CrossRef]
- Nagamune, K.; Hicks, L.M.; Fux, B.; Brossier, F.; Chini, E.N.; Sibley, L.D. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 2008, 451, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Su, C.-Y.; Schaber, C.; Crowley, J.R.; Hsu, F.-F.; Carlson, J.R.; Odom, A.R. Malaria parasites produce volatile mosquito attractants. MBio 2015, 6, e00235-15. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, H.K.; Derbyshire, E.R. Investigating the Role of Class I Adenylate-Forming Enzymes in Natural Product Biosynthesis. ACS Chem. Biol. 2019, 15, 17–27. [Google Scholar] [CrossRef]
- Zhu, G.; LaGier, M.J.; Stejskal, F.; Millership, J.J.; Cai, X.; Keithly, J.S. Cryptosporidium parvum: The first protist known to encode a putative polyketide synthase. Gene 2002, 298, 79–89. [Google Scholar] [CrossRef]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B.; et al. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genomics 2015, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, J.; Striepen, B. Make it or take it: Fatty acid metabolism of apicomplexan parasites. Eukaryot. Cell 2007, 6, 1727–1735. [Google Scholar] [CrossRef] [Green Version]
- Marrakchi, H.; Lanéelle, M.-A.; Daffé, M. Mycolic Acids: Structures, Biosynthesis, and Beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef] [Green Version]
- Tandel, J.; English, E.D.; Sateriale, A.; Gullicksrud, J.A.; Beiting, D.P.; Sullivan, M.C.; Pinkston, B.; Striepen, B. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019, 4, 2226–2236. [Google Scholar] [CrossRef] [Green Version]
- Suarez, C.E.; Bishop, R.P.; Alzan, H.F.; Poole, W.A.; Cooke, B.M. Advances in the application of genetic manipulation methods to apicomplexan parasites. Int. J. Parasitol. 2017, 47, 701–710. [Google Scholar] [CrossRef]
- Tran, P.N.; Yen, M.-R.; Chiang, C.-Y.; Lin, H.-C.; Chen, P.-Y. Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi. Appl. Microbiol. Biotechnol. 2019, 103, 3277–3287. [Google Scholar] [CrossRef] [Green Version]
- Harvey, C.J.B.; Tang, M.; Schlecht, U.; Horecka, J.; Fischer, C.R.; Lin, H.-C.; Li, J.; Naughton, B.; Cherry, J.; Miranda, M.; et al. HEx: A heterologous expression platform for the discovery of fungal natural products. Sci. Adv. 2018, 4, eaar5459. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganley, J.G.; Derbyshire, E.R. Linking Genes to Molecules in Eukaryotic Sources: An Endeavor to Expand Our Biosynthetic Repertoire. Molecules 2020, 25, 625. https://doi.org/10.3390/molecules25030625
Ganley JG, Derbyshire ER. Linking Genes to Molecules in Eukaryotic Sources: An Endeavor to Expand Our Biosynthetic Repertoire. Molecules. 2020; 25(3):625. https://doi.org/10.3390/molecules25030625
Chicago/Turabian StyleGanley, Jack G., and Emily R. Derbyshire. 2020. "Linking Genes to Molecules in Eukaryotic Sources: An Endeavor to Expand Our Biosynthetic Repertoire" Molecules 25, no. 3: 625. https://doi.org/10.3390/molecules25030625




