A New Valuable Synthesis of Polyfunctionalized Furans Starting from β-Nitroenones and Active Methylene Compounds
Abstract
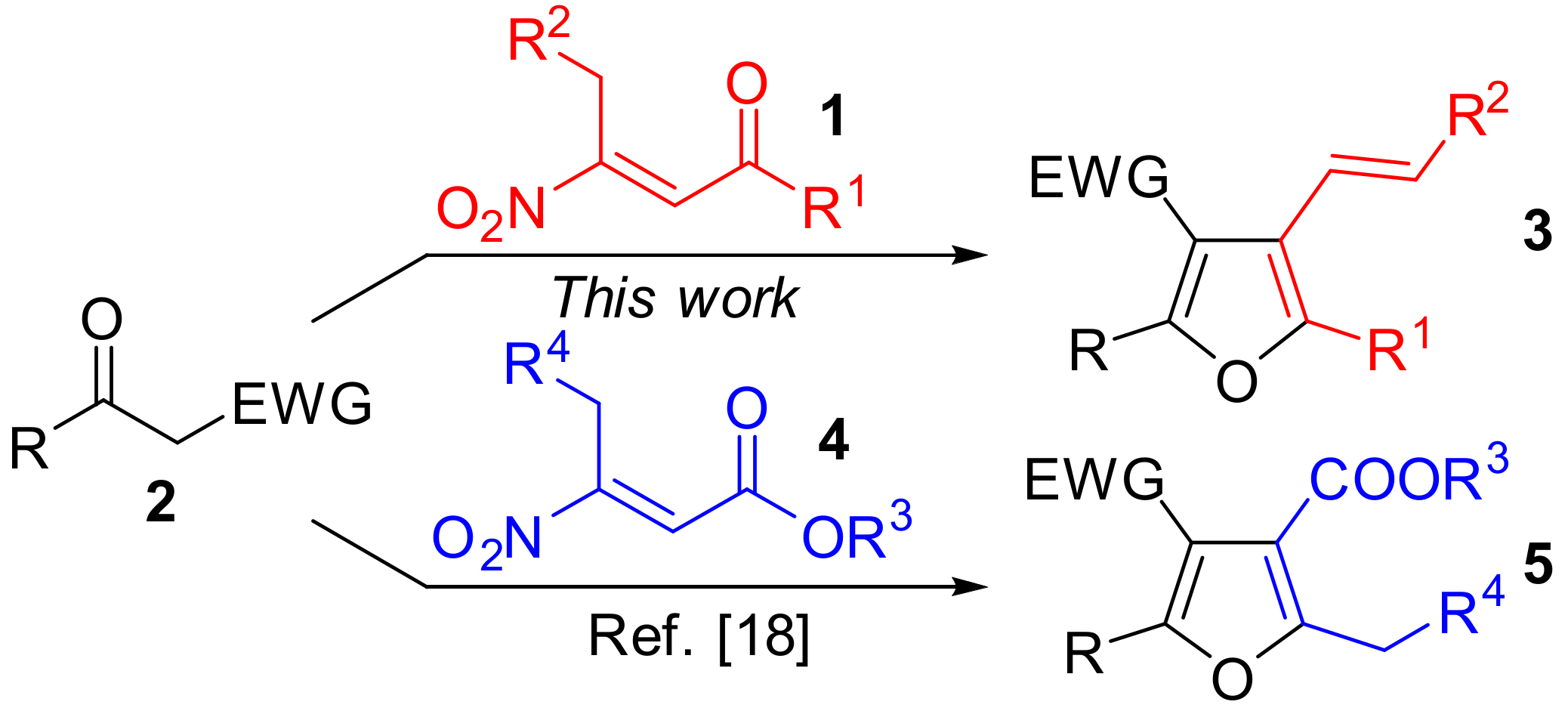
1. Introduction
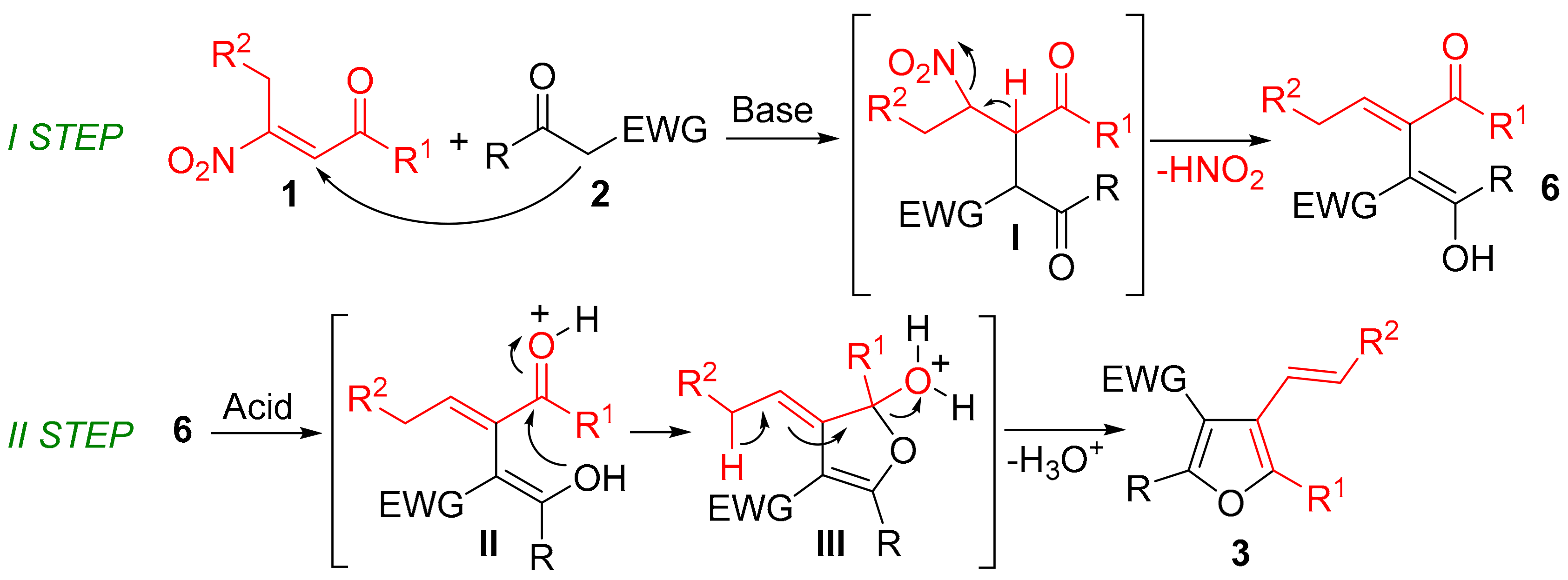
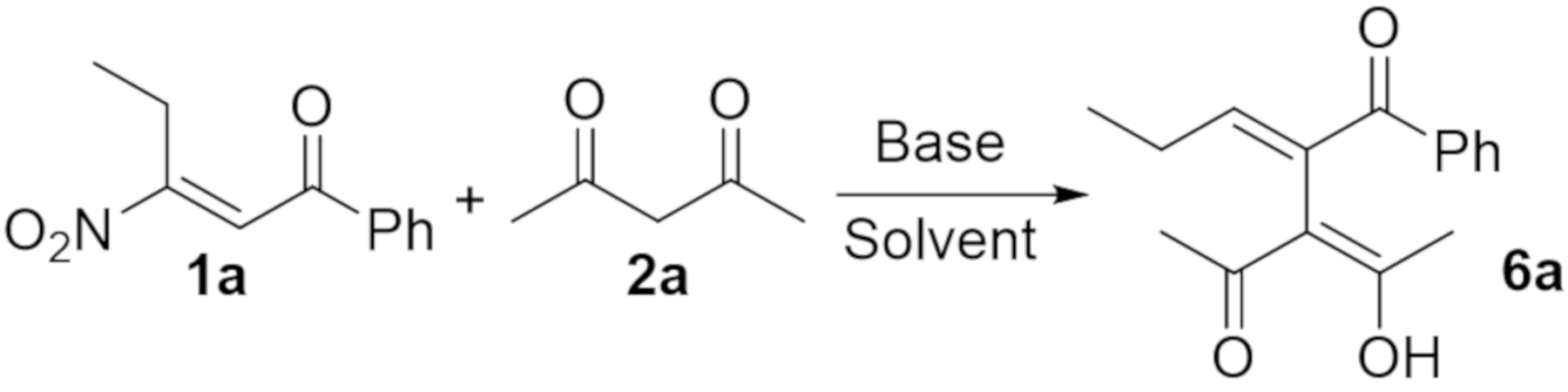
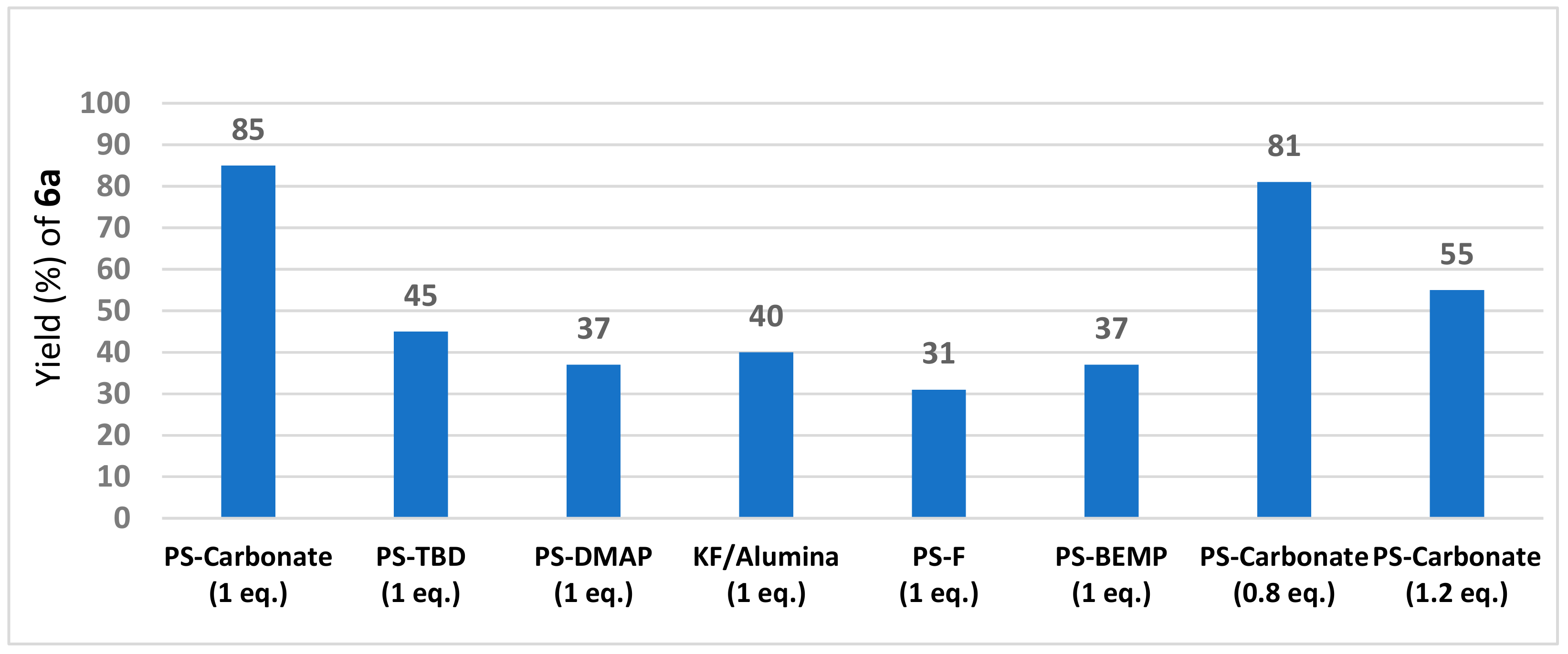
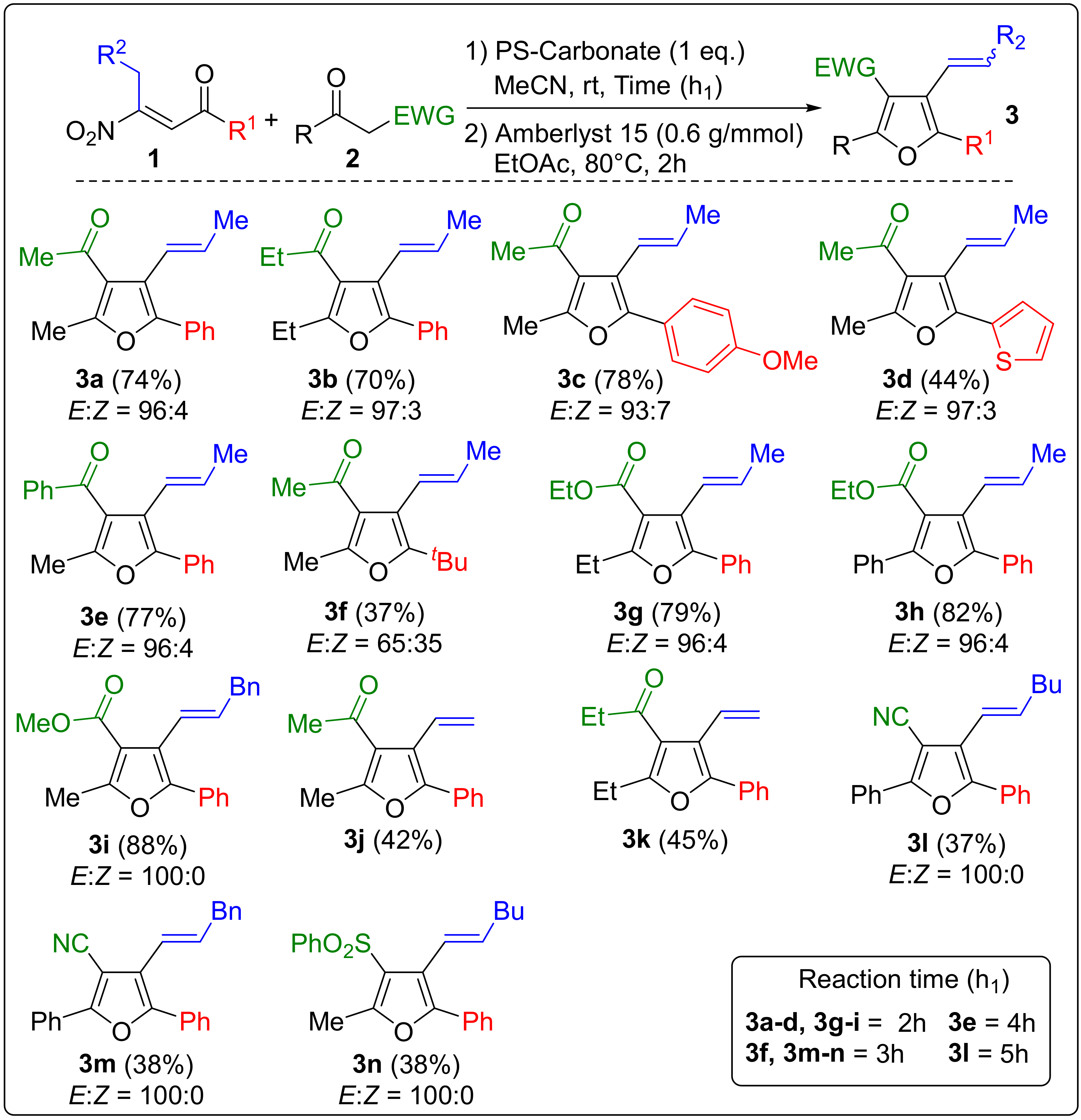
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Section
4.2. Chemistry Section
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PS-carbonate | carbonate on polymer support (Sigma-Aldrich code: 21850: loading: 3.5 mmol/g) |
| PS-DMAP | 4-(dimethylamino)pyridine, polymer-bound (Sigma-Aldrich code: 39410, loading: 3 mmol/g) |
| PS-BEMP | 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine, polymer-bound (Sigma-Aldrich code: 20026, loading: 2.2 mmol/g) |
| PS-TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene bound to polystyrene (Sigma-Aldrich code: 01961, loading: 3.0 mmol/g) |
| PS-F | fluoride on polymer support (Sigma-Aldrich code: 47060, loading: 3.0 mmol/g) |
| KF/alumina | potassium fluoride on aluminum oxide (Sigma-Aldrich code: 60244, loading: 5.5 mmol/g) |
References
- Lipshutz, B.H. Five-membered heteroaromatic rings as intermediates in organic synthesis. Chem. Rev. 1986, 865, 795–819. [Google Scholar] [CrossRef]
- Merritt, A.T.; Ley, S.V. Clerodane diterpenoids. Nat. Prod. Rep. 1992, 9, 243–287. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, D.S.; Rodriguez, A.L.; Carlson, K.E.; Sun, J.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Synthesis and biological evaluation of a novel series of furans: ligands selective for estrogen receptor α. J. Med. Chem. 2001, 44, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.L.; Carvalho, A.J.F.; Trovatti, E. Progress of polymers from renewable resources: Furans, vegetable oils, and polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Ramasastry, S.S.V. Taming furfuryl cations for the synthesis of privileged structures and novel scaffolds. Org. Biomol. Chem. 2013, 11, 4299–4303. [Google Scholar] [CrossRef] [PubMed]
- Yet, L. Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Hou, X.L.; Cheung, H.Y.; Hon, T.Y.; Kwan, P.L.; Lo, T.H.; Tong, S.Y.; Wong, H.N.C. Regioselective syntheses of substituted furans. Tetrahedron 1998, 51, 1955–2020. [Google Scholar] [CrossRef]
- Melzig, L.; Rauhut, C.B.; Knochel, P. 2,3-Functionalization of furans, benzofurans and thiophenes via magnesiation and sulfoxide-magnesium exchange. Chem. Commun. 2009, 3536–3538. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.; Krüger, L. The preparation of 2,3,5-tri- and 2,3-disubstituted furans by regioselective palladium(0)-catalyzed coupling reactions: Application to the syntheses of rosefuran and the F5 furan fatty acid. Eur. J. Org. Chem. 1999, 2045–2057. [Google Scholar] [CrossRef]
- Moran, W.J.; Rodríguez, A. Metal-catalyzed furan synthesis. A review. Org. Prep. Proced. Int. 2012, 44, 103–130. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Ballini, R. Efficient two-step sequence for the synthesis of 2,5-disubstituted furanderivatives from functionalized nitroalkanes: Successive AmberlystA21- and Amberlyst 15-catalyzed processes. Chem. Commun. 2010, 46, 6165–6167. [Google Scholar] [CrossRef] [PubMed]
- Minkler, S.R.K.; Isley, N.A.; Lippincott, D.J.; Krause, N.; Lipshutz, B.H. Leveraging the micellar effect: Gold-catalyzed dehydrative cyclizations in water at room temperature. Org. Lett. 2014, 16, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Palisse, A.; Kirsch, S.F. Synthesis of furans through silver-catalyzed propargyl–Claisen rearrangement followed by cyclocondensation. Eur. J. Org. Chem. 2014, 32, 7095–7098. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Gabrielli, S.; Ballini, R.; Palmieri, A. Two-Step synthesis of polysubstituted 6-nitroindoles under flow chemical and microwave conditions. Adv. Synth. Catal. 2017, 359, 3407–3413. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Parlapiano, M.; Ballini, R. One-pot synthesis of alkyl pyrrole-2-carboxylates starting from β-nitroacrylates and primary amines. RSC Adv. 2015, 5, 4210–4213. [Google Scholar] [CrossRef]
- Chiurchiù, E.; Patehebieke, Y.; Gabrielli, S.; Ballini, R.; Palmieri, A. β-Nitroacrylates as starting materials of thiophene-2-carboxylates under continuous flow conditions. Adv. Synth. Catal. 2019, 361, 2042–2047. [Google Scholar] [CrossRef]
- Gabrielli, S.; Chiurchiù, E.; Palmieri, A. β-Nitroacrylates: A versatile and growing class of functionalized nitroalkenes. Adv. Synth. Catal. 2019, 361, 630–653. [Google Scholar] [CrossRef]
- Ballini, R.; Gabrielli, S.; Palmieri, A. β-Nitroacrylates as precursors of tetrasubstituted furans in a one-pot process and under acidic solvent-free conditions. Synlett 2010, 2468–2470. [Google Scholar] [CrossRef]
- Ballini, R.; Palmieri, A. Formation of carbon-carbon double bonds: Recent developments via nitrous acid elimination (NAE) from aliphatic nitro compounds. Adv. Synth. Catal. 2019, 361, 5070–5097. [Google Scholar] [CrossRef]
- Ballini, R.; Gabrielli, S.; Palmieri, A.; Petrini, M. A two-step synthesis of unsymmetrical 1,4-disubstituted carbazoles from sulfonylindoles under heterogeneous catalysis. Adv. Synth. Catal. 2010, 352, 2459–2462. [Google Scholar] [CrossRef]
- Kadota, J.; Chatani, N.; Murai, S. A platinum complex-catalyzed reaction of 3-chloro-1,3-diene monoepoxides with carbon nucleophiles involving nucleophilic substitution at the central carbon atom of the π-allyl ligand in the intermediate complex. Dependency of regioselectivity upon the added Lewis acids. Tetrahedron 2000, 56, 2231–2237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–n are available from the authors. |
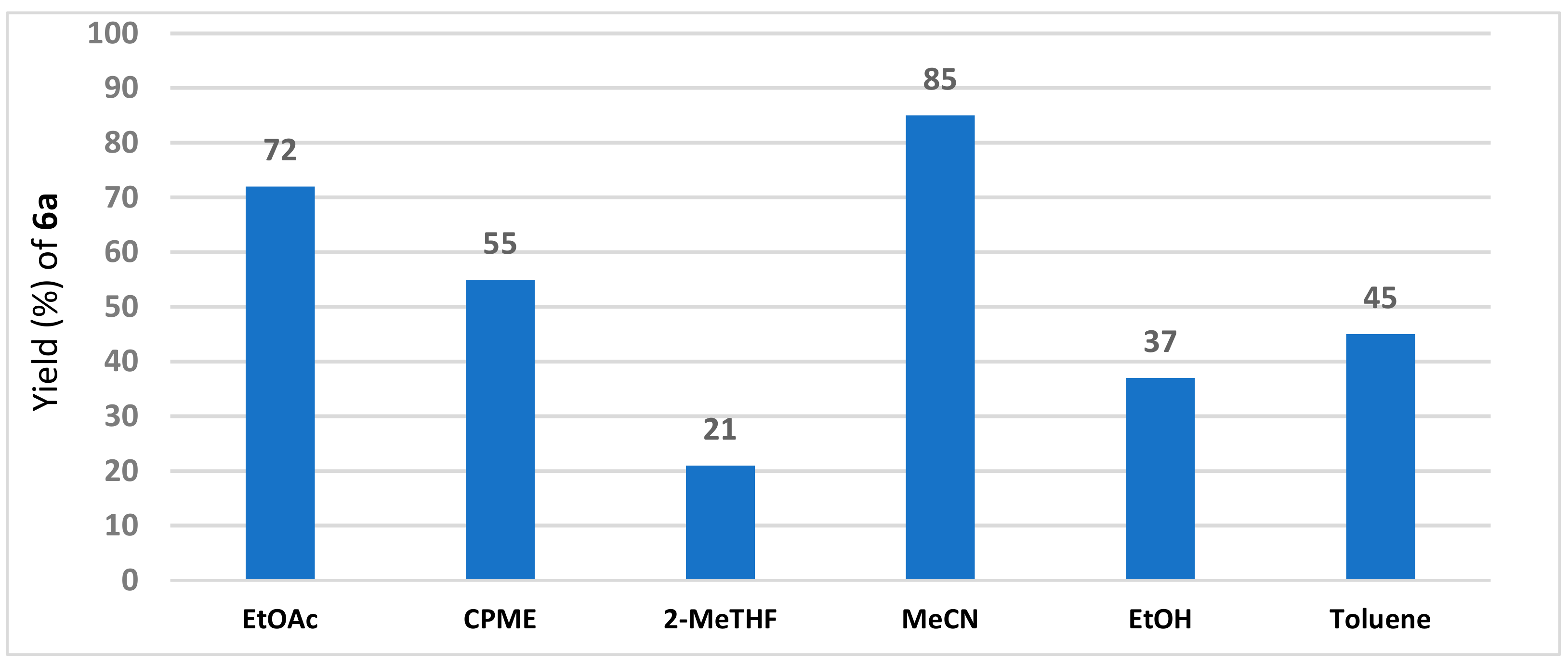
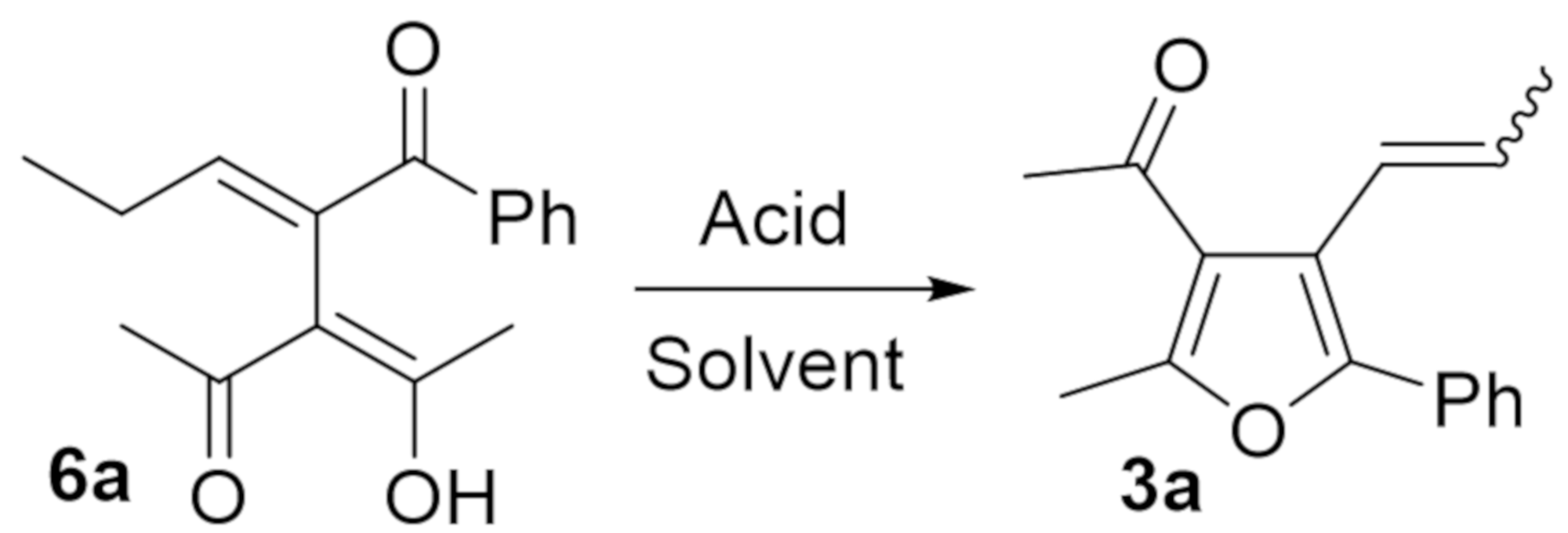
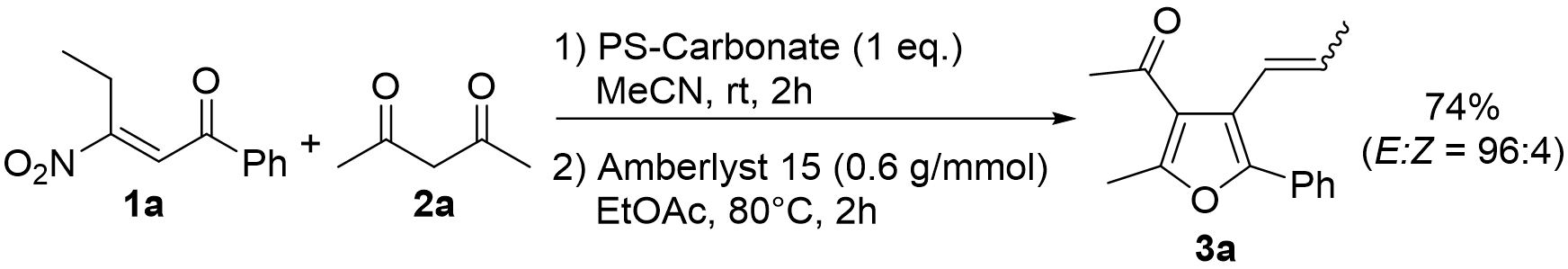
| Entry | Amberlyst 15 (g/mmol) | Solvent | Temperature (°C) 1 | Time (h) | Yield (%) of 3a 2 | E:Z |
|---|---|---|---|---|---|---|
| a | 1 | MeCN | 80 | 2 | 53 | 96:4 |
| b | 1 | EtOAc | 80 | 2 | 76 | 96:4 |
| c | 1 | Toluene | 80 | 2 | 68 | 82:18 |
| d | 1 | 2-MeTHF | 80 | 2 | 58 | 90:10 |
| e | 1 | EtOAc | 100 | 1 | 74 | 90:10 |
| f | 1 | EtOAc | 60 | 4 | 45 | 96:4 |
| g | 1.2 | EtOAc | 80 | 2 | 82 | 96:4 |
| h | 0.8 | EtOAc | 80 | 2 | 89 | 96:4 |
| i | 0.6 | EtOAc | 80 | 2 | 91 | 96:4 |
| j | 0.4 | EtOAc | 80 | 2 | 85 | 96:4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiurchiù, E.; Gabrielli, S.; Ballini, R.; Palmieri, A. A New Valuable Synthesis of Polyfunctionalized Furans Starting from β-Nitroenones and Active Methylene Compounds. Molecules 2019, 24, 4575. https://doi.org/10.3390/molecules24244575
Chiurchiù E, Gabrielli S, Ballini R, Palmieri A. A New Valuable Synthesis of Polyfunctionalized Furans Starting from β-Nitroenones and Active Methylene Compounds. Molecules. 2019; 24(24):4575. https://doi.org/10.3390/molecules24244575
Chicago/Turabian StyleChiurchiù, Elena, Serena Gabrielli, Roberto Ballini, and Alessandro Palmieri. 2019. "A New Valuable Synthesis of Polyfunctionalized Furans Starting from β-Nitroenones and Active Methylene Compounds" Molecules 24, no. 24: 4575. https://doi.org/10.3390/molecules24244575
APA StyleChiurchiù, E., Gabrielli, S., Ballini, R., & Palmieri, A. (2019). A New Valuable Synthesis of Polyfunctionalized Furans Starting from β-Nitroenones and Active Methylene Compounds. Molecules, 24(24), 4575. https://doi.org/10.3390/molecules24244575







