Application of Erythromycin and/or Raoultella sp. Strain MC3 Alters the Metabolic Activity of Soil Microbial Communities as Revealed by the Community Level Physiological Profiling Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Analyses
2.2. Analysis of the Data
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Devries, S.L.; Zhang, P. Antibiotics and the Terrestrial Nitrogen Cycle: A Review. Curr. Pollut. Rep. 2016, 2, 51–67. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total. Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Wang, J.; Zhou, H.; Jiang, C. The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotoxicol. Environ. Saf. 2016, 134, 43–52. [Google Scholar] [CrossRef]
- Orlewska, K.; Piotrowska-Seget, Z.; Cycoń, M. Use of the PCR-DGGE Method for the Analysis of the Bacterial Community Structure in Soil Treated with the Cephalosporin Antibiotic Cefuroxime and/or Inoculated with a Multidrug-Resistant Pseudomonas putida Strain MC1. Front. Microbiol. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.; Fu, J.; Zhou, Z.-Q.; Zhang, N.; Guo, L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fertil. Soils 2014, 50, 939–947. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlatesTM. Eur. J. Soil Biol. 2015, 68, 69–76. [Google Scholar] [CrossRef]
- Cycoń, M.; Orlewska, K.; Markowicz, A.; Żmijowska, A.; Smoleń-Dzirba, J.; Bratosiewicz-Wąsik, J.; Wąsik, T.J.; Piotrowska-Seget, Z. Vancomycin and/or Multidrug-Resistant Citrobacter Freundii Altered the Metabolic Pattern of Soil Microbial Community. Front. Microbiol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- On behalf of the ESAC Project Group; Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, vi37–vi45. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Liang, J.-H.; Han, X. Structure-activity relationships and mechanism of action of macrolides derived from erythromycin as antibacterial agents. Curr. Top. Med. Chem. 2013, 13, 3131–3164. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Antolović, R. From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials. Antibiotics 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Dong, D.; Zhang, X.; Sun, C.; Wang, C.; Hua, X.; Zhang, L.; Guo, Z. Occurrence and ecological risk assessment of 22 emerging contaminants in the Jilin Songhua River (Northeast China). Environ. Sci. Pollut. Res. 2018, 25, 24003–24012. [Google Scholar] [CrossRef] [PubMed]
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total. Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total. Environ. 2018, 446–459. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Li, W.; Liu, J.; Cai, Y. Investigation of Fluoroquinolones, Sulfonamides and Macrolides in Long-Term Wastewater Irrigation Soil in Tianjin, China. Bull. Environ. Contam. Toxicol. 2012, 89, 857–861. [Google Scholar] [CrossRef]
- Bin Ho, Y.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total. Environ. 2014, 261–267. [Google Scholar] [CrossRef]
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of Antibiotics in Wastewater-Irrigated Soils and Their Accumulation in Vegetable Crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Y.; Lihong, G.; Liu, J.; Cai, Y. Occurrence and distribution of antibiotics in urban soil in Beijing and Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 11360–11371. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Bester, K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ. Pollut. 2006, 143, 565–571. [Google Scholar] [CrossRef]
- Topp, E.; Renaud, J.; Sumarah, M.W.; Sabourin, L. Reduced persistence of the macrolide antibiotics erythromycin, clarithromycin and azithromycin in agricultural soil following several years of exposure in the field. Sci. Total. Environ. 2016, 562, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Orlewska, K.; Piotrowska-Seget, Z.; Bratosiewicz-Wąsik, J.; Cycoń, M. Characterization of bacterial diversity in soil contaminated with the macrolide antibiotic erythromycin and/or inoculated with a multidrug-resistant Raoultella sp. strain using the PCR-DGGE approach. Appl. Soil Ecol. 2018, 126, 57–64. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Biochemical and microbial soil functioning after application of the insecticide imidacloprid. J. Environ. Sci. 2015, 27, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Borymski, S.; Orlewska, K.; Wąsik, T.J.; Piotrowska-Seget, Z. An Analysis of the Effects of Vancomycin and/or Vancomycin-Resistant Citrobacter freundii Exposure on the Microbial Community Structure in Soil. Front. Microbiol. 2016, 7, 1015. [Google Scholar] [CrossRef]
- Insam, H. A New Set of Substrates Proposed for Community Characterization in Environmental Samples. In Microbial Communities; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1997; pp. 259–260. [Google Scholar]
- Orlewska, K.; Markowicz, A.; Piotrowska-Seget, Z.; Smoleń-Dzirba, J.; Cycoń, M. Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1. Sustainability 2018, 10, 3549. [Google Scholar] [CrossRef]
- Orwin, K.; Wardle, D. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.; Dou, Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Ma, T.; Pan, X.; Chen, L.; Liu, W.; Christie, P.; Luo, Y.; Wu, L. Effects of different concentrations and application frequencies of oxytetracycline on soil enzyme activities and microbial community diversity. Eur. J. Soil Biol. 2016, 76, 53–60. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.; Sun, W.; Xia, Y.; Ma, J.; Fu, J.; Zhang, Z.; Wu, H.; Qian, M. Variations in the fate and biological effects of sulfamethoxazole, norfloxacin and doxycycline in different vegetable-soil systems following manure application. J. Hazard. Mater. 2016, 304, 49–57. [Google Scholar] [CrossRef]
- Kong, W.-D.; Zhu, Y.; Fu, B.-J.; Marschner, P.; He, J.-Z. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environ. Pollut. 2006, 143, 129–137. [Google Scholar] [CrossRef]
- Liu, W.; Pan, N.; Chen, W.; Jiao, W.; Wang, M. Effect of veterinary oxytetracycline on functional diversity of soil microbial community. Plant Soil Environ. 2012, 58, 295–301. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Muñiz, S.; Val, J.; Navarro, E. Effects of 18 pharmaceuticals on the physiological diversity of edaphic microorganisms. Sci. Total. Environ. 2017, 595, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Semedo, M.; Song, B.; Sparrer, T.; Phillips, R.L. Antibiotic Effects on Microbial Communities Responsible for Denitrification and N2O Production in Grassland Soils. Front. Microbiol. 2018, 9, 2121. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Lakzian, A.; Haghnia, G.; Astaraei, A.; Rasouli-Sadaghiani, M.; Ceccherini, M.T.; Datta, R. Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: An incubation study. PLoS ONE 2017, 12, e0180663. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Han, L.; Cui, Y.; Xue, Y.; Cai, L.; Yu, Y. Changes in soil microbial community structure and function associated with degradation and resistance of carbendazim and chlortetracycline during repeated treatments. Sci. Total. Environ. 2016, 572, 1203–1212. [Google Scholar] [CrossRef]
- Demoling, L.A.; Bååth, E.; Greve, G.; Wouterse, M.; Schmitt, H. Effects of sulfamethoxazole on soil microbial communities after adding substrate. Soil Biol. Biochem. 2009, 41, 840–848. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Ying, G.-G.; Luo, Z.; Feng, H. Changes in functional diversity of soil microbial community with addition of antibiotics sulfamethoxazole and chlortetracycline. Appl. Microbiol. Biotechnol. 2011, 95, 1615–1623. [Google Scholar] [CrossRef]
- Cycoń, M.; Żmijowska, A.; Piotrowska-Seget, Z. Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int. J. Environ. Sci. Technol. 2014, 11, 1305–1316. [Google Scholar] [CrossRef]
- Hirth, N.; Topp, E.; Dörfler, U.; Stupperich, E.; Munch, J.C.; Schroll, R. An effective bioremediation approach for enhanced microbial degradation of the veterinary antibiotic sulfamethazine in an agricultural soil. Chem. Biol. Technol. Agric. 2016, 3, 29. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Huang, Y.; Wu, L.; Liu, X.; Luo, Y. Residues and risks of veterinary antibiotics in protected vegetable soils following application of different manures. Chemosphere 2016, 152, 229–237. [Google Scholar] [CrossRef]
- Chen, S.; Chang, C.; Deng, Y.; An, S.; Dong, Y.H.; Zhou, J.; Hu, M.; Zhong, G.; Zhang, L.-H. Fenpropathrin Biodegradation Pathway inBacillussp. DG-02 and Its Potential for Bioremediation of Pyrethroid-Contaminated Soils. J. Agric. Food Chem. 2014, 62, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Karpouzas, D.G.; Walker, A. Factors influencing the ability of Pseudomonas putida epI to degrade ethoprophos in soil. Soil Biol. Biochem. 2000, 32, 1753–1762. [Google Scholar] [CrossRef]
- Ding, G.-C.; Radl, V.; Schloter-Hai, B.; Jechalke, S.; Heuer, H.; Smalla, K.; Schloter, M. Dynamics of Soil Bacterial Communities in Response to Repeated Application of Manure Containing Sulfadiazine. PLoS ONE 2014, 9, e92958. [Google Scholar] [CrossRef] [PubMed]
- Chessa, L.; Pusino, A.; Garau, G.; Mangia, N.P.; Pinna, M.V. Soil microbial response to tetracycline in two different soils amended with cow manure. Environ. Sci. Pollut. Res. 2015, 23, 5807–5817. [Google Scholar] [CrossRef]
- Dunkle, J.A.; Xiong, L.; Mankin, A.S.; Cate, J.H. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. USA 2010, 107, 17152–17157. [Google Scholar] [CrossRef]
- Wei, L.; Qin, K.; Zhao, N.; Noguera, D.R.; Qiu, W.; Zhao, Q.; Kong, X.; Zhang, W.; Kabutey, F.T. Transformation of erythromycin during secondary effluent soil aquifer recharging: Removal contribution and degradation path. J. Environ. Sci. 2017, 51, 173–180. [Google Scholar] [CrossRef]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with azoxystrobin. Environ. Monit. Assess. 2015, 187, 615. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Song, H.-S.; Renslow, R.S.; Fredrickson, J.K.; Lindemann, S.R. Integrating ecological and engineering concepts of resilience in microbial communities. Front. Microbiol. 2015, 6, 1298. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, A.; Amelung, W.; Hollert, H.; Kaestner, M.; Kandeler, E.; Kruse, J.; Miltner, A.; Ottermanns, R.; Pagel, H.; Peth, S.; et al. The impact of chemical pollution on the resilience of soils under multiple stresses: A conceptual framework for future research. Sci. Total Environ. 2016, 568, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Wilmes, P.; Schrader, S. Measuring soil sustainability via soil resilience. Sci. Total. Environ. 2018, 626, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
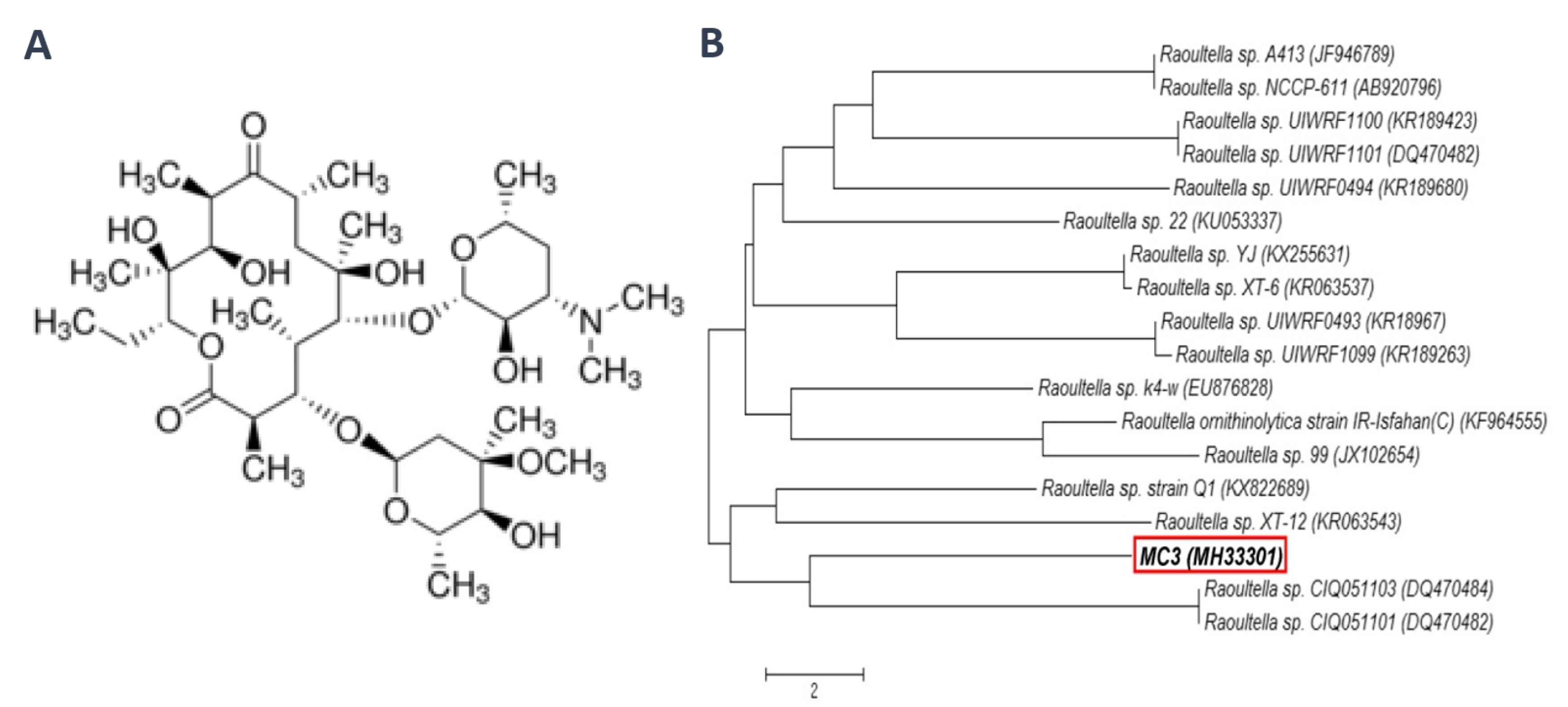

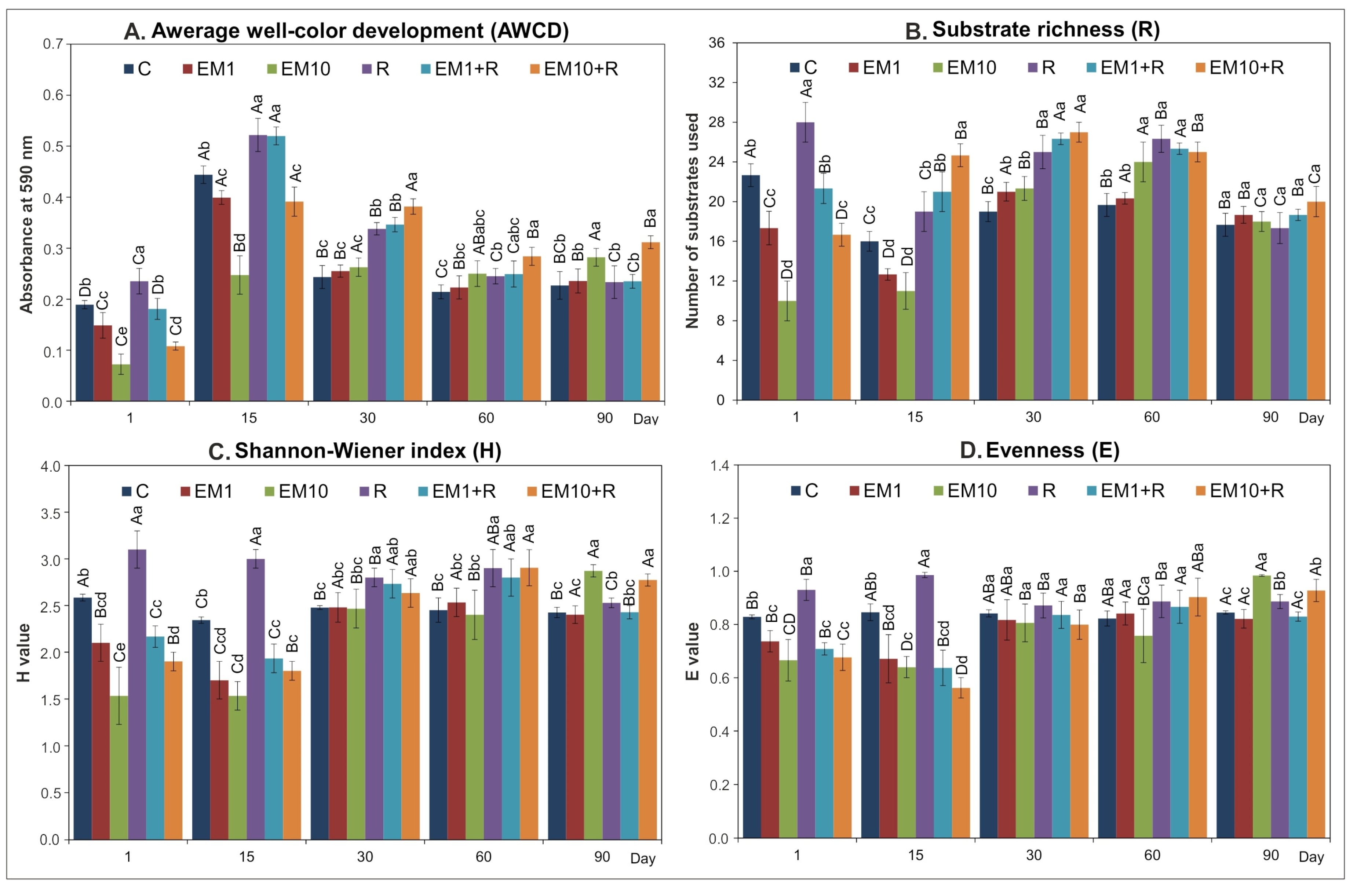
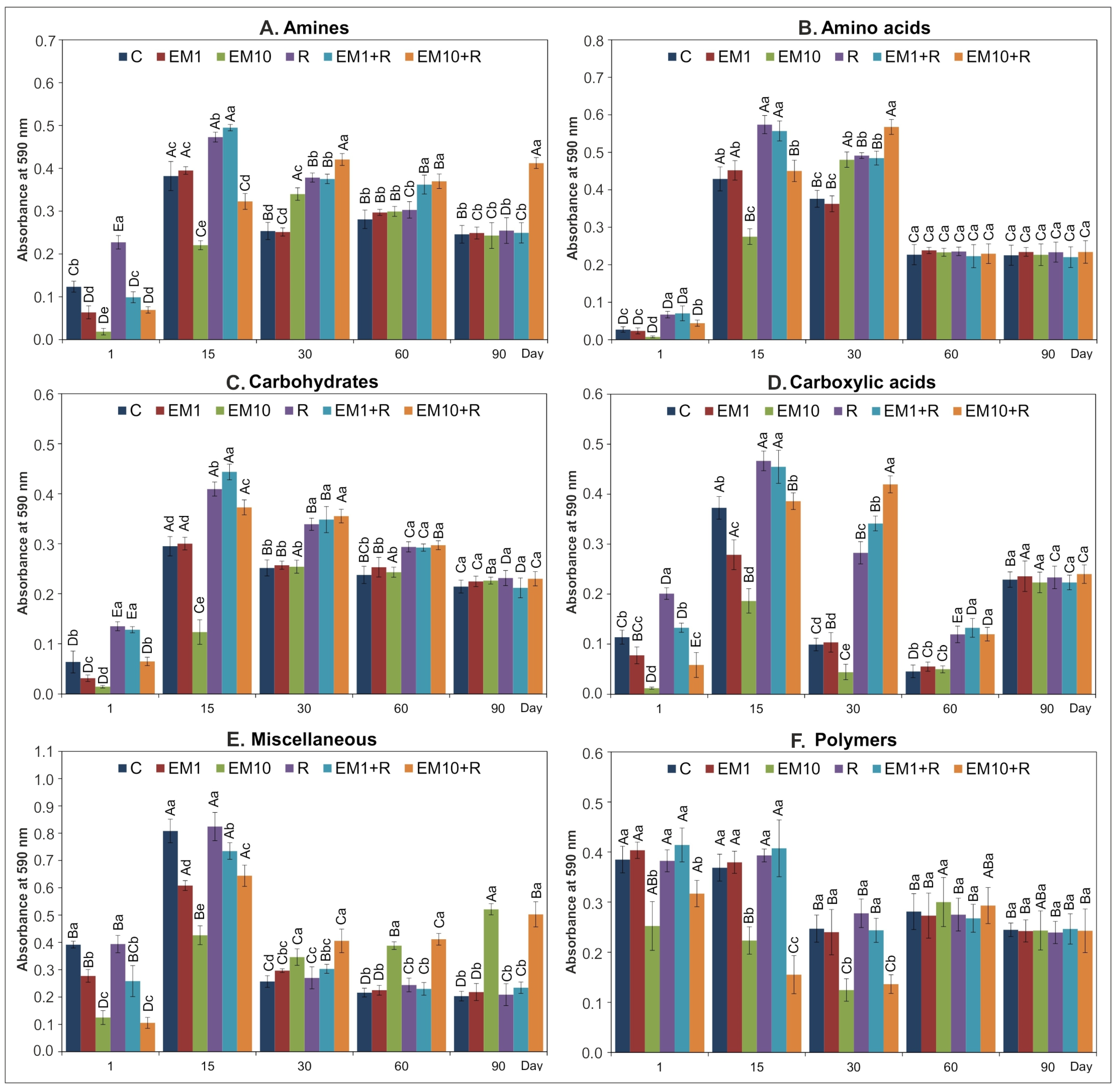
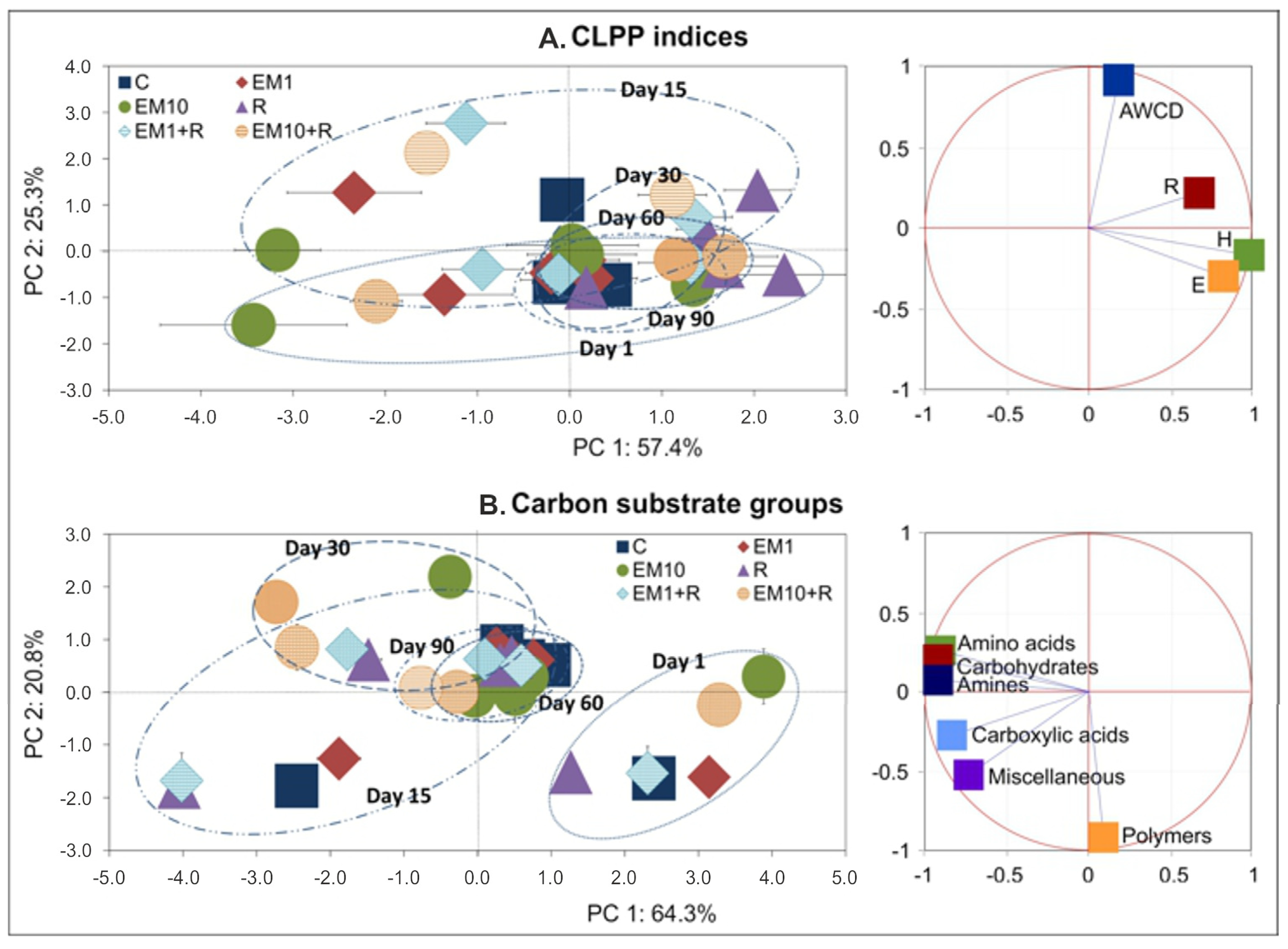

| SV | AWCD | R | H | E | ||||
|---|---|---|---|---|---|---|---|---|
| VE | p | VE | p | VE | p | VE | p | |
| S | <1 | 0.004 ** | <1 | 0.403 | 2 | <0.001 *** | 3 | <0.001 *** |
| C | 11 | <0.001 *** | 2 | <0.001 *** | 5 | <0.001 *** | 8 | <0.001 *** |
| T | 3 | <0.001 *** | 38 | <0.001 *** | 40 | <0.001 *** | 25 | <0.001 *** |
| S × C | <1 | 0.020 * | <1 | 0.586 | <1 | 0.063 | <1 | 0.211 |
| S × T | <1 | <0.001 *** | 3 | <0.001 *** | 1 | 0.001 ** | 4 | 0.005 ** |
| C × T | 55 | <0.001 *** | 47 | <0.001 *** | 46 | <0.001 *** | 36 | <0.001 *** |
| S × C × T | <1 | 0.866 | 2 | 0.029 * | 2 | 0.002 ** | 9 | <0.001 *** |
| SV | Amines | Amino Acids | Carbohydrates | |||
| VE | p | VE | p | VE | p | |
| S | 70 | <0.001 *** | <1 | 0.084 | <1 | <0.001 *** |
| C | <1 | <0.001 *** | 8 | <0.001 *** | 9 | <0.001 *** |
| T | 2 | <0.001 *** | 41 | <0.001 *** | 53 | <0.001 *** |
| S × C | 13 | <0.001 *** | <1 | <0.001 *** | <1 | 0.077 |
| S × T | <1 | <0.001 *** | 2 | <0.001 *** | 1 | <0.001 *** |
| C × T | <1 | <0.001 *** | 47 | <0.001 *** | 36 | <0.001 *** |
| S × C × T | 14 | <0.001 *** | <1 | <0.001 *** | <1 | 0.006 ** |
| SV | Carboxylic Acids | Miscellaneous | Polymers | |||
| VE | p | VE | p | VE | p | |
| S | <1 | 0.001 ** | <1 | 0.002 ** | <1 | 0.013 * |
| C | 6 | <0.001 *** | 18 | <0.001 *** | 11 | <0.001 *** |
| T | 44 | <0.001 *** | 9 | <0.001 *** | 26 | <0.001 *** |
| S × C | <1 | 0.025 * | <1 | <0.001 *** | <1 | 0.001 ** |
| S × T | <1 | <0.001 *** | <1 | 0.080 | 1 | <0.001 *** |
| C × T | 47 | <0.001 *** | 72 | <0.001 *** | 58 | <0.001 *** |
| S × C × T | 1 | <0.001 *** | <1 | <0.001 *** | 1 | 0.002 ** |
| SV | CLPP Indices | Carbon Substrate Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | |||||
| VE | p | VE | p | VE | p | VE | p | |
| S | 1 | <0.001 *** | 1 | 0.072 | <1 | 0.003 ** | <1 | <0.001 *** |
| C | 8 | <0.001 *** | 4 | 0.005 ** | 11 | <0.001 *** | 2 | <0.001 *** |
| T | 35 | <0.001 *** | 25 | <0.001 *** | 34 | <0.001 *** | 46 | <0.001 *** |
| S × C | <1 | 0.268 | <1 | 0.456 | <1 | 0.045 * | <1 | <0.001 *** |
| S × T | 1 | 0.003 ** | 7 | 0.001 ** | <1 | <0.001 *** | 4 | <0.001 *** |
| C × T | 50 | <0.001 *** | 32 | <0.001 *** | 5 | <0.001 *** | 41 | <0.001 *** |
| S × C × T | 1 | 0.005 ** | 10 | 0.002 ** | <1 | 0.446 | 4 | <0.001 *** |
| Day | SV | CLPP Indices | Carbon Substrate Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | ||||||
| VE | p | VE | p | VE | p | VE | p | ||
| 1 | S | 8 | <0.001 *** | <1 | 0.758 | 2 | 0.032 * | <1 | 0.942 |
| C | 86 | <0.001 *** | 10 | 0.379 | 94 | <0.001 *** | 45 | 0.002 ** | |
| S × C | 3 | 0.014 * | 35 | 0.051 | <1 | 0.955 | 31 | 0.007 ** | |
| 15 | S | 5 | 0.009 ** | <1 | 0.849 | 3 | <0.001 *** | 40 | <0.001 *** |
| C | 86 | <0.001 *** | 4 | 0.725 | 96 | <0.001 *** | 33 | <0.001 *** | |
| S × C | 4 | 0.057 | 26 | 0.154 | <1 | 0.080 | 22 | <0.001 *** | |
| 30 | S | 11 | <0.001 *** | 27 | 0.007 ** | <1 | 0.500 | 7 | 0.033 * |
| C | 81 | <0.001 *** | 39 | 0.007 ** | 98 | <0.001 *** | 33 | 0.001 ** | |
| S × C | 3 | 0.066 | 3 | 0.569 | <1 | 0.194 | 47 | <0.001 *** | |
| 60 | S | <1 | 0.592 | 5 | 0.201 | <1 | 0.438 | 13 | 0.027 * |
| C | 92 | <0.001 *** | <1 | 0.959 | 8 | <0.001 *** | 47 | 0.002 ** | |
| S × C | <1 | 0.907 | 66 | <0.001 *** | 3 | 0.214 | 16 | 0.050 * | |
| 90 | S | <1 | 0.332 | 9 | 0.114 | <1 | 0.514 | 59 | <0.001 *** |
| C | 93 | <0.001 *** | 46 | 0.010 * | 99 | <0.001 *** | 1 | 0.210 | |
| S × C | 1 | 0.334 | 6 | 0.435 | <1 | 0.780 | 36 | <0.001 *** | |
| Parameter | Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| EM1 | EM10 | R | EM1+R | EM10+R | |||
| AWCD | 1 | 0.650Bab | 0.237Cc | 0.617BCb | 0.889Aa | 0.399BCbc | 0.558B |
| 15 | 0.817ABa | 0.386BCb | 0.703ABa | 0.382Bb | 0.788Aa | 0.615B | |
| 30 | 0.688ABab | 0.774Aa | 0.438Cbc | 0.403Bc | 0.273Cc | 0.515B | |
| 60 | 0.925Aa | 0.770Aa | 0.907Aa | 0.859Aa | 0.670ABa | 0.826A | |
| 90 | 0.838ABa | 0.592ABb | 0.431Cb | 0.863Aa | 0.574ABb | 0.659B | |
| Substrate richness (R) | 1 | 0.619Cb | 0.283Ec | 0.621Bb | 0.888Aa | 0.581Bb | 0.598B |
| 15 | 0.656Ca | 0.523Db | 0.689Ba | 0.527Bb | 0.297Dc | 0.538B | |
| 30 | 0.814Ba | 0.780Ba | 0.522Cb | 0.442Cc | 0.407Cc | 0.593B | |
| 60 | 0.933Aa | 0.641Cb | 0.493Cc | 0.552Bbc | 0.573Bb | 0.638B | |
| 90 | 0.892ABa | 0.962Aa | 0.947Aa | 0.892Aa | 0.771Ab | 0.893A | |
| Shannon-Wiener index (H) | 1 | 0.687Ba | 0.426Cc | 0.671Cab | 0.722BCa | 0.581Db | 0.617B |
| 15 | 0.572Cbc | 0.487Cc | 0.563Dbc | 0.704Ca | 0.624CDab | 0.590B | |
| 30 | 0.929Aa | 0.903Aab | 0.771Bc | 0.816Bbc | 0.885Aab | 0.861A | |
| 60 | 0.935Aa | 0.917Aa | 0.691BCb | 0.752BCb | 0.688BCb | 0.797A | |
| 90 | 0.973Aa | 0.689Bb | 0.918Aa | 0.951Aa | 0.749Bb | 0.856A | |
| Evenness (E) | 1 | 0.801Ba | 0.676Bb | 0.784BCab | 0.747Bab | 0.691Bab | 0.740B |
| 15 | 0.659Ca | 0.607Bab | 0.716Ca | 0.603Cab | 0.497Cb | 0.616C | |
| 30 | 0.889ABab | 0.769ABb | 0.928Aa | 0.938Aa | 0.906Aa | 0.886A | |
| 60 | 0.957Aa | 0.847Aab | 0.858ABab | 0.902Aab | 0.826Ab | 0.878A | |
| 90 | 0.938Aab | 0.717Bc | 0.908ABab | 0.964Aa | 0.824Abc | 0.870A | |
| Parameter | Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| EM1 | EM10 | R | EM1+R | EM10+R | |||
| AWCD amines | 1 | 0.757Ba | 0.281Cc | 0.414Cb | 0.282Cc | 0.224Dc | 0.392B |
| 15 | 0.344Cb | 0.080Dc | 0.086Dc | 0.666Aa | 0.389Cb | 0.313B | |
| 30 | 0.893Aa | 0.408Bd | 0.614Bc | 0.542Bc | 0.734Ab | 0.638A | |
| 60 | 0.941Aa | 0.491Bb | 0.340Cc | 0.350Cc | 0.205Dd | 0.465B | |
| 90 | 0.891Aa | 0.876Aa | 0.854Aa | 0.543Bb | 0.519Bb | 0.736A | |
| AWCD amino acids | 1 | 0.749Ba | 0.161Cb | −0.194Cc | −0.222Cc | 0.219Cb | 0.143C |
| 15 | 0.897Aa | 0.472Bb | 0.493Bb | 0.539Bb | 0.904Aa | 0.661B | |
| 30 | 0.932Aa | 0.565Bb | 0.530Bb | 0.551Bb | 0.324Bc | 0.580B | |
| 60 | 0.863Ab | 0.889Aab | 0.885Aab | 0.963Aa | 0.976Aa | 0.915A | |
| 90 | 0.886Ab | 0.984Aa | 0.928Aab | 0.956Aab | 0.925Aab | 0.936A | |
| AWCD carbohydrates | 1 | 0.348Bb | 0.137Bc | −0.068Dd | −0.014Dcd | 0.738ABa | 0.228D |
| 15 | 0.957Aa | 0.263Bd | 0.440Cbc | 0.329Cc | 0.582Bb | 0.514C | |
| 30 | 0.945Aa | 0.978Aa | 0.484BCb | 0.444Cb | 0.416Cb | 0.653BC | |
| 60 | 0.879Aa | 0.945Aa | 0.617Bb | 0.625Bb | 0.599Bb | 0.733AB | |
| 90 | 0.907Aa | 0.893Aa | 0.853Aa | 0.950Aa | 0.864Aa | 0.893A | |
| AWCD carboxylic acids | 1 | 0.512CDab | 0.057Cd | 0.128Ccd | 0.709Ba | 0.343Bbc | 0.350C |
| 15 | 0.596Cb | 0.333Bc | 0.596Bb | 0.640Bb | 0.930Aa | 0.619B | |
| 30 | 0.890Aa | 0.285BCb | −0.301Dc | −0.421Cc | −0.529Dc | −0.015C | |
| 60 | 0.306Db | 0.777Aa | −0.244Dc | −0.318Cc | −0.246Cc | 0.055C | |
| 90 | 0.901Aa | 0.950Aa | 0.950Aa | 0.952Aa | 0.909Aa | 0.933A | |
| AWCD miscellaneous | 1 | 0.548Cb | 0.190Cc | 0.934ABa | 0.498Cb | 0.156Cc | 0.465AB |
| 15 | 0.604Cc | 0.358Bd | 0.961Aa | 0.834Ab | 0.662Ac | 0.684A | |
| 30 | 0.731Bb | 0.484Ac | 0.883ABa | 0.693Bb | 0.269Bd | 0.612AB | |
| 60 | 0.923Aa | 0.113Cc | 0.774Cb | 0.886Aa | 0.050Dc | 0.550AB | |
| 90 | 0.876Aa | −0.220Dc | 0.853BCa | 0.739Bb | −0.189Ec | 0.412B | |
| AWCD polymers | 1 | 0.906Aab | 0.488Bd | 0.980Aa | 0.860Bb | 0.700Bc | 0.787AB |
| 15 | 0.942Aa | 0.433BCc | 0.875Bab | 0.821Bb | 0.266Cd | 0.668B | |
| 30 | 0.887Aa | 0.336Cc | 0.778Bb | 0.975Aa | 0.380Cc | 0.671B | |
| 60 | 0.935Aa | 0.881Aa | 0.960ABa | 0.913ABa | 0.917Aa | 0.921A | |
| 90 | 0.948Aa | 0.870Aa | 0.939ABa | 0.910ABa | 0.848Aa | 0.903A | |
| SV/Parameter | AWCD | R | H | E | Amines | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| Tr | 58 | <0.01 ** | 50 | <0.01 ** | 73 | <0.001 *** | 13 | 0.019 * | 37 | <0.01 ** |
| T | 16 | <0.01 ** | 19 | <0.01 ** | 3 | <0.001 *** | 5 | 0.306 | 15 | <0.01 ** |
| Tr × T | 26 | <0.01 ** | 28 | <0.01 ** | 21 | <0.001 *** | 30 | 0.060 | 46 | <0.01 ** |
| SV/Parameter | Amino acids | Carbohydrates | Carboxylic Acids | Miscellaneous | Polymers | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| Tr | 36 | <0.01 ** | 26 | <0.01 ** | 60 | <0.01 ** | 47 | <0.001 *** | 51 | <0.01 ** |
| T | 34 | <0.01 ** | 35 | <0.01 ** | 14 | <0.01 ** | 10 | <0.001 *** | 15 | <0.01 ** |
| Tr × T | 30 | <0.01 ** | 37 | <0.01 ** | 24 | <0.01 ** | 36 | <0.001 *** | 33 | <0.01 ** |
| Parameter | Treatment | |||||
|---|---|---|---|---|---|---|
| EM1 | EM10 | R | EM1+R | EM10+R | ||
| AWCD | 0.294a | 0.338a | −0.325b | −0.148ab | 0.140ab | 0.060 |
| Substrate richness (RS) | 0.702b | 0.950a | 0.841ab | 0.190d | 0.450c | 0.627 |
| Shannon−Wiener index (H) | 0.881a | 0.391b | 0.649ab | 0.750a | 0.324b | 0.599 |
| Evenness (E) | 0.614ab | 0.044c | 0.441ab | 0.791a | 0.291bc | 0.436 |
| AWCD amines | 0.266ab | 0.673a | 0.506ab | 0.073b | 0.104ab | 0.324 |
| AWCD amino acids | −0.486c | 0.841a | 0.654a | 0.777a | 0.323b | 0.422 |
| AWCD carbohydrates | 0.457a | 0.565a | 0.607a | 0.849a | −0.350b | 0.426 |
| AWCD carboxylic acids | 0.516b | 0.890a | 0.872a | 0.521b | 0.658ab | 0.691 |
| AWCD miscellaneous | 0.776a | −0.087b | −0.192b | 0.602a | −0.020b | 0.216 |
| AWCD polymers | 0.462a | 0.775a | −0.248b | 0.469a | 0.572a | 0.406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cycoń, M.; Markowicz, A.; Wąsik, T.J.; Piotrowska-Seget, Z. Application of Erythromycin and/or Raoultella sp. Strain MC3 Alters the Metabolic Activity of Soil Microbial Communities as Revealed by the Community Level Physiological Profiling Approach. Microorganisms 2020, 8, 1860. https://doi.org/10.3390/microorganisms8121860
Cycoń M, Markowicz A, Wąsik TJ, Piotrowska-Seget Z. Application of Erythromycin and/or Raoultella sp. Strain MC3 Alters the Metabolic Activity of Soil Microbial Communities as Revealed by the Community Level Physiological Profiling Approach. Microorganisms. 2020; 8(12):1860. https://doi.org/10.3390/microorganisms8121860
Chicago/Turabian StyleCycoń, Mariusz, Anna Markowicz, Tomasz J. Wąsik, and Zofia Piotrowska-Seget. 2020. "Application of Erythromycin and/or Raoultella sp. Strain MC3 Alters the Metabolic Activity of Soil Microbial Communities as Revealed by the Community Level Physiological Profiling Approach" Microorganisms 8, no. 12: 1860. https://doi.org/10.3390/microorganisms8121860
APA StyleCycoń, M., Markowicz, A., Wąsik, T. J., & Piotrowska-Seget, Z. (2020). Application of Erythromycin and/or Raoultella sp. Strain MC3 Alters the Metabolic Activity of Soil Microbial Communities as Revealed by the Community Level Physiological Profiling Approach. Microorganisms, 8(12), 1860. https://doi.org/10.3390/microorganisms8121860








