Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Data Collection
2.3. Serology
2.4. Statistical Analysis
3. Results
3.1. Participants
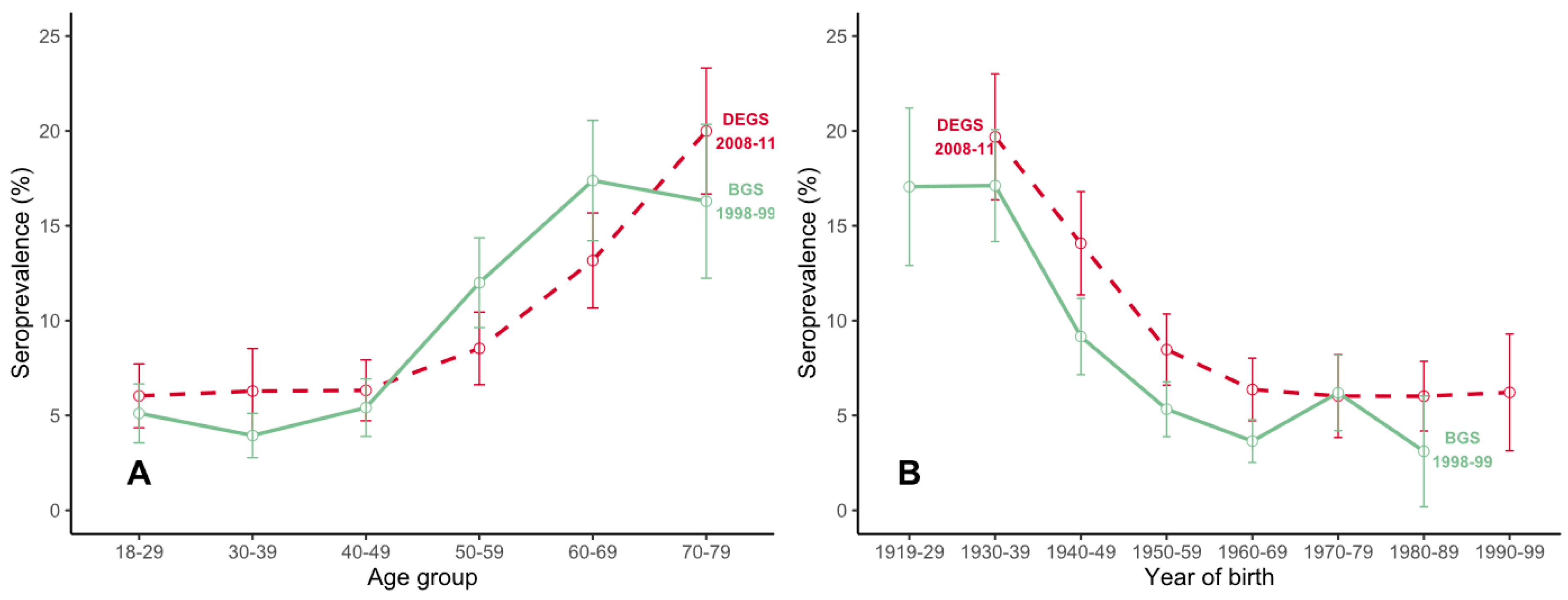
3.2. Trend Analyses between Two Cross-Sectional Studies
3.3. Rates of Seroconversion and Seroreversion
3.4. Seroconversion
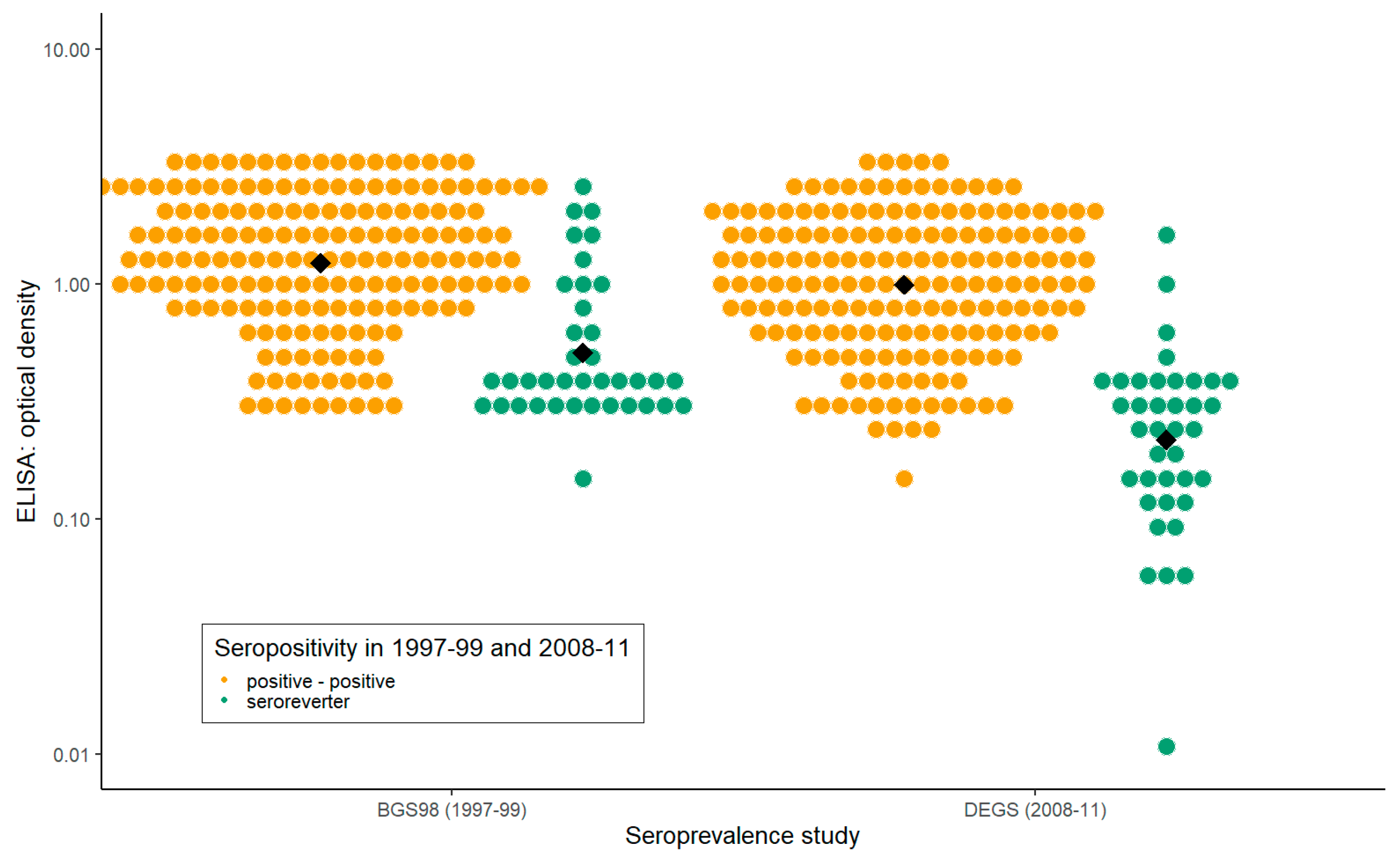
3.5. Seroreversion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanek, G.; Gray, J.; Strle, F.; Wormser, G. Lyme borreliosis. Lancet Infect. Dis. 2004, 4, 197–198. [Google Scholar] [CrossRef]
- Stanek, G.; Fingerle, V.; Hunfeld, K.-P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, H.I.; Böhme, M.; Standaert, S.M.; Karch, H.; Plotkin, S.A. Incidence of Lyme Borreliosis in the Würzburg Region of Germany. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Lyme Disease Data Tables. 2019. Available online: https://www.cdc.gov/lyme/datasurveillance/tables-recent.html (accessed on 19 December 2019).
- Lindgren, E.; Jaenson, T.G.; Menne, B.; WHO. Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures; WHO Regional Office for Europe: Copenhagen, Denmark, 2006. [Google Scholar]
- Enkelmann, J.; Böhmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.-S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. CDC Estimates 300 000 US Cases of Lyme Disease Annually. JAMA 2013, 310, 1110. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Hauffe, H.; Carpi, G.; Vourc’h, G.I.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar]
- Hubálek, Z. Epidemiology of Lyme Borreliosis. Curr. Probl. Dermatol. 2009, 37, 31–50. [Google Scholar] [CrossRef]
- Guy, E.; Martyn, C.; Bateman, D.; Heckels, J.; Lawton, N. Lyme disease: Prevalence and clinical importance of Borrelia burgdorferi specific IgG in forestry workers. Lancet 1989, 333, 484–486. [Google Scholar] [CrossRef]
- Rath, P.M.; Ibershoff, B.; Mohnhaupt, A.; Albig, J.; Eljaschewitsch, B.; Jürgens, D.; Horbach, I.; Fehrenbach, F.J. Seroprevalence of lyme borreliosis in forestry workers from Brandenburg, Germany. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 372–377. [Google Scholar] [CrossRef]
- Zhioua, E.; Rodhain, F.; Binet, P.; Perez-Eid, C. Prevalence of antibodies to Borrelia burgdorferi in forestry workers of Ile de France, France. Eur. J. Epidemiol. 1997, 13, 959–962. [Google Scholar] [CrossRef]
- De Keukeleire, M.; Robert, A.; Luyasu, V.; Kabamba, B.; Vanwambeke, S.O. Seroprevalence of Borrelia burgdorferi in Belgian forestry workers and associated risk factors. Parasites Vectors 2018, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Jurke, A.; Bannert, N.; Brehm, K.; Fingerle, V.; Kempf, V.A.; Kömpf, D.; Lunemann, M.; Mayer-Scholl, A.; Niedrig, M.; Nöckler, K.; et al. Serological survey of Bartonella spp., Borrelia burgdorferi, Brucella spp., Coxiella burnetii, Francisella tularensis, Leptospira spp., Echinococcus, Hanta-, TBE- and XMR-virus infection in employees of two forestry enterprises in North Rhine-Westphalia, Germany, 2011–2013. Int. J. Med. Microbiol. 2015, 305, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Dehnert, M.; Fingerle, V.; Klier, C.; Talaska, T.; Schlaud, M.; Krause, G.; Wilking, H.; Poggensee, G. Seropositivity of Lyme Borreliosis and Associated Risk Factors: A Population-Based Study in Children and Adolescents in Germany (KiGGS). PLoS ONE 2012, 7, e41321. [Google Scholar] [CrossRef] [PubMed]
- Wilking, H.; Fingerle, V.; Klier, C.; Thamm, M.; Stark, K. Antibodies againstBorrelia burgdorferisensu lato among Adults, Germany, 2008–2011. Emerg. Infect. Dis. 2015, 21, 107–110. [Google Scholar] [CrossRef]
- Wilske, B.; Fingerle, V.; Schulte-Spechtel, U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 2007, 49, 13–21. [Google Scholar] [CrossRef]
- Hauser, U.; Lehnert, G.; Lobentanzer, R.; Wilske, B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 1997, 35, 1433–1444. [Google Scholar] [CrossRef]
- Hauser, U.; Lehnert, G.; Wilske, B. Validity of Interpretation Criteria for Standardized Western Blots (Immunoblots) for Serodiagnosis of Lyme Borreliosis Based on Sera Collected throughout Europe. J. Clin. Microbiol. 1999, 37, 2241–2247. [Google Scholar] [CrossRef]
- Thefeld, W.; Stolzenberg, H.; Bellach, B.M. The Federal Health Survey: Response, composition of participants and non-responder analysis. Das Gesundheitswesen 1999, 61, S57–S61. [Google Scholar]
- Scheidt-Nave, C.; Kamtsiuris, P.; Goesswald, A.; Hölling, H.; Lange, M.; Busch, M.A.; Dahm, S.; Dölle, R.; Ellert, U.; Fuchs, J.; et al. German health interview and examination survey for adults (DEGS)—Design, objectives and implementation of the first data collection wave. BMC Public Health 2012, 12, 730. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Fingerle, V.; Hunfeld, K.-P.; Huppertz, H.-I.; Dersch, R. Lyme Neuroborreliosis. Deutsches Ärzteblatt Int. 2018, 115, 751–756. [Google Scholar] [CrossRef]
- Fingerle, V.; Eiffert, H.; Gessner, A.; Göbel, U.B.; Hofmann, H.; Hunfeld, K.P.; Krause, A.; Pfister, H.W.; Sing, A. MiQ12 Lyme-Borreliose: MiQ: Qualitätsstandards in der Mikrobiologisch-Infektiologischen Diagnostik; Urban & Fischer: Jena/Munich, Germany, 2017; pp. 1–68. [Google Scholar]
- Dressler, F.; Ackermann, R.; Steere, A.C. Antibody Responses to the Three Genomic Groups of Borrelia burgdorferi in European Lyme Borreliosis. J. Infect. Dis. 1994, 169, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Schoenborn, C.A. Age adjustment using the 2000 projected US population. Healthy People Stat. Notes 2001, 20, 1–10. [Google Scholar]
- Lumley, T. Survey: Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Boršič, K.; Blagus, R.; Cerar, T.; Strle, F.; Stupica, D. Clinical Course, Serologic Response, and Long-Term Outcome in Elderly Patients with Early Lyme Borreliosis. J. Clin. Med. 2018, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Spielman, A.; Burke, G.S.; Closter, L.; Foley, D.T.; Christianson, D. Reinfection and relapse in early Lyme disease. Am. J. Trop. Med. Hyg. 2006, 75, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Hanincová, K.; Mukherjee, P.; Liveris, D.; Nowakowski, J.; McKenna, D.; Brisson, D.; Cooper, D.; Bittker, S.; Madison, G.; et al. Differentiation of Reinfection from Relapse in Recurrent Lyme Disease. N. Engl. J. Med. 2012, 367, 1883–1890. [Google Scholar] [CrossRef]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin. Infect. Dis. 2001, 33, 780–785. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Nowakowski, J.; Bittker, S.; Cooper, D.; Nadelman, R.B.; Wormser, G.P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 1996, 34, 1–9. [Google Scholar] [CrossRef]
- Branda, J.A.; Body, B.A.; Boyle, J.; Branson, B.M.; Dattwyler, R.J.; Fikrig, E.; Gerald, N.J.; Gomes-Solecki, M.; Kintrup, M.; Ledizet, M.; et al. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin. Infect. Dis. 2017, 66, 1133–1139. [Google Scholar] [CrossRef]
- Robertson, J.; Guy, E.; Andrews, N.; Wilske, B.; Anda, P.; Granström, M.; Hauser, U.; Moosmann, Y.; Sambri, V.; Schellekens, J.; et al. A European multicenter study of immunoblotting in serodiagnosis of lyme borreliosis. J. Clin. Microbiol. 2000, 38, 2097–2102. [Google Scholar] [CrossRef]
- Zhioua, E.; Gern, L.; Aeschlimann, A.; Sauvain, M.; Van Der Linden, S.; Fahrer, H. Longitudinal study of Lyme borreliosis in a high risk population in Switzerland. Parasite 1998, 5, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; McHugh, G.; Damle, N.; Sikand, V.K. Prospective Study of Serologic Tests for Lyme Disease. Clin. Infect. Dis. 2008, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Rebman, A.W.; Crowder, L.A.; Kirkpatrick, A.; Aucott, J.N. Characteristics of seroconversion and implications for diagnosis of post-treatment Lyme disease syndrome: Acute and convalescent serology among a prospective cohort of early Lyme disease patients. Clin. Rheumatol. 2014, 34, 585–589. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number of Seronegative Participants in BGS98 | % Seronegative Participants in BGS98 | Number of Seroconversions | % of Seroconversions | p-Value * | Univariable Analyses | Multivariable Analyses | ||
|---|---|---|---|---|---|---|---|---|---|
| N = 2223 | N = 117 | OR | 95% CI | OR | 95% CI | ||||
| Sex | <0.01 | ||||||||
| Female | 1155 | 52 | 30 | 26 | Ref | ||||
| Male | 1068 | 48 | 87 | 74 | 3.33 | 2.2–5.16 | 3.55 | 2.33–5.57 | |
| Age ° | 0.42 | 1.01 | 0.99–1.02 | 1.01 | 0.99–1.02 | ||||
| 18–29 | 277 | 13 | 11 | 9 | |||||
| 30–39 | 587 | 26 | 30 | 26 | |||||
| 40–49 | 542 | 24 | 31 | 27 | |||||
| 50–59 | 529 | 24 | 29 | 25 | |||||
| 60–69 | 241 | 11 | 13 | 11 | |||||
| 70–79 | 47 | 2 | 3 | 3 | |||||
| Place of residence § | 0.05 | ||||||||
| Baden-Württemberg | 225 | 10 | 14 | 12 | Ref | ||||
| Bavaria | 244 | 11 | 16 | 14 | 1.06 | 0.5–2.25 | 1.01 | 0.47–2.21 | |
| Central | 260 | 12 | 10 | 9 | 0.60 | 0.26–1.38 | 0.58 | 0.24–1.33 | |
| Northwest | 318 | 14 | 15 | 13 | 0.75 | 0.35–1.59 | 0.80 | 0.37–1.75 | |
| North Rhine-Westphalia | 357 | 16 | 10 | 9 | 0.43 | 0.18–0.99 | 0.47 | 0.20–1.10 | |
| East (north) | 416 | 19 | 20 | 17 | 0.76 | 0.38–1.57 | 0.73 | 0.35–1.54 | |
| East (south) | 403 | 18 | 32 | 27 | 1.30 | 0.69–2.57 | 1.28 | 0.66–2.59 | |
| Population | 0.03 | ||||||||
| <5000 | 550 | 25 | 39 | 33 | Ref | ||||
| 5,000–50,000 | 840 | 38 | 48 | 41 | 0.79 | 0.51–1.23 | 0.96 | 0.59–1.54 | |
| 50,000–500,000 | 562 | 25 | 19 (16) | 16 | 0.46 | 0.26–0.79 | 0.57 | 0.31–1.03 | |
| >500,000 | 271 | 12 | 11 | 9 | 0.55 | 0.27–1.06 | 0.79 | 0.36–1.61 | |
| Socio-economic status | 0.50 | ||||||||
| High | 573 | 26 | 28 | 24 | Ref | ||||
| Middle | 1374 | 63 | 78 | 67 | 1.17 | 0.76–1.85 | 1.30 | 0.83–2.09 | |
| Low | 249 | 11 | 10 | 9 | 0.81 | 0.37–1.65 | 0.85 | 0.38–1.76 | |
| Characteristic | Number of Seropositive Participants in 1997–1999 | % of Seropositive Participants in 1997–1999 | Number of Seroreversion | % of All Seroreversions | p-Value * | Univariable Analyses | Multivariable Analyses | ||
|---|---|---|---|---|---|---|---|---|---|
| N = 215 | N = 38 | OR | 95% CI | OR | 95% CI | ||||
| Sex | <0.01 | ||||||||
| Female | 72 | 33 | 21 | 55 | Ref | - | - | - | |
| Male | 143 | 67 | 17 | 45 | 0.33 | 0.16–0.67 | 0.21 | 0.08–0.53 | |
| Age | 0.31 | 0.99 | 0.96–1.01 | 0.97 | 0.94–1.00 | ||||
| 18–29 | 18 | 8 | 6 | 16 | |||||
| 30–39 | 26 | 12 | 6 | 16 | |||||
| 40–49 | 30 | 14 | 3 | 8 | |||||
| 50–59 | 75 | 35 | 11 | 29 | |||||
| 60–69 | 56 | 26 | 11 | 29 | |||||
| 70–79 | 10 | 5 | 1 | 3 | |||||
| Optical density (1997–1999) $ | <0.01 | 0.38 | 0.25–0.54 | 0.39 | 0.24–0.55 | ||||
| <0.582 | 54 | 25 | 27 | 71 | |||||
| 0.582–1.2 | 54 | 25 | 5 | 13 | |||||
| 1.2–2.08 | 53 | 25 | 4 | 11 | |||||
| >=2.08 | 54 | 25 | 2 | 5 | |||||
| Place of residence § | 0.16 | ||||||||
| Baden-Württemberg | 32 | 15 | 7 | 18 | Ref | - | - | - | |
| Bavaria | 32 | 15 | 5 | 13 | 0.66 | 0.18–2.34 | 0.49 | 0.09–2.35 | |
| Central | 35 | 16 | 6 | 16 | 0.74 | 0.21–2.51 | 0.42 | 0.09–1.77 | |
| Northwest | 28 | 13 | 2 | 5 | 0.27 | 0.04–1.27 | 0.25 | 0.03–1.49 | |
| North Rhine-Westphalia | 19 | 9 | 6 | 16 | 1.65 | 0.45–6.01 | 1.62 | 0.29–9.24 | |
| East (north) | 28 | 13 | 8 | 21 | 1.43 | 0.44–4.73 | 1.74 | 0.42–7.39 | |
| East (south) | 41 | 19 | 4 | 11 | 0.39 | 0.09–1.42 | 0.29 | 0.06–1.33 | |
| Population | 0.91 | ||||||||
| <5000 | 62 | 29 | 10 | 26 | Ref | - | - | - | |
| 5000–50,000 | 88 | 41 | 17 | 45 | 1.25 | 0.53–3.03 | 0.63 | 0.20–1.96 | |
| 50,000–500,000 | 51 | 24 | 8 | 21 | 0.97 | 0.34–2.67 | 0.33 | 0.07–1.35 | |
| >500,000 | 14 | 7 | 3 | 8 | 1.42 | 0.28-5.59 | 0.45 | 0.06–3.02 | |
| Socio-economic status | 0.57 | ||||||||
| High | 56 | 26 | 11 | 29 | |||||
| Middle | 132 | 62 | 21 | 55 | 0.77 | 0.35–1.79 | 0.49 | 0.17–1.39 | |
| Low | 25 | 25 | 6 | 16 | 1.29 | 0.4–3.93 | 0.90 | 0.21–3.60 | |
| Cumulative Number of Reactive Antigens or Specific Antigen | Number of Seropositive Participants in 1997–99 | % of all Seropositive | Number of Seroreversions | % of All Seroreversions | p-Value $ | Univariable Analyses | Multivariable Analyses § | ||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||||||
| OspC | 48 | 22 | 3 | 8 | 0.02 | 0.25 | 0.06–0.74 | 0.39 | 0.08–1.38 |
| VlsE | 206 | 96 | 34 | 90 | 0.03 | 0.25 | 0.06–1.04 | 0.39 | 0.08–1.99 |
| p39 | 122 | 57 | 16 | 42 | 0.04 | 0.49 | 0.24–0.99 | 0.97 | 0.42–2.26 |
| p58 | 134 | 62 | 12 | 32 | <0.01 | 0.21 | 0.09–0.43 | 0.51 | 0.20–1.26 |
| p83 | 91 | 42 | 4 | 11 | <0.01 | 0.12 | 0.04–0.32 | 0.27 | 0.06–0.84 |
| DbpA | 133 | 62 | 15 | 40 | <0.01 | 0.33 | 0.16–0.67 | 0.71 | 0.23–2.05 |
| Nr of antigens | <0.01 | ||||||||
| 1 | 26 | 12 | 11 | 29 | Ref | ||||
| 2 | 48 | 22 | 16 | 42 | 0.68 | 0.25–1.83 | |||
| 3 | 39 | 18 | 7 | 18 | 0.30 | 0.09–0.90 | |||
| 4 | 41 | 19 | 1 | 3 | 0.03 | 0.00–0.20 | |||
| 5 | 35 | 16 | 2 | 5 | 0.08 | 0.01–0.36 | |||
| 6 | 26 | 12 | 1 | 3 | 0.05 | 0.00–0.32 | |||
| Total | 215 | 38 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woudenberg, T.; Böhm, S.; Böhmer, M.; Katz, K.; Willrich, N.; Stark, K.; Kuhnert, R.; Fingerle, V.; Wilking, H. Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany. Microorganisms 2020, 8, 1859. https://doi.org/10.3390/microorganisms8121859
Woudenberg T, Böhm S, Böhmer M, Katz K, Willrich N, Stark K, Kuhnert R, Fingerle V, Wilking H. Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany. Microorganisms. 2020; 8(12):1859. https://doi.org/10.3390/microorganisms8121859
Chicago/Turabian StyleWoudenberg, Tom, Stefanie Böhm, Merle Böhmer, Katharina Katz, Niklas Willrich, Klaus Stark, Ronny Kuhnert, Volker Fingerle, and Hendrik Wilking. 2020. "Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany" Microorganisms 8, no. 12: 1859. https://doi.org/10.3390/microorganisms8121859
APA StyleWoudenberg, T., Böhm, S., Böhmer, M., Katz, K., Willrich, N., Stark, K., Kuhnert, R., Fingerle, V., & Wilking, H. (2020). Dynamics of Borrelia burgdorferi-Specific Antibodies: Seroconversion and Seroreversion between Two Population-Based, Cross-Sectional Surveys among Adults in Germany. Microorganisms, 8(12), 1859. https://doi.org/10.3390/microorganisms8121859





