In Vitro Immune Response of Mononuclear Cells to Multidrug-Resistant Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Growth Conditions
2.2. Bacterial Extracts Isolations
2.2.1. Surface Protein Isolation
2.2.2. Bacterial DNA Isolation
2.3. Peripheral Blood Mononuclear Cells (PBMC) Isolation and Stimulation with Bacterial Extracts
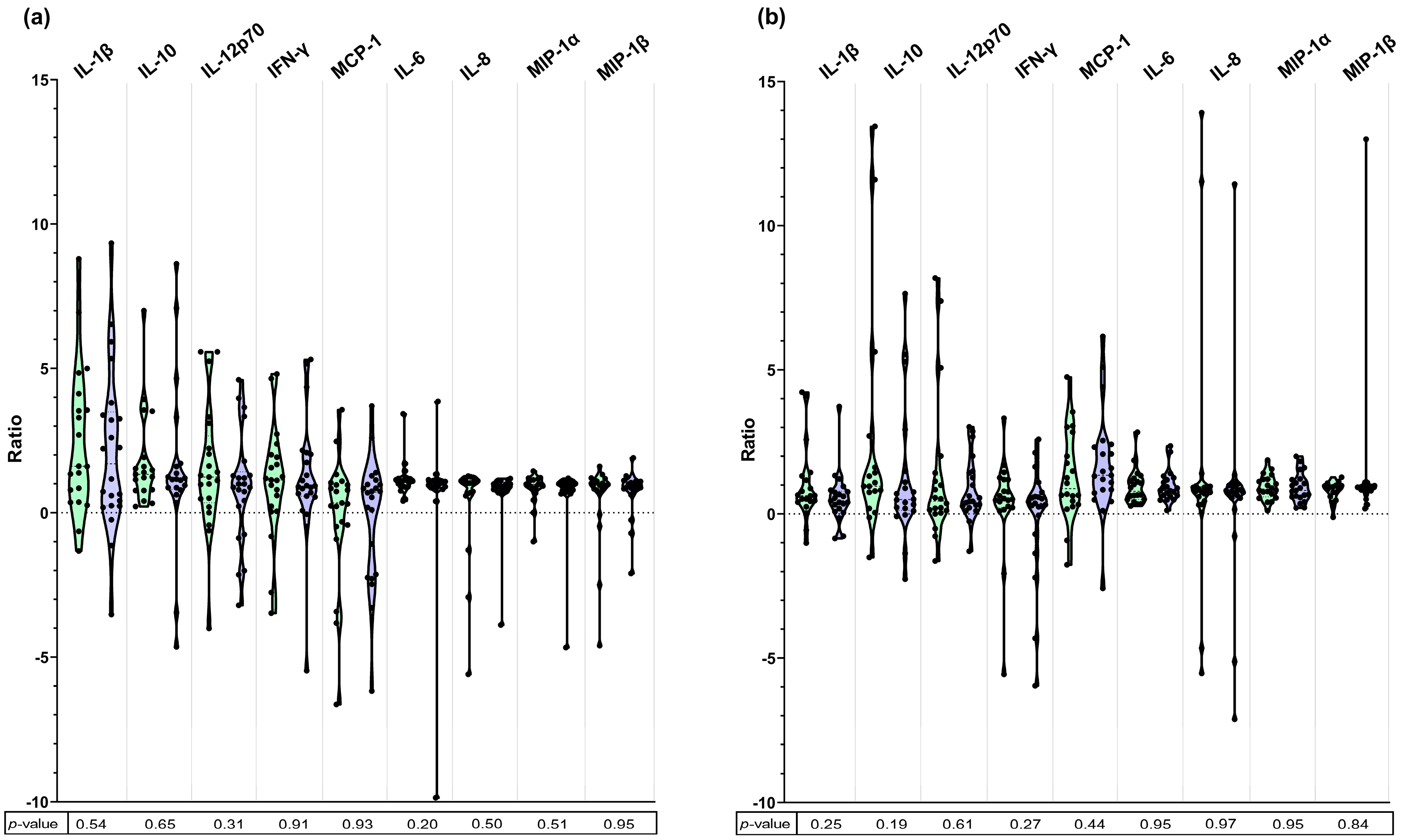
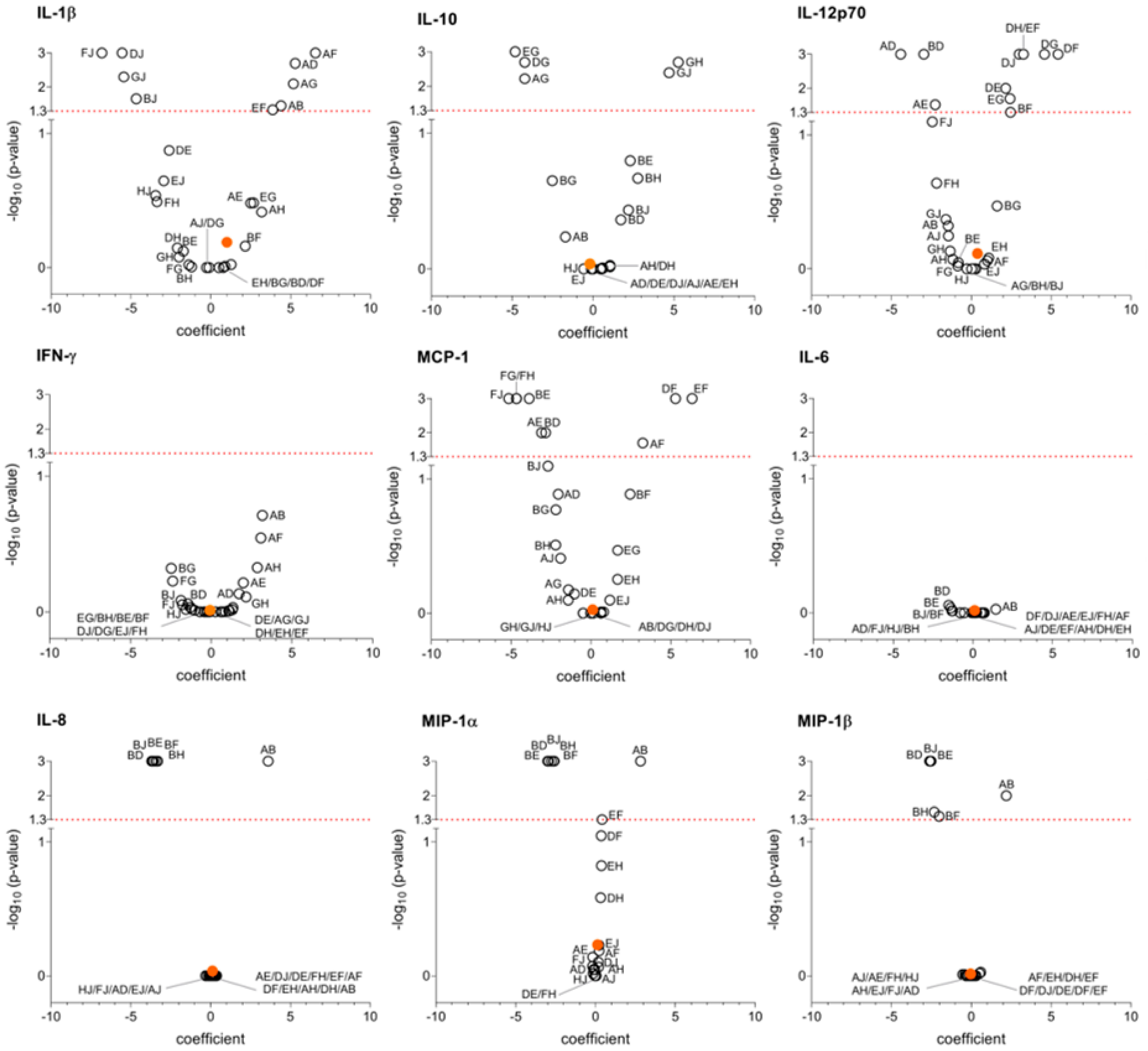
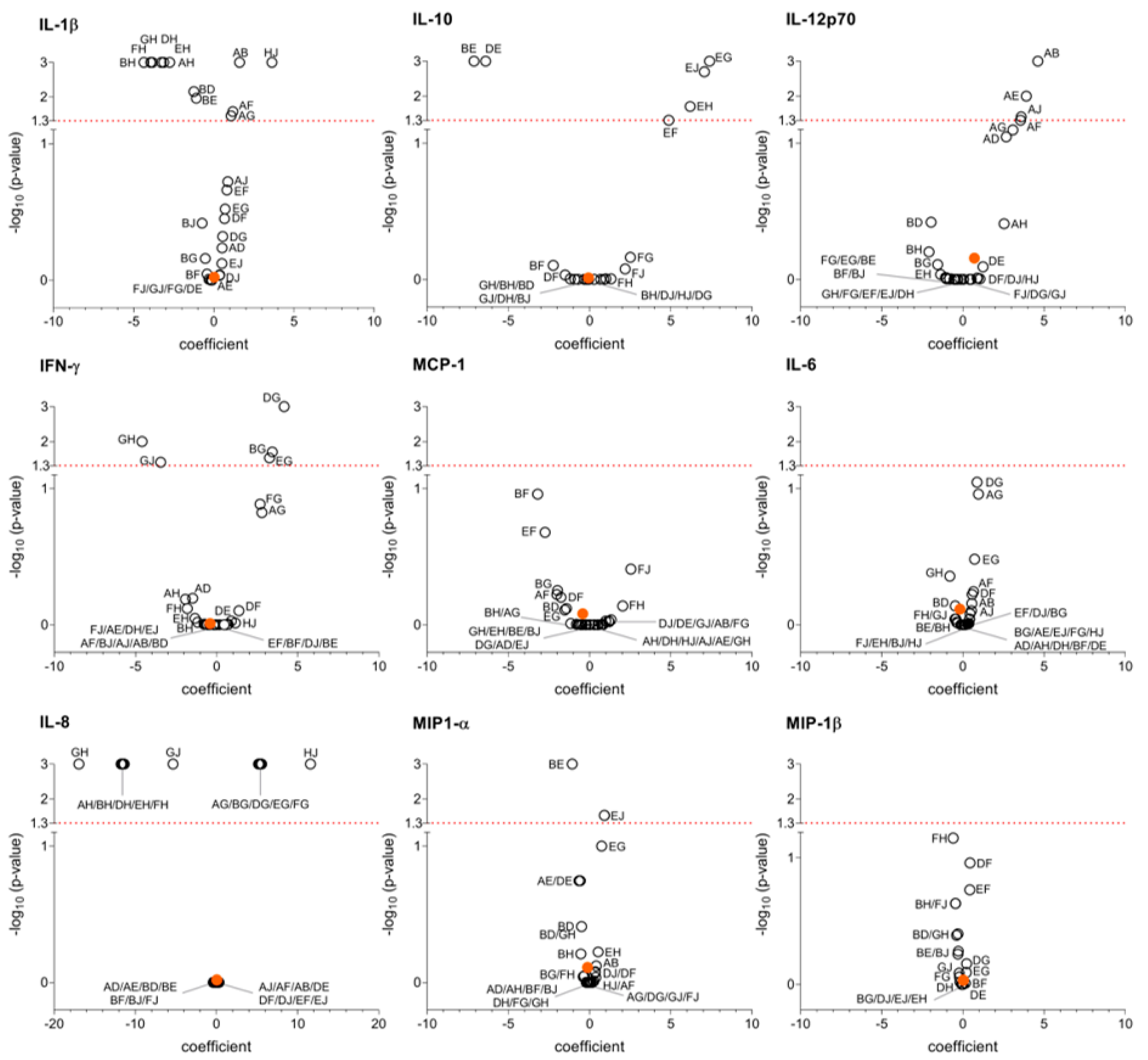
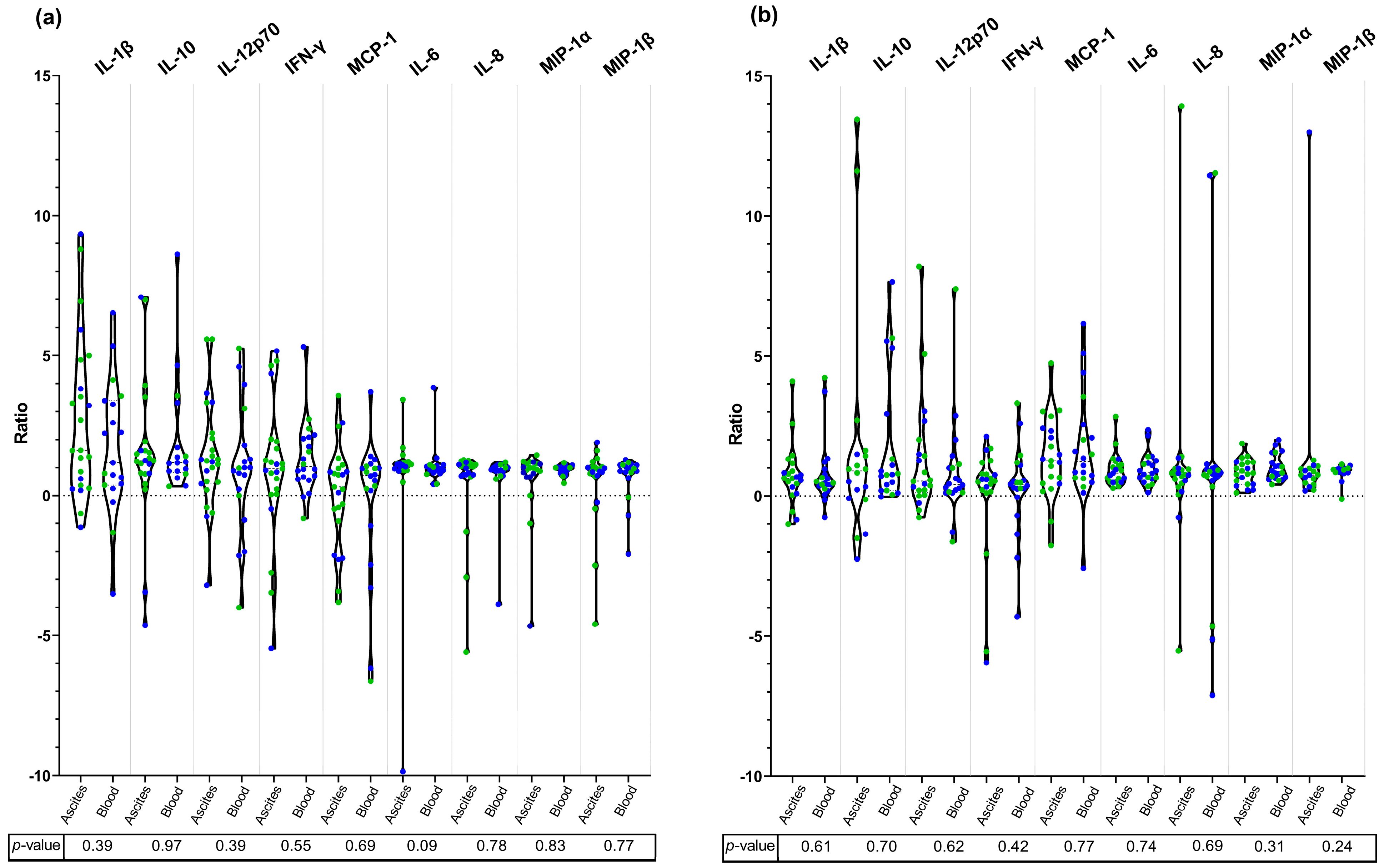
2.4. Determination of Cytokine Concentration and Ratio
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLF | Acute-on-chronic liver failure; |
| CAID | Cirrhosis-associated immune dysfunction; |
| HE | Hepatic encephalopathy; |
| ESBL | Extended-spectrum—betalactamases; |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing; |
| IFN-γ | Interferon-gamma; |
| MASLD | Metabolic dysfunction-associated steatotic liver disease; |
| MCP-1 | Monocyte chemoattractant protein-1; |
| MDR | Multidrug-resistant; |
| MDRO | Multidrug-resistant organisms; |
| MetALD | Metabolic and alcohol-related liver disease; |
| MIP | Macrophage inflammatory protein; |
| PBMC | Peripheral blood mononuclear cells; |
| SBP | Spontaneous bacterial peritonitis; |
| TLR | Toll-like receptor. |
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Martin-Mateos, R.; Van der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 112–134. [Google Scholar] [CrossRef]
- Jalan, R.; Fernandez, J.; Wiest, R.; Schnabl, B.; Moreau, R.; Angeli, P.; Stadlbauer, V.; Gustot, T.; Bernardi, M.; Canton, R.; et al. Bacterial Infections in Cirrhosis: A Position Statement Based on the EASL Special Conference 2013. J. Hepatol. 2014, 60, 1310–1324. [Google Scholar] [CrossRef]
- Piano, S.; Bunchorntavakul, C.; Marciano, S.; Rajender Reddy, K. Infections in Cirrhosis. Lancet Gastroenterol. Hepatol. 2024, 9, 745–757. [Google Scholar] [CrossRef]
- Gallaher, C.E.; Shawcross, D.L. Management of Multidrug-Resistant Infections in Cirrhosis. Semin. Liver. Dis. 2022, 42, 173–187. [Google Scholar] [CrossRef]
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Epidemiology and Effects of Bacterial Infections in Patients with Cirrhosis Worldwide. Gastroenterology 2019, 156, 1368–1380.e10. [Google Scholar] [CrossRef]
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Weist, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and Fungal Infections in Acute-on-Chronic Liver Failure: Prevalence, Characteristics and Impact on Prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Fernández, J.; Prado, V.; Trebicka, J.; Amoros, A.; Gustot, T.; Wiest, R.; Deulofeu, C.; Garcia, E.; Acevedo, J.; Fuhrmann, V.; et al. Multidrug-Resistant Bacterial Infections in Patients with Decompensated Cirrhosis and with Acute-on-Chronic Liver Failure in Europe. J. Hepatol. 2019, 70, 398–411. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients with Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Song, K.H.; Jeon, J.H.; Park, W.B.; Park, S.W.; Bin Kim, H.; Oh, M.D.; Lee, H.S.; Kim, N.J.; Choe, K.W. Clinical Outcomes of Spontaneous Bacterial Peritonitis Due to Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli and Klebsiella Species: A Retrospective Matched Case-Control Study. BMC Infect. Dis. 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amor, A.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic Inflammation in Decompensated Cirrhosis: Characterization and Role in Acute-on-Chronic Liver Failure. Hepatology 2016, 64, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Juanola, A.; Ma, A.T.; de Wit, K.; Gananandan, K.; Roux, O.; Zaccherini, G.; Jiménez, C.; Tonon, M.; Solé, C.; Villaseca, C.; et al. Novel Prognostic Biomarkers in Decompensated Cirrhosis: A Systematic Review and Meta-Analysis. Gut 2024, 73, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Bristianou, M.; Panagou, C.; Adamis, T.; Raftogiannis, M.; Antonopoulou, A.; Chrisofos, M.; Galani, I.; Kanellakopoulou, K.; Tsaganos, T.; Giamarellos-Bourboulis, E.J. The Impact of Multidrug Resistance on the Pathogenicity of Escherichia Coli: An Experimental Study. Int. J. Antimicrob. Agents 2008, 31, 216–223. [Google Scholar] [CrossRef]
- Auger, G.; Corvec, S.; Roquilly, A.; Segain, J.P.; Lepelletier, D.; Reynaud, A.; Asehnoune, K. Escherichia Coli-Induced Productions of pro-Inflammatory Cytokines Are Regulated by MAP Kinases and G-Protein but Not by Akt: Relationship with Phylogenetic Groups and Resistance Patterns. Cytokine 2011, 56, 290–297. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing Tables for Interpretation of MICs and Zone Diameters 8.1. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf (accessed on 20 December 2018).
- European Committee on Antimicrobial Susceptibility Testing Tables for Interpretation of MICs and Zone Diameters 9.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf (accessed on 10 December 2019).
- European Committee on Antimicrobial Susceptibility Testing Tables for Interpretation of MICs and Zone Diameters 10.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 12 December 2020).
- EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 12 December 2020).
- Sánchez, B.; Arias, S.; Chaignepain, S.; Denayrolles, M.; Schmitter, J.M.; Bressollier, P.; Urdaci, M.C. Identification of Surface Proteins Involved in the Adhesion of a Probiotic Bacillus Cereus Strain to Mucin and Fibronectin. Microbiology 2009, 155, 1708–1716. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Lucena-Prieto, M.R.; Moro-García, M.A.; Alonso-Arias, R.; Sánchez, B. Whole Fractions from Probiotic Bacteria Induce In Vitro Th17 Responses in Human Peripheral Blood Mononuclear Cells. J. Funct. Foods 2018, 48, 367–373. [Google Scholar] [CrossRef]
- Tabah, A.; Koulenti, D.; Laupland, K.; Misset, B.; Valles, J.; Bruzzi de Carvalho, F.; Paiva, J.A.; Çakar, N.; Ma, X.; Eggimann, P.; et al. Characteristics and Determinants of Outcome of Hospital-Acquired Bloodstream Infections in Intensive Care Units: The EUROBACT International Cohort Study. Intensive Care Med. 2012, 38, 1930–1945. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernández-Barat, L.; Li Bassi, G.L.; Ferrer, M. Resistance Patterns and Outcomes in Intensive Care Unit (ICU)-Acquired Pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) Classification of Multidrug Resistant Organisms. J. Infect. 2015, 70, 213–222. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of Mortality in Patients with Infections Due to Multi-Drug Resistant Gram Negative Bacteria: The Study, the Patient, the Bug or the Drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Recker, M.; Laabei, M.; Toleman, M.S.; Reuter, S.; Saunderson, R.B.; Blane, B.; Török, M.E.; Ouadi, K.; Stevens, E.; Yokoyama, M.; et al. Clonal Differences in Staphylococcus Aureus Bacteraemia-Associated Mortality. Nat. Microbiol. 2017, 2, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Jacob, S.A.; Thoppil, J. Targeting Sepsis through Inflammation and Oxidative Metabolism. World J. Crit. Care Med. 2025, 14, 101499. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Francés, R.; Zapater, P.; González-Navajas, J.M.; Muñoz, C.; Caño, R.; Moreu, R.; Pascual, S.; Bellot, P.; Pérez-Mateo, M.; Such, J. Bacterial DNA in Patients with Cirrhosis and Noninfected Ascites Mimics the Soluble Immune Response Established in Patients with Spontaneous Bacterial Peritonitis. Hepatology 2008, 47, 978–985. [Google Scholar] [CrossRef]
- Zapater, P.; Francés, R.; González-Navajas, J.M.; de la Hoz, M.A.; Moreu, R.; Pascual, S.; Monfort, D.; Montoliu, S.; Vila, C.; Escudero, A.; et al. Serum and Ascitic Fluid Bacterial DNA: A New Independent Prognostic Factor in Noninfected Patients with Cirrhosis. Hepatology 2008, 48, 1924–1931. [Google Scholar] [CrossRef]
- Frasinariu, O.E.; Ceccarelli, S.; Alisi, A.; Moraru, E.; Nobili, V. Gut-Liver Axis and Fibrosis in Nonalcoholic Fatty Liver Disease: An Input for Novel Therapies. Dig. Liver Dis. 2013, 45, 543–551. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like Receptor Recognizes Bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Spohn, R.; Buwitt-Beckmann, U.; Brock, R.; Jung, G.; Ulmer, A.J.; Wiesmüller, K.-H. Synthetic Lipopeptide Adjuvants and Toll-like Receptor 2—Structure–Activity Relationships. Vaccine 2004, 22, 2494–2499. [Google Scholar] [CrossRef]
- Matsuura, M. Structural Modifications of Bacterial Lipopolysaccharide That Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Collaborative Action of Toll-Like and Nod-Like Receptors as Modulators of the Inflammatory Response to Pathogenic Bacteria. Mediators Inflamm. 2014, 2014, 432785. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.; Hasan, M.; Bergstedt, J.; Rouilly, V.; Libri, V.; Urrutia, A.; Alanio, C.; Scepanovic, P.; Hammer, C.; Jönsson, F.; et al. Natural Variation in the Parameters of Innate Immune Cells Is Preferentially Driven by Genetic Factors. Nat. Immunol. 2018, 19, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Kronsten, V.T.; Woodhouse, C.A.; Zamalloa, A.; Lim, T.Y.; Edwards, L.A.; Martinez-Llordella, M.; Sanchez-Fueyo, A.; Shawcross, D.L. Exaggerated Inflammatory Response to Bacterial Products in Decompensated Cirrhotic Patients Is Orchestrated by Interferons IL-6 and IL-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G489–G499. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The Multifaceted Nature of IL-10: Regulation, Role in Immunological Homeostasis and Its Relevance to Cancer, COVID-19 and Post-COVID Conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Guarner, C.; Soriano, G. Bacterial Translocation and Its Consequences in Patients with Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2005, 17, 27–31. [Google Scholar] [CrossRef]
| Infection by MDR E. coli n = 6 | Infection by Susceptible E. coli n = 6 | p-Value | |
|---|---|---|---|
| Sex, M/F, n (%) | 6 (100%)/0 | 4 (66.7%)/2 (33.3%) | 0.45 |
| Age, (years) | 69 ± 10 | 68 ± 13 | 0.48 |
| Etiology of cirrhosis, n (%) | 0.46 | ||
| Alcohol-related liver disease | 2 (33.3%) | 1 (16.7%) | |
| MASLD | 3 (50%) | 2 (33.3%) | |
| MetALD | 1 (16.7%) | 1 (16.7%) | |
| Alcohol + Autoimmune | 0 | 2 (33.3%) | |
| Child–Pugh score at infection diagnosis, (points) | 9 ± 2.3 | 9 ± 1.7 | 0.50 |
| MELD score at infection diagnosis, (points) | 23 ± 5.1 | 20 ± 9.3 | 0.23 |
| C-reactive protein (mg/dL) | 103.0 ± 63.4 | 93.7 ± 50.0 | 0.78 |
| White blood cell count (×109/L) | 10.9 ± 5.5 | 8.2 ± 4.4 | 0.38 |
| Antibiotic prophylaxis, n (%) | 4 (66.7%) | 2 (33.3%) | 0.57 |
| Type of infection, n (%) | 0.56 | ||
| Bacteremia | 4 (66.7%) | 2 (33.3%) | |
| SBP | 2 (33.3%) | 4 (66.7%) | |
| Site of acquisition, n (%) | 0.07 | ||
| Community-acquired | 2 (33.3%) | 3 (50%) | |
| Healthcare associated | 2 (33.3%) | 3 (50%) | |
| Nosocomial | 2 (33.3%) | 0 | |
| Acute kidney injury at infection diagnosis, n (%) | 2 (33.3%) | 3 (50%) | 1 |
| Decompensation of cirrhosis, n (%) | 6 (100%), of which: | 5 (83.3%), of which: | 1 |
| Ascites | 3 (50%) | 5 (100%) | |
| Variceal bleeding | 1 (16.7%) | 0 | |
| Ascites + HE | 2 (33.3%) | 0 | |
| ACLF, n (%) | 1 (16.7%) | 2 (33.3%) | 1 |
| 30-day mortality, n (%) | 1 (16.7%) | 0 | 1 |
| Surface Protein Extract | DNA Extract | ||||
|---|---|---|---|---|---|
| Antibiotic-Susceptible vs. MDR E. coli | Healthy Donors | Antibiotic-Susceptible vs. MDR E. coli | Healthy Donors | ||
| IL-1β | coefficient (95% CI) | 1.01 (−3.51–5.53) | - | 0.03 (−1.15–1.21) | - |
| p-value | 0.65 | <0.001 * | 0.96 | <0.001 * | |
| IL-10 | coefficient (95% CI) | −0.19 (−3.84–3.47) | - | −0.14 (−5.78–5.48) | - |
| p-value | 0.92 | 0.01 * | 0.96 | 0.002 * | |
| IL-12p70 | coefficient (95% CI) | 0.39 (−2.28–3.05) | - | 0.71 (−3.07–4.49) | - |
| p-value | 0.77 | <0.001 * | 0.70 | 0.01 * | |
| IFN-γ | coefficient (95% CI) | −0.07 (−4.61–4.47) | - | −0.04 (−3.6–3.53) | - |
| p-value | 0.97 | 0.18 | 0.98 | 0.008 * | |
| MCP-1 | coefficient (95% CI) | 0.11 (−2.95–3.18) | - | −0,42 (−4.32–3.48) | - |
| p-value | 0.94 | <0.001 * | 0,83 | 0.40 | |
| IL-6 | coefficient (95% CI) | 0.11 (−4.81–5.02) | - | −0.18 (−1.40–1.05) | - |
| p-value | 0.96 | 0.93 | 0.77 | 0.045 * | |
| IL-8 | coefficient (95% CI) | 0.11 (−2.08–2.31) | - | 0.06 (−2.36–2.50) | - |
| p-value | 0.92 | <0.001 * | 0.96 | <0.001 * | |
| MIP-1α | coefficient (95% CI) | 0.13 (−0.36–0.62) | - | −0.13 (−1.10–0.84) | - |
| p-value | 0.58 | <0.001 * | 0.78 | 0.01 * | |
| MIP-1β | coefficient (95% CI) | −0.04 (−2.19–2.11) | - | 0.03 (−0.59–0.64) | - |
| p-value | 0.97 | 0.004 * | 0.93 | 0.01 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuyàs, B.; Cantó, E.; Sanchez-Ardid, E.; Miró, E.; Alvarado-Tapias, E.; Román, E.; Poca, M.; Navarro, F.; Ferrero-Gregori, A.; Escorsell, M.À.; et al. In Vitro Immune Response of Mononuclear Cells to Multidrug-Resistant Escherichia coli. Microorganisms 2025, 13, 1164. https://doi.org/10.3390/microorganisms13051164
Cuyàs B, Cantó E, Sanchez-Ardid E, Miró E, Alvarado-Tapias E, Román E, Poca M, Navarro F, Ferrero-Gregori A, Escorsell MÀ, et al. In Vitro Immune Response of Mononuclear Cells to Multidrug-Resistant Escherichia coli. Microorganisms. 2025; 13(5):1164. https://doi.org/10.3390/microorganisms13051164
Chicago/Turabian StyleCuyàs, Berta, Elisabet Cantó, Elisabet Sanchez-Ardid, Elisenda Miró, Edilmar Alvarado-Tapias, Eva Román, Maria Poca, Ferran Navarro, Andreu Ferrero-Gregori, Maria Àngels Escorsell, and et al. 2025. "In Vitro Immune Response of Mononuclear Cells to Multidrug-Resistant Escherichia coli" Microorganisms 13, no. 5: 1164. https://doi.org/10.3390/microorganisms13051164
APA StyleCuyàs, B., Cantó, E., Sanchez-Ardid, E., Miró, E., Alvarado-Tapias, E., Román, E., Poca, M., Navarro, F., Ferrero-Gregori, A., Escorsell, M. À., Vidal, S., & Soriano, G. (2025). In Vitro Immune Response of Mononuclear Cells to Multidrug-Resistant Escherichia coli. Microorganisms, 13(5), 1164. https://doi.org/10.3390/microorganisms13051164








