Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. X-Ray Data Collection and Refinement
2.3. Cell Cultures of L. tarentolae
2.4. MTT Cell Viability Assay
2.5. Dose-Dependent Effect of Test Compounds on Cell Viability
2.6. SAP Enzyme Assay
2.7. Dose-Dependent Direct Effect on SAP Activity
2.8. Comparing Secreted and Intracellular Leishmania Acid Phosphatase Activity
3. Results and Discussion
3.1. Synthesis and Characterization of Compounds
3.2. Crystallographic Analysis of ZZ1-04 and ZZ1-13
3.3. Growth Curves Following Addition of ZZ1-10 and ZZ1-13
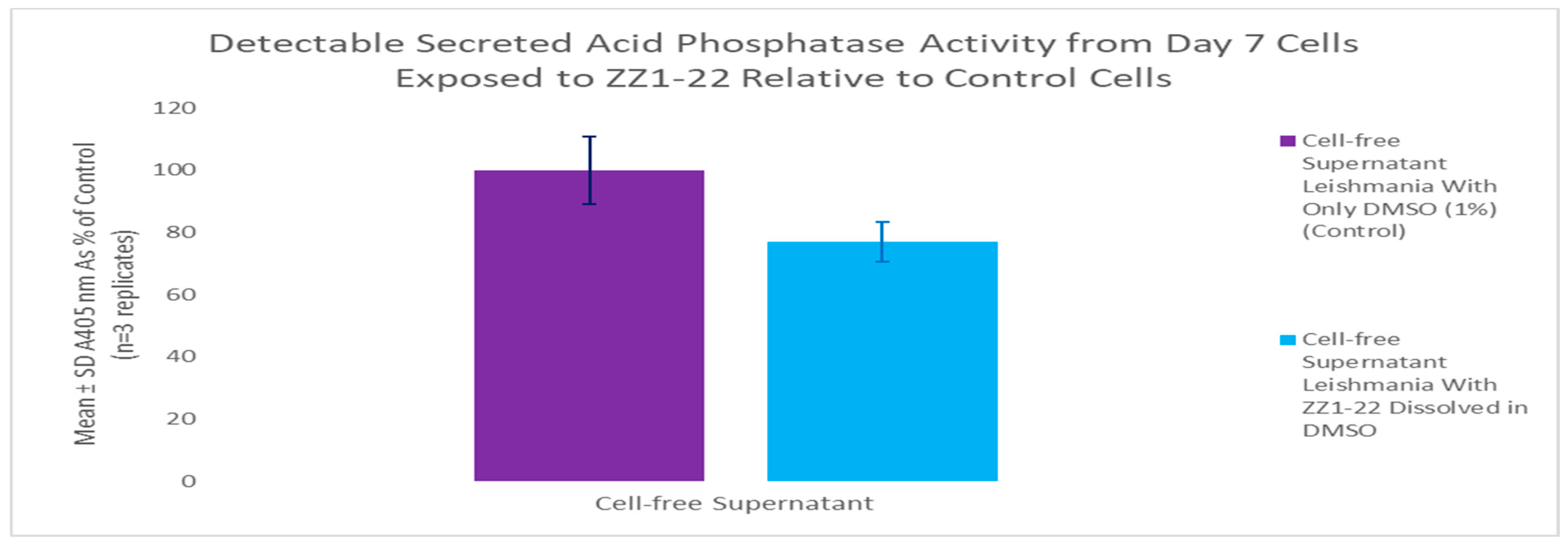
3.4. ZZ1-22 MTT and SAP Assays
3.5. ZZ1-04 and Assays
3.5.1. ZZ1-04 and Cell Viability
3.5.2. ZZ1-04 and Secreted Acid Phosphatase Activity
3.6. ZZ1-20 MTT and SAP Assays
3.7. Stability in Solution Study for Direct Effect on SAP
4. Conclusions
Limitations of These Studies
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Leishmaniasis. 12 January 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 1 February 2024).
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Ovung, A.; Bhattacharyya, J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Li, E.W.; Katinas, J.M.; Jones, M.A.; Hamaker, C.G. Structural characterization of naphthalene sulfonamides and a sulfonate ester and their in vitro efficacy against Leishmania tarentolae promastigotes. New J. Chem. 2021, 45, 4791–4801. [Google Scholar] [CrossRef]
- Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, İ. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics 2023, 15, 779. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Sriprasanthi, R.B.; Shamsudeen, S.; Adam, F.; Bhawani, S.A. A concise review of the natural existance, synthesis, properties, and applications of syringaldehyde. BioResources 2012, 7, 4377. [Google Scholar]
- Shahzad, S.; Mateen, S.; Mariyath, P.M.M.; Naeem, S.S.; Akhtar, K.; Rizvi, W.; Moin, S. Protective effect of syringaldehyde on biomolecular oxidation, inflammation and histopathological alterations in isoproterenol induced cardiotoxicity in rats. Biomed. Pharmacother. 2018, 108, 625. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; McColl, J.M.; Wonko, S.L.; Thayer, R.S.; Hancock, D.S. Evaluation of the thiosemicarbazones of some aryl alkyl ketones and related compounds for anticonvulsant activities. Eur. J. Med. Chem. 1991, 26, 529. [Google Scholar] [CrossRef]
- Scarim, C.B.; Jornada, D.H.; Machado, M.G.M.; Ferreira, C.M.R.; dos Santos, J.L.; Chung, M.C. Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. Eur. J. Med. Chem. 2019, 162, 378. [Google Scholar] [CrossRef] [PubMed]
- Katinas, J.; Epplin, R.; Hamaker, C.; Jones, M.A. Sulfonamides as Inhibitors of Leishmania—Potential New Treatments for Leishmaniasis. Anti-Infect. Agents 2017, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Soares, D.C.; Saraiva, E.M.; Meyer-Fernandes, J.R.; Souto-Padrón, T. Different secreted phosphatase activities in Leishmania amazonensis. FEMS Microbiol. Lett. 2013, 340, 117. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. SAINT, version V8.38A; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.M.; Muñoz, D.L.; Cedeño, D.L.; Vélez, I.D.; Jones, M.A.; Robledo, S.M. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 2010, 126, 471. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, J.B.; Peter, S.J.; Cedeño, D.L.; Constantino, M.H.; Edwards, K.A.; Kamowski, E.M.; Jones, M.A. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorg. Med. Chem. 2008, 16, 7033. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, B.M.; Cass, C.L.; Cedeño, D.L.; Vallejo, R.; Jones, M.A. Effects of specific electric field stimulation on the kinetics of secreted acid phosphatases from Leishmania tarentolae and implications for therapy. Pathogens 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, C.G.; Oberts, B.P. Synthesis and crystal structures of the bis-Schiff bases of 2-(methylthio)aniline with isophthaldehyde, terephthaldehyde, and para-diacetylbenzene. J. Chem. Crystallogr. 2006, 36, 735. [Google Scholar] [CrossRef]
- Hamaker, C.G.; Germann, S.M. Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde. Crystals 2024, 14, 99. [Google Scholar] [CrossRef]

















| Compound | ZZ1-04 | ZZ1-13 |
|---|---|---|
| CCDC Deposit No. | 2364214 | 2364215 |
| Chemical formula | C11H14N2O3S2 | C16H17N3O3S |
| Mr | 286.36 | 331.38 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 100 (2) | 100 (2) |
| a, b, c (Å) | 5.1702 (2) 17.4274 (7) 14.5938 (6) | 11.6522 (7) 5.6318 (3) 24.0789 (14) |
| β (°) | 96.097 (2) | 103.799 (4) |
| V (Å3) | 1307.51 (9) | 1534.52 (15) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm–1) | 0.409 | 0.230 |
| Crystal size (mm) | 0.47 × 0.18 × 0.04 | 0.21 × 0.12 × 0.05 |
| Diffractometer | Bruker APEX-II CCD | |
| Absorption correction | Multi-scan SADABS | |
| Tmin, Tmax | 0.90, 0.98 | 0.92, 0.99 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 37399, 2792, 2501 | 43034, 2369, 2703 |
| Rint | 0.0292 | 0.0592 |
| (sin θ/λ)max (Å–1) | 0.634 | 0.634 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0238, 0.0635, 1.051 | 0.0333, 0.0820, 1.032 |
| No. of reflections | 2792 | 3269 |
| No. of parameters | 171 | 220 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | |
| Δρmax, Δρmin (e Å–3) | 0.325, –0.206 | 0.297, –0.226 |
| Bond | ZZ1-04 | ZZ1-13 |
|---|---|---|
| C6–C7 | 1.4608 (17) | 1.456 (2) |
| C7–N8 | 1.2817 (16) | 1.284 (2) |
| N8–N9 | 1.3842 (14) | 1.3835 (18) |
| N9–C10 | 1.3333 (16) | 1.344 (2) |
| C10–S11 | 1.6726 (12) | 1.6846 (16) |
| C10–S12/N12 | 1.7475 (13) | 1.351 (2) |
| Bond | ZZ1-04 | ZZ1-13 |
|---|---|---|
| C6–C7–N8 | 121.93 (11) | 123.08 (14) |
| C7–N8–N9 | 114.23 (10) | 114.38 (13) |
| N8–N9–C10 | 120.75 (11) | 121.15 (13) |
| N9–C10–S11 | 120.54 (9) | 119.08 (12) |
| N9–C10–S12/N12 | 114.15 (9) | 115.72 (14) |
| C10–S12/N12–C18 | 101.38 (6) | 125.16 (14) |
| C3–O13–H13 | 107.2 (15) | 109.1 (17) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| O13–H13…S11 i | 0.76 (2) | 2.66 (2) | 3.3192 (10) | 145.9 (18) |
| O13–H13…O15 | 0.76 (2) | 2.226 (19) | 2.6698 (13) | 118.2 (17) |
| N9–H9…O13 ii | 0.859 (18) | 2.108 (18) | 3.0342 (14) | 173.2 (16) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| N9–H9…S11 i | 0.83 (2) | 2.64 (2) | 3.4410 (14) | 161.2 (18) |
| O13–O13…O14 ii | 0.81 (3) | 2.35 (3) | 2.9606 (16) | 133 (2) |
| N12–H12…S11 iii | 0.83 (2) | 2.78 (2) | 3.4724 (14) | 142.7 (17) |
| Test Compound | A405 nm as % of Control |
|---|---|
| Only DMSO (1%) (Control Supernatant) | 100% |
| Aged ZZ1-04 | 4.20% |
| Aged ZZ1-20 | 24.07% |
| Aged ZZ1-22 | 4.69% |
| Freshly Prepared ZZ1-04 | 9.35% |
| Freshly Prepared ZZ1-20 | 43.81% |
| Freshly Prepared ZZ1-22 | 114.47% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.H.; Zelaya, Z.Z.; Hamaker, C.G.; Jones, M.A. Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro. Microorganisms 2024, 12, 2641. https://doi.org/10.3390/microorganisms12122641
Shang HH, Zelaya ZZ, Hamaker CG, Jones MA. Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro. Microorganisms. 2024; 12(12):2641. https://doi.org/10.3390/microorganisms12122641
Chicago/Turabian StyleShang, Henry H., Zaryna Z. Zelaya, Christopher G. Hamaker, and Marjorie A. Jones. 2024. "Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro" Microorganisms 12, no. 12: 2641. https://doi.org/10.3390/microorganisms12122641
APA StyleShang, H. H., Zelaya, Z. Z., Hamaker, C. G., & Jones, M. A. (2024). Inhibitory Effects of Sulfur Derivatives on Leishmania tarentolae Cell Viability and Secreted Acid Phosphatase In Vitro. Microorganisms, 12(12), 2641. https://doi.org/10.3390/microorganisms12122641








