Nitrogen-Fixing Paenibacillus haidiansis and Paenibacillus sanfengchensis: Two Novel Species from Plant Rhizospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Strains
2.2. Nitrogenase Activity Assay
2.3. nifH Gene Sequence and Phylogenetic Analysis
2.4. 16S rRNA Gene Sequence and Phylogenetic Analysis
2.5. Genome Sequencing and Analysis
2.6. Morphological, Physiological and Biochemical Analysis
2.7. Chemotaxonomic Characterization
3. Results
3.1. Strain Isolation, Nitrogenase Activity and nifH Genes
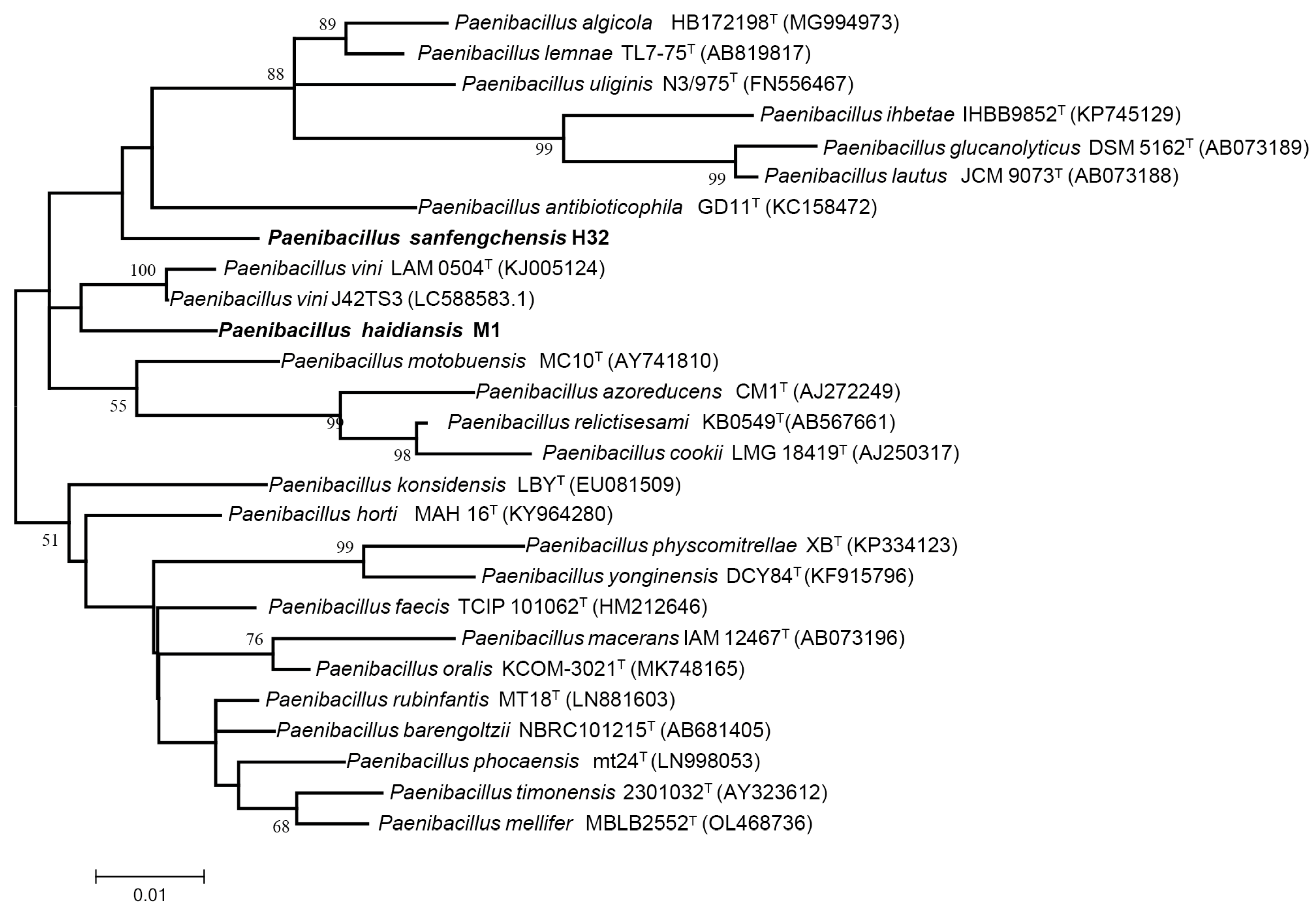
3.2. Phylogenetic Analysis of 16S rRNA Gene
3.3. Genome Sequence and Similarity Analysis
3.4. Analysis of Nitrogen Fixation and Nitrogen Metabolism Genes
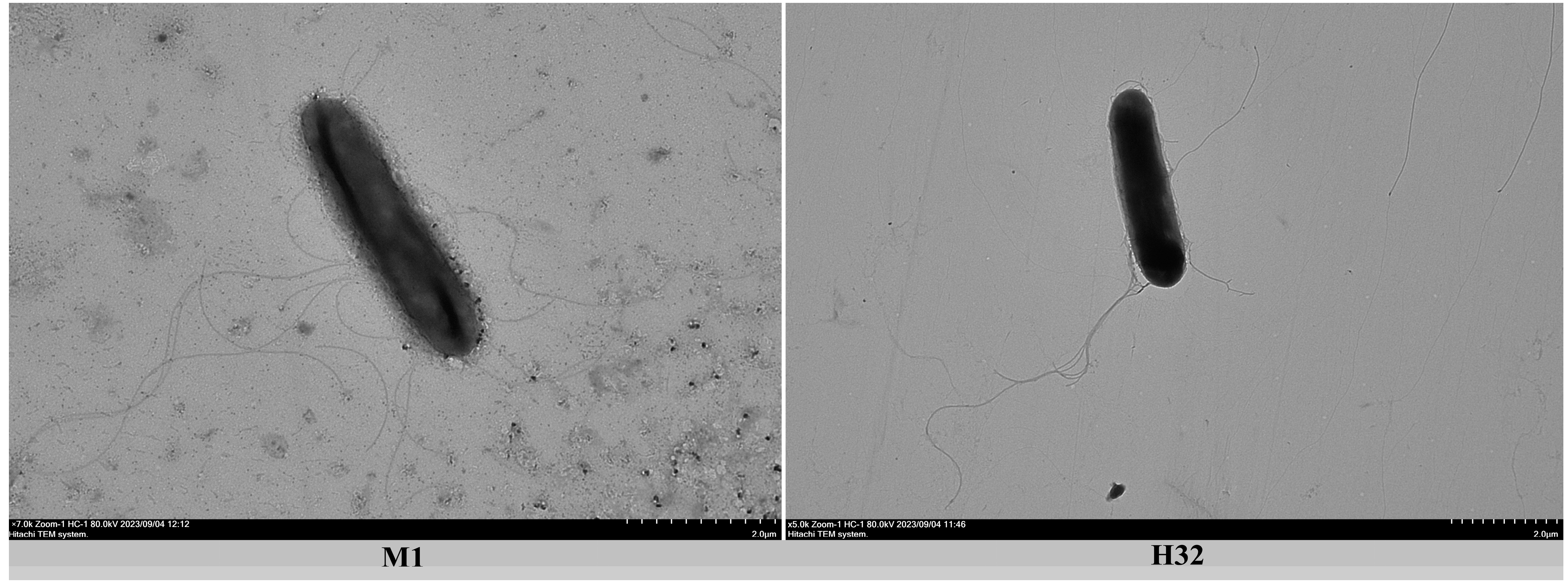
3.5. Phenotypic Characteristics
3.6. Chemotaxonomic Analyses
3.7. Description of Paenibacillus haidiansis sp. nov
3.8. Description of Paenibacillus sanfengchensis nov sp. nov
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ash, C.; Priest, F.G.; Collins, M.D. Molecular identification of rRNA group 3 Bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek 1993, 64, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Liu, X.; Chen, S.F. Paenibacillus sinensis sp. nov., a nitrogen-fixing species isolated from plant rhizospheres. Antonie Van Leeuwenhoek 2022, 115, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zuo, Y.Z.; Gao, M.; Chen, S.F. Paenibacillus caui sp. nov., a nitrogen-fixing Species Isolated from the Rhizosphere Soil of a Peach Tree. Int. J. Syst. Evol. Microbiol. 2022, 72, 005216. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.Y.; Ma, Y.C.; Zhou, Y.G.; Gao, F.; Liu, H.C.; Chen, S.F. Paenibacillus sonchi sp nov., a nitrogen-fixing species isolated from the rhizosphere of Sonchus oleraceus. Int. J. Syst. Evol. Microbiol. 2009, 59, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Zhou, Y.G.; Liu, H.C.; Chen, S.F. Paenibacillus jilunlii sp nov., a nitrogen-fixing species isolated from the rhizosphere of Begonia semperflorens. Int. J. Syst. Evol. Microbiol. 2011, 61, 1350–1355. [Google Scholar] [CrossRef]
- Jin, H.J.; Lv, J.; Chen, S.F. Paenibacillus sophorae sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Sophora japonica. Int. J. Syst. Evol. Microbiol. 2011, 61, 767–771. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhang, J.; Chen, S.F. Paenibacillus zanthoxyli sp nov, a novel nitrogen-fixing species isolated from the rhizosphere of Zanthoxylum simulans. Int. J. Syst. Evol. Microbiol. 2007, 57, 873–877. [Google Scholar] [CrossRef]
- Ma, Y.C.; Xia, Z.Q.; Liu, X.M.; Chen, S.F. Paenibacillus sabinae sp nov., a nitrogen-fixing species isolated from the rhizosphere soils of shrubs. Int. J. Syst. Evol. Microbiol. 2007, 57, 6–11. [Google Scholar] [CrossRef]
- Ma, Y.C.; Chen, S.F. Paenibacillus forsythiae sp nov., a nitrogen-fixing species isolated from the rhizosphere soil of Forsythia mira. Int. J. Syst. Evol. Microbiol. 2008, 58, 319–323. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, J.; Li, Q.X.; Chen, S.F. Paenibacillus triticisoli, sp. nov., a nitrogen-fixing species isolated from wheat rhizosphere soil. Antonie Van Leeuwenhoek 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Xie, J.B.; Zhang, L.H.; Zhou, Y.G.; Liu, H.C.; Chen, S.F. Paenibacillus taohuashanense sp. nov., a nitrogen-fixing species isolated from rhizosphere soil of the root of Caragana kansuensis pojark. Antonie Van Leeuwenhoek 2012, 102, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Mülner, P.; Schwarz, E.; Dietel, K.; Herfort, S.; Jähne, J.; Lasch, P.; Cernava, T.; Berg, G.; Vater, J. Fusaricidins, polymyxins and volatiles produced by Paenibacillus polymyxa strains DSM 32871 and M1. Pathogens 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.F. Fusaricidin Produced by Paenibacillus polymyxa WLY78 induces systemic resistance against Fusarium wilt of cucumber. Int. J. Mol. Sci. 2019, 20, 5240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Li, Y.; Chen, S.F. Fusaricidin biosynthesis is controlled via a KinB-Spo0A-AbrB signal pathway in Paenibacillus polymyxa WLY78. Mol. Plant Microbe Interact. 2021, 34, 1378–1389. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Chen, S.F. Application of N2-fixing Paenibacillus triticisoli BJ-18 changes the compositions and functions of the bacterial, diazotrophic, and fungal microbiomes in the rhizosphere and root/shoot endosphere of wheat under field conditions. Biol. Fertil. Soils 2021, 51, 347–362. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Chen, S.F. Diazotroph Paenibacillus triticisoli BJ-18 drives the variation in bacterial, diazotrophic and fungal communities in the rhizosphere and root/shoot endosphere of Maize. Int. J. Mol. Sci. 2021, 22, 1460. [Google Scholar] [CrossRef]
- Xie, J.B.; Du, Z.L.; Bai, L.Q.; Tian, C.F.; Zhang, Y.Z.; Xie, J.Y.; Wang, T.S.; Liu, X.M.; Chen, X.; Cheng, Q.; et al. Comparative genomic analysis of N2-fixing and non-N2-fixing Paenibacillus spp.: Organization, evolution and expression of the nitrogen fixation genes. PLoS Genet. 2014, 10, e1004231. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthof, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Minnikin, D.E.; O’donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Komagata, K.; Suzuki, K. Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol. 1987, 19, 161–207. [Google Scholar]
- Sasser, M.; Kunitsky, C.; Jackoway, G.; Ezzell, J.W.; Teska, J.D.; Harper, B.; Parker, S.; Barden, D.; Blair, H.; Breezee, J.; et al. Identification of Bacillus anthracis from culture using gas chromatographic analysis of fatty acid methyl esters. J. AOAC Int. 2005, 88, 178–181. [Google Scholar] [CrossRef]
- Collins, M.D.; Goodfellow, M.; Minnikin, D.E. Fatty acid, isoprenoid quinone and polar lipid composition in the classification of Curtobacterium and related taxa. J. Gen. Microbiol. 1980, 118, 29–37. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Tiedje, J.M. Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, X.; Shi, H.; Sun, L.; Li, Y.; Li, Q.; Zhang, H.; Chen, S.; Li, J. Positive and negative regulation of transferred nif genes mediated by indigenous GlnR in Gram-positive Paenibacillus polymyxa. PLoS Genet. 2018, 14, e1007629. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, X.; Wu, X.; Chen, S.F. Genome-wide mapping of GlnR-binding sites reveals the global regulatory role of GlnR in controlling the metabolism of nitrogen and carbon in Paenibacillus polymyxa WLY78. BMC Genom. 2023, 24, 85. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Y.; Wang, T.; Hua, C.; Hu, R.; Shang, Y.; Shi, H.; Chen, S.F. Glutamine synthetase and GlnR regulate nitrogen metabolism in Paenibacillus polymyxa WLY78. Appl. Environ. Microbiol. 2023, 89, e0013923. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108541. [Google Scholar] [CrossRef] [PubMed]
- Eskin, N.; Vessey, K.; Tian, L. Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in monocot plants. Int. J. Agron. 2014, 2014, 208383. [Google Scholar] [CrossRef]
- Bageshwar, U.K.; Srivastava, M.; Pardha-Saradhi, P.; Paul, S.; Gothandapani, S.; Jaat, R.S.; Shankar, P.; Yadav, R.; Biswas, D.R.; Kumar, P.A.; et al. An environmentally friendly engineered azotobacter strain that replaces a substantial amount of urea fertilizer while sustaining the same wheat yield. Appl. Environ. Microbiol. 2017, 83, e00590-17. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, Y.L.; Zhang, H.W.; Wang, M.Y.; Chen, S.F. Diazotrophic Paenibacillus beijingensis BJ-18 provides nitrogen for plant and promotes plant growth, nitrogen uptake and metabolism. Front. Microbiol. 2019, 10, 1119. [Google Scholar] [CrossRef]

| Strains | Accession No. | Genome Size (Mb) | DNA GC (%) | Gene Number | RNAs | ||
|---|---|---|---|---|---|---|---|
| tRNA | 16S rRNAs | ncRNA (Non-Coding RNA) | |||||
| Test strain M1 | GCA_036553665.1 | 5.7 | 52 | 5462 | 87 | 2 | 4 |
| Type strain Paenibacillus vini strain J42TS3 | GCA_018403325.1 | 5.6 | 49 | 5274 | 93 | 2 | 4 |
| Type strain Paenibacillus vini strain CENA-BCM001 | GCA_030412165.1 | 5.7 | 49 | 5293 | 93 | 2 | 4 |
| Test strain H32 | GCA_036864765.1 | 6.46 | 52.5 | 5511 | 77 | 1 | 8 |
| Type strain Paenibacillus faecis strain J25TS5 | GCA_008084145.1 | 6.3 | 53 | 5743 | 83 | 2 | 4 |
| Type strain Paenibacillus faecis DSM 23593(T) | GCA_008084145.1 | 6.3 | 53 | 5699 | 64 | 4 | 4 |
| Characteristic | Test Strain | Reference Strain | Test Strain | Reference Strain |
|---|---|---|---|---|
| M1 | P. vini LAM0504T | H32 | P. faecis 65684T | |
| pH range | 6–8 | 6–8 | 5–9 | 6–9 |
| NaCl | 0–1.0% | 0–3% | 0–1.0% | 0–3% |
| Growth temperature (°C) | 25–35 | 30 | 20–40 | 30 |
| Nitrate reduction | + | + | + | + |
| Starch hydrolysis | + | + | + | + |
| Dihydroxyacetone production | − | − | − | − |
| Mobility | +++ | + | + | + |
| Flagellum | + | + | + | + |
| Production of acid from following substrates: | ||||
| D-fructose | + | − | + | + |
| D-xylose | + | + | + | + |
| D-glucose | + | + | + | + |
| Trehalose | + | + | + | + |
| D-galactose | + | + | + | + |
| Maltose | + | + | + | + |
| D-mannitol | − | − | − | + |
| L-rhamnose | − | − | − | + |
| D-sorbitol | − | − | − | − |
| Inositol | − | − | − | − |
| Fatty Acid | M1 | P. vini LAM0504T | H32 | P. faecis DSM 23593T |
|---|---|---|---|---|
| Saturated | ||||
| C12:0 | 0.62 | 0.5 | 1.61 | 1.60 |
| C14:0 | 3.16 | 3.30 | 1.10 | 1.77 |
| C16:0 | 24.39 | 9.73 | 6.47 | 21.56 |
| C17:0 | TR | - | TR | 1.50 |
| Unsaturated | ||||
| C14:1 ω5c | - | 0.17 | TR | TR |
| C19:0 cyclo ω8c | - | - | TR | TR |
| C18:1 ω9c | TR | TR | 1.63 | 1.04 |
| C20:4 ω6,9,12,15c | TR | - | - | - |
| Branched saturated | ||||
| iso-C14:0 | 1.28 | 2.39 | TR | TR |
| iso-C15:0 | 3.79 | 8.80 | 7.89 | 3.75 |
| iso-C16:0 | 7.53 | 8.42 | 2.77 | 3.57 |
| iso-C17:0 | 2.05 | 3.24 | 2.01 | 2.84 |
| anteiso-C15:0 | 45.71 | 50.30 | 61.41 | 45.42 |
| anteiso-C17:0 | 7.52 | 5.21 | 7.92 | 12.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Gao, M.; Hu, R.; Shang, Y.; Liu, M.; Lan, P.; Jiao, S.; Wei, G.; Chen, S. Nitrogen-Fixing Paenibacillus haidiansis and Paenibacillus sanfengchensis: Two Novel Species from Plant Rhizospheres. Microorganisms 2024, 12, 2561. https://doi.org/10.3390/microorganisms12122561
Zhang W, Gao M, Hu R, Shang Y, Liu M, Lan P, Jiao S, Wei G, Chen S. Nitrogen-Fixing Paenibacillus haidiansis and Paenibacillus sanfengchensis: Two Novel Species from Plant Rhizospheres. Microorganisms. 2024; 12(12):2561. https://doi.org/10.3390/microorganisms12122561
Chicago/Turabian StyleZhang, Weilong, Miao Gao, Rui Hu, Yimin Shang, Minzhi Liu, Peichun Lan, Shuo Jiao, Gehong Wei, and Sanfeng Chen. 2024. "Nitrogen-Fixing Paenibacillus haidiansis and Paenibacillus sanfengchensis: Two Novel Species from Plant Rhizospheres" Microorganisms 12, no. 12: 2561. https://doi.org/10.3390/microorganisms12122561
APA StyleZhang, W., Gao, M., Hu, R., Shang, Y., Liu, M., Lan, P., Jiao, S., Wei, G., & Chen, S. (2024). Nitrogen-Fixing Paenibacillus haidiansis and Paenibacillus sanfengchensis: Two Novel Species from Plant Rhizospheres. Microorganisms, 12(12), 2561. https://doi.org/10.3390/microorganisms12122561






