Differential Transcriptomic Profile of Piscirickettsia salmonis LF-89 and EM-90 During an In Vivo Spatial Separation Co-Culture in Atlantic Salmon
Abstract
:1. Introduction
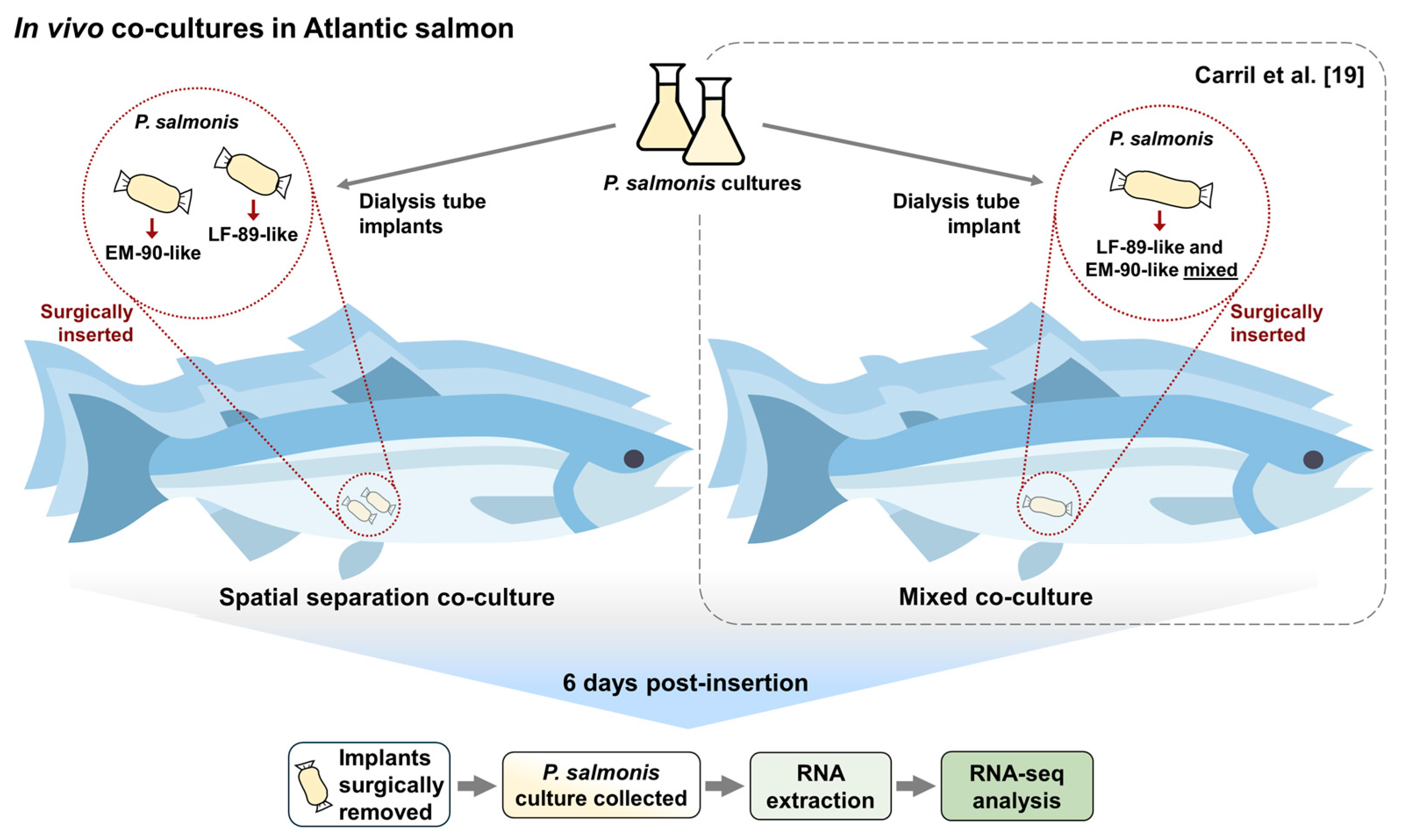
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fryer, J.L.; Hedrick, R.P. Piscirickettsia salmonis: A Gram-negative intracellular bacterial pathogen of fish. J. Fish Dis. 2003, 26, 251–262. [Google Scholar] [CrossRef]
- Sernapesca. Informe con Antecedentes Sanitarios de Agua Dulce y Mar; Primer Semestre–Año 2023; Servicio Nacional de Pesca y Acuicultura (Sernapesca); Ministerio de Economía, Fomento y Turismo, Gobierno de Chile: Valparaíso, Chile, 2013.
- Maisey, K.; Montero, R.; Christodoulides, M. Vaccines for piscirickettsiosis (salmonid rickettsial septicaemia, SRS): The Chile perspective. Expert Rev. Vaccines 2017, 16, 215–228. [Google Scholar] [CrossRef]
- SAG. Productos Biológicos Inmunológicos con Registro Provisional uso en Salmónidos 2024. Available online: https://www.sag.gob.cl/sites/default/files/lista_salmonidos_registro_provisional_02072024.pdf (accessed on 1 September 2024).
- Figueroa, C.; Torrealba, D.; Morales-Lange, B.; Mercado, L.; Dixon, B.; Conejeros, P.; Silva, G.; Soto, C.; Gallardo, J.A. Commercial Vaccines Do Not Confer Protection Against Two Genogroups of Piscirickettsia salmonis, LF-89 and EM-90, in Atlantic Salmon. Biology 2022, 11, 993. [Google Scholar] [CrossRef]
- Valenzuela-Aviles, P.; Torrealba, D.; Figueroa, C.; Mercado, L.; Dixon, B.; Conejeros, P.; Gallardo-Matus, J. Why vaccines fail against Piscirickettsiosis in farmed salmon and trout and how to avoid it: A review. Front. Immunol. 2022, 13, 1019404. [Google Scholar] [CrossRef] [PubMed]
- Bohle, H.; Henriquez, P.; Grothusen, H.; Navas, E.; Sandoval, A.; Bustamante, F.; Bustos, P.; Mancilla, M. Comparative Genome Analysis of Two Isolates of the Fish Pathogen Piscirickettsia salmonis from Different Hosts Reveals Major Differences in Virulence-Associated Secretion Systems. Genome Announc. 2014, 2, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bravo, C.; Martinez, V. Whole-genome comparative analysis of the pathogen Piscirickettsia salmonis. Vet. Microbiol. 2016, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nourdin-Galindo, G.; Sanchez, P.; Molina, C.F.; Espinoza-Rojas, D.A.; Oliver, C.; Ruiz, P.; Vargas-Chacoff, L.; Carcamo, J.G.; Figueroa, J.E.; Mancilla, M.; et al. Comparative Pan-Genome Analysis of Piscirickettsia salmonis Reveals Genomic Divergences within Genogroups. Front. Cell. Infect. Microbiol. 2017, 7, 459. [Google Scholar] [CrossRef]
- Isla, A.; Saldarriaga-Cordoba, M.; Fuentes, D.E.; Albornoz, R.; Haussmann, D.; Mancilla-Schulz, J.; Martinez, A.; Figueroa, J.; Avendano-Herrera, R.; Yanez, A. Multilocus sequence typing detects new Piscirickettsia salmonis hybrid genogroup in Chilean fish farms: Evidence for genetic diversity and population structure. J. Fish Dis. 2019, 42, 721–737. [Google Scholar] [CrossRef]
- Otterlei, A.; Brevik, O.J.; Jensen, D.; Duesund, H.; Sommerset, I.; Frost, P.; Mendoza, J.; McKenzie, P.; Nylund, A.; Apablaza, P. Phenotypic and genetic characterization of Piscirickettsia salmonis from Chilean and Canadian salmonids. BMC Vet. Res. 2016, 12, 55. [Google Scholar] [CrossRef]
- Saavedra, J.; Hernandez, N.; Osses, A.; Castillo, A.; Cancino, A.; Grothusen, H.; Navas, E.; Henriquez, P.; Bohle, H.; Bustamante, F. Prevalence, geographic distribution and phenotypic differences of Piscirickettsia salmonis EM-90-like isolates. J. Fish Dis. 2017, 40, 1055–1063. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Ildefonso, R.; Pena, A.; Enriquez, R.; Barrientos, S.; Maldonado, L. Comparative pathogenesis of piscirickettsiosis in Atlantic salmon (Salmo salar L.) post-smolt experimentally challenged with LF-89-like and EM-90-like Piscirickettsia salmonis isolates. J. Fish Dis. 2017, 40, 1451–1472. [Google Scholar] [CrossRef] [PubMed]
- Rozas-Serri, M.; Pena, A.; Arriagada, G.; Enriquez, R.; Maldonado, L. Comparison of gene expression in post-smolt Atlantic salmon challenged by LF-89-like and EM-90-like Piscirickettsia salmonis isolates reveals differences in the immune response associated with pathogenicity. J. Fish Dis. 2018, 41, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.I.; Griffen, A.A.; Birkbeck, T.H. Isolates of Piscirickettsia salmonis from Scotland and Ireland show evidence of clonal diversity. Appl. Environ. Microbiol. 2004, 70, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Tandberg, J.I.; Lagos, L.X.; Langlete, P.; Berger, E.; Rishovd, A.L.; Roos, N.; Varkey, D.; Paulsen, I.T.; Winther-Larsen, H.C. Comparative Analysis of Membrane Vesicles from Three Piscirickettsia salmonis Isolates Reveals Differences in Vesicle Characteristics. PLoS ONE 2016, 11, e0165099. [Google Scholar] [CrossRef]
- Schober, I.; Bunk, B.; Carril, G.; Freese, H.M.; Ojeda, N.; Riedel, T.; Meier-Kolthoff, J.P.; Goker, M.; Sproer, C.; Flores-Herrera, P.A.; et al. Ongoing diversification of the global fish pathogen Piscirickettsia salmonis through genetic isolation and transposition bursts. ISME J. 2023, 17, 2247–2258. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Pena, A.; Gardner, I.; Penaloza, E.; Maldonado, L.; Munoz, A.; Mardones, F.O.; Rodriguez, C.; Ildefonso, R.; Senn, C.; et al. Co-Infection by LF-89-Like and EM-90-Like Genogroups of Piscirickettsia salmonis in Farmed Atlantic Salmon in Chile: Implications for Surveillance and Control of Piscirickettsiosis. Pathogens 2023, 12, 450. [Google Scholar] [CrossRef]
- Carril, G.; Winther-Larsen, H.C.; Løvoll, M.; Sørum, H. Cohabitation of Piscirickettsia salmonis genogroups (LF-89 and EM-90): Synergistic effect on growth dynamics. Front. Cell. Infect. Microbiol. 2023, 13, 1253577. [Google Scholar] [CrossRef]
- Kinnula, H.; Mappes, J.; Sundberg, L.R. Coinfection outcome in an opportunistic pathogen depends on the inter-strain interactions. BMC Evol. Biol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Sundberg, L.R.; Ketola, T.; Laanto, E.; Kinnula, H.; Bamford, J.K.; Penttinen, R.; Mappes, J. Intensive aquaculture selects for increased virulence and interference competition in bacteria. Proc. Biol. Sci. 2016, 283, 20153069. [Google Scholar] [CrossRef]
- Carril, G.; Morales-Lange, B.; Løvoll, M.; Inami, M.; Winther-Larsen, H.C.; Øverland, M.; Sørum, H. Salmonid Rickettsial Septicemia (SRS) disease dynamics and Atlantic salmon immune response to Piscirickettsia salmonis LF-89 and EM-90 co-infection. Vet. Res. 2024, 55, 102. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-culturing microbial consortia: Approaches for applications in biomanufacturing and bioprocessing. Crit. Rev. Biotechnol. 2022, 42, 46–72. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.J.; Sørum, H.N. Outer membrane protein expression during in vivo cultivation of Vibrio salmonicida. Fish Shellfish Immun. 1998, 8, 367–377. [Google Scholar] [CrossRef]
- Morales-Lange, B.; Agboola, J.O.; Hansen, J.O.; Lagos, L.; Oyas, O.; Mercado, L.; Mydland, L.T.; Øverland, M. The Spleen as a Target to Characterize Immunomodulatory Effects of Down-Stream Processed Cyberlindnera jadinii Yeasts in Atlantic Salmon Exposed to a Dietary Soybean Meal Challenge. Front. Immunol. 2021, 12, 708747. [Google Scholar] [CrossRef] [PubMed]
- Gebrie, A. Transposable elements as essential elements in the control of gene expression. Mobile DNA 2023, 14, 9. [Google Scholar] [CrossRef]
- De Palmenaer, D.; Siguier, P.; Mahillon, J. IS4 family goes genomic. BMC Evol. Biol. 2008, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Lysnyansky, I.; Calcutt, M.J.; Ben-Barak, I.; Ron, Y.; Levisohn, S.; Methe, B.A.; Yogev, D. Molecular characterization of newly identified IS3, IS4 and IS30 insertion sequence-like elements in Mycoplasma bovis and their possible roles in genome plasticity. FEMS Microbiol. Lett. 2009, 294, 172–182. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef]
- Olasz, F.; Szabó, M.; Veress, A.; Bibó, M.; Kiss, J. The dynamic network of IS30 transposition pathways. PLoS ONE 2022, 17, e0271414. [Google Scholar] [CrossRef]
- Saavedra, J.; Grandon, M.; Villalobos-Gonzalez, J.; Bohle, H.; Bustos, P.; Mancilla, M. Isolation, Functional Characterization and Transmissibility of p3PS10, a Multidrug Resistance Plasmid of the Fish Pathogen Piscirickettsia salmonis. Front. Microbiol. 2018, 9, 923. [Google Scholar] [CrossRef]
- Li, P.; Zong, W.; Zhang, Z.; Lv, W.; Ji, X.; Zhu, D.; Du, X.; Wang, S. Effects and molecular mechanism of flagellar gene flgK on the motility, adhesion/invasion, and desiccation resistance of Cronobacter sakazakii. Food Res. Int. 2023, 164, 112418. [Google Scholar] [CrossRef]
- Holyoake, L.V.; Poole, R.K.; Shepherd, M. The CydDC Family of Transporters and Their Roles in Oxidase Assembly and Homeostasis. Adv. Microb. Physiol. 2015, 66, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Meibom, K.L.; Charbit, A. Francisella tularensis metabolism and its relation to virulence. Front. Microbiol 2010, 1, 140. [Google Scholar] [CrossRef] [PubMed]
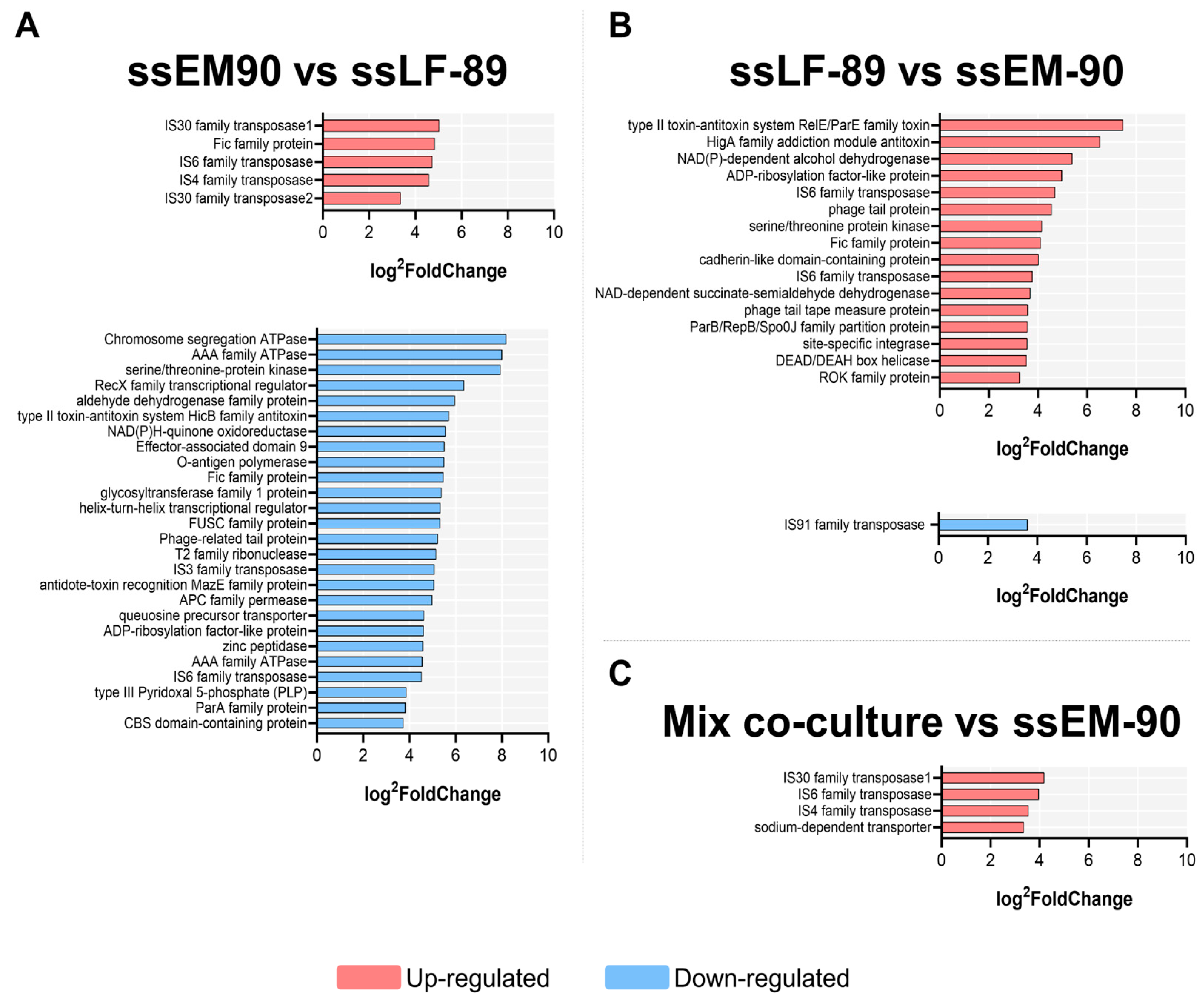

| Pairwise Comparison | Up | Down |
|---|---|---|
| ssEM-90 vs. ssLF-89 a | 7 | 50 |
| ssLF-89 vs. ssEM-90 b | 31 | 1 |
| Mixed co-culture vs. ssEM-90 c | 4 | 2 |
| Mixed co-culture vs. ssLF-89 d | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carril, G.; Winther-Larsen, H.C.; Løvoll, M.; Sørum, H. Differential Transcriptomic Profile of Piscirickettsia salmonis LF-89 and EM-90 During an In Vivo Spatial Separation Co-Culture in Atlantic Salmon. Microorganisms 2024, 12, 2480. https://doi.org/10.3390/microorganisms12122480
Carril G, Winther-Larsen HC, Løvoll M, Sørum H. Differential Transcriptomic Profile of Piscirickettsia salmonis LF-89 and EM-90 During an In Vivo Spatial Separation Co-Culture in Atlantic Salmon. Microorganisms. 2024; 12(12):2480. https://doi.org/10.3390/microorganisms12122480
Chicago/Turabian StyleCarril, Gabriela, Hanne C. Winther-Larsen, Marie Løvoll, and Henning Sørum. 2024. "Differential Transcriptomic Profile of Piscirickettsia salmonis LF-89 and EM-90 During an In Vivo Spatial Separation Co-Culture in Atlantic Salmon" Microorganisms 12, no. 12: 2480. https://doi.org/10.3390/microorganisms12122480
APA StyleCarril, G., Winther-Larsen, H. C., Løvoll, M., & Sørum, H. (2024). Differential Transcriptomic Profile of Piscirickettsia salmonis LF-89 and EM-90 During an In Vivo Spatial Separation Co-Culture in Atlantic Salmon. Microorganisms, 12(12), 2480. https://doi.org/10.3390/microorganisms12122480






