Screening for STIs: Results of a Health-Promotion Programme in a Portuguese University
Abstract
:1. Introduction
- How common are STIs among university students?
- What are the most common causes of STIs in the university population?
- Are the implemented treatments effective?
- Are the university members interested in STI screening?
- Is there an increase in participation in STI screening as a result of the awareness campaign?
2. Material and Methods
2.1. Set-Up of Protection+ Health-Promotion Programme
2.2. Protection+ Participants
2.3. Samples Collection and Processing
2.3.1. Urogenital Swabs
2.3.2. Urine Samples
2.3.3. Serum Samples
2.4. STI Screening
2.5. Statistical Analysis
3. Results
3.1. Protection+ Health-Promotion Programme Outcomes
3.2. Infection with C. trachomatis
3.3. Infection with M. genitalium
3.4. Infection with T. pallidum
3.5. Infection with N. gonorrhoeae
3.6. Infection with HIV, HBV and HCV
3.7. Infection with U. parvum, U. urealyticum and M. hominis
3.8. Co-Detection of Multiple Agents
3.9. Immunity to HAV
3.10. Follow-Up of STI Diagnosis
3.11. Impact of Awareness Campaigns on the Participation in STI Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, Gonorrhoea, Trichomoniasis and Syphilis: Global Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- McHaro, R.D.; Kisinda, A.; Njovu, L.; McHaro, M.; Mbwilo, F.; Mihale, G.; Komba, B.; Andrew, E.; Mayaud, P.; Kroidl, A.; et al. Prevalence of and Risk Factors Associated with HIV, Herpes Simplex Virus-Type 2, Chlamydia trachomatis and Neisseria gonorrhoeae Infections Among 18–24 Year Old Students Attending Higher Learning Institutions in Mbeya-Tanzania. PLoS ONE 2022, 17, e0266596. [Google Scholar] [CrossRef] [PubMed]
- Cannovo, N.; Bianchini, E.; Gironacci, L.; Garbati, E.; Di Prospero, F.; Cingolani, M.; Scendoni, R.; Fedeli, P. Sexually Transmitted Infections in Adolescents and Young Adults: A Cross Section of Public Health. Int. J. Environ. Res. Public Health 2024, 21, 501. [Google Scholar] [CrossRef] [PubMed]
- WHO Sexually Transmitted Infections (STIs). World Health Organization: WHO. Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Sexually-Transmitted-Infections-(Stis) (accessed on 20 September 2024).
- Tuddenham, S.; Hamill, M.M.; Ghanem, K.G. Diagnosis and Treatment of Sexually Transmitted Infections. JAMA 2022, 327, 161. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, N.; Chapagain, S. Need for Hepatitis B Vaccination in Medical Students. J. Nepal Med. Assoc. 2023, 61, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Rahangdale, L.; Mungo, C.; O’Connor, S.; Chibwesha, C.J.; Brewer, N.T. Human Papillomavirus Vaccination and Cervical Cancer Risk. BMJ 2022, 379, e070115. [Google Scholar] [CrossRef]
- Cole, S. Herpes Simplex Virus. Nurs. Clin. N. Am. 2020, 55, 337–345. [Google Scholar] [CrossRef]
- Barbier, F.; Mer, M.; Szychowiak, P.; Miller, R.F.; Mariotte, É.; Galicier, L.; Bouadma, L.; Tattevin, P.; Azoulay, É. Management of HIV-Infected Patients in the Intensive Care Unit. Intensive Care Med. 2020, 46, 329–342. [Google Scholar] [CrossRef]
- Sienkiewicz, L.; Thomas, Y.; Reynoso, A.; Munson, E. Incidence and Laboratory Diagnosis of Sexually-Transmitted Infections among University Students in a High-Prevalence Community. J. Am. Coll. Health 2023, 71, 571–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Zong, X.; Bai, H.; Fan, L.; Li, T.; Liu, Z. Prevalence of Mycoplasma genitalium and Chlamydia trachomatis in Chinese Female with Lower Reproductive Tract Infection: A Multicenter Epidemiological Survey. BMC Infect. Dis. 2023, 23, 2. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (Mpox) Virus: Classification, Origin, Transmission, Genome Organization, Antiviral Drugs, and Molecular Diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Al-Tammemi, A.B.; Sallam, M.; Rebhi, A.; Soliman, L.; Al Sarayrih, L.; Tarhini, Z.; Abutaima, R.; Aljaberi, M.A.; Barakat, M. The Outbreak of Ebola Virus Disease in 2022: A Spotlight on a Re-Emerging Global Health Menace. Narra J. 2022, 2, e97. [Google Scholar] [CrossRef] [PubMed]
- Major, C.G.; Paz-Bailey, G.; Hills, S.L.; Rodriguez, D.M.; Biggerstaff, B.J.; Johansson, M. Risk Estimation of Sexual Transmission of Zika Virus—United States, 2016–2017. J. Infect. Dis. 2021, 224, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Van Gerwen, O.T.; Muzny, C.A.; Marrazzo, J.M. Sexually Transmitted Infections and Female Reproductive Health. Nat. Microbiol. 2022, 7, 1116–1126. [Google Scholar] [CrossRef]
- Foschi, C.; Zagarrigo, M.; Belletti, M.; Marangoni, A.; Re, M.C.; Gaspari, V. Genital and Extra-Genital Chlamydia trachomatis and Neisseria gonorrhoeae Infections in Young Women Attending a Sexually Transmitted Infections (STI) Clinic. New Microbiol. 2020, 43, 115–120. [Google Scholar]
- Grad, A.I.; Vică, M.L.; Ungureanu, L.; Siserman, C.V.; Tătaru, A.D.; Matei, H.V. Assessment of STI Screening in Romania Using a Multiplex PCR Technique. J. Infect. Dev. Ctries. 2020, 14, 341–348. [Google Scholar] [CrossRef]
- Spindola, T.; de Melo, L.D.; Brandão, J.d.L.; de Oliveira, D.C.; Marques, S.C.; Arreguy-Sena, C.; Pinto, P.F. Social Representation of Young People in Higher Education about Sexually Transmitted Infections. Rev. Bras. Enferm. 2023, 76, e20220406. [Google Scholar] [CrossRef]
- Rodrigues, R.; Sousa, C.; Vale, N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics 2022, 12, 1795. [Google Scholar] [CrossRef]
- Scaglione, E.; Mantova, G.; Caturano, V.; Fanasca, L.; Carraturo, F.; Farina, F.; Pagliarulo, C.; Vitiello, M.; Pagliuca, C.; Salvatore, P.; et al. Molecular Epidemiology of Genital Infections in Campania Region: A Retrospective Study. Diagnostics 2022, 12, 1798. [Google Scholar] [CrossRef]
- ECDC European Centre for Disease Prevention and Control. Technical Report: Technologies, Strategies and Approaches for Testing Populations at Risk of Sexually Transmitted Infections in the EU/EEA; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021. [Google Scholar]
- Ezeanya-Bakpa, C.C.; Agbakoba, N.R.; Oguejiofor, C.B.; Enweani-Nwokelo, I.B. Sequence Analysis Reveals Asymptomatic Infection with Mycoplasma hominis and Ureaplasma urealyticum Possibly Leads to Infertility in Females: A Cross-Sectional Study. Int. J. Reprod. Biomed. 2021, 19, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, T.; Kong, Y.; Xie, X.; Ruan, Z. Ureaplasma Infections: Update on Epidemiology, Antimicrobial Resistance, and Pathogenesis. Crit. Rev. Microbiol. 2024, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Stol, K.; Jans, J.; De Bruin, L.O.; Unger, W.; Van Rossum, A. Perinatal Infections with Ureaplasma. Pediatr. Infect. Dis. J. 2021, 40, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- DGS Direção Geral de Saúde. Norma 058/2011; Diagnóstico e Rastreio Laboratorial Da Infeção Pelo Vírus Da Imunodeficiência Humana (VIH); Direção Geral de Saúde: Lisboa, Portugal, 2014. [Google Scholar]
- DGS Direção Geral de Saúde. Norma 027/2017; Avaliação Diagnóstica Da Infeção Por Vírus Da Hepatite C; Direção Geral de Saúde: Lisboa, Portugal, 2017. [Google Scholar]
- DGS Direção Geral de Saúde. Norma 003/2017; Hepatite A; Direção Geral de Saúde: Lisboa, Portugal, 2017. [Google Scholar]
- Cutoiu, A.; Boda, D. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis and Chlamydia trachomatis in Symptomatic and Asymptomatic Patients. Biomed. Rep. 2023, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Leli, C.; Mencacci, A.; Latino, M.A.; Clerici, P.; Rassu, M.; Perito, S.; Castronari, R.; Pistoni, E.; Luciano, E.; De Maria, D.; et al. Prevalence of Cervical Colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in Childbearing Age Women by a Commercially Available Multiplex Real-Time PCR: An Italian Observational Multicentre Study. J. Microbiol. Immunol. Infect. 2018, 51, 220–225. [Google Scholar] [CrossRef]
- Tadera, K.; Kitagawa, H.; Kitano, H.; Hara, T.; Kashiyama, S.; Nomura, T.; Omori, K.; Shigemoto, N.; Yokozaki, M.; Ohge, H. Prevalence of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum Detection in Urine and Respiratory Tract Samples in Hiroshima, Japan. Heliyon 2023, 9, e14543. [Google Scholar] [CrossRef]
- Karim, S.; Bouchikhi, C.; Bouchikhi, C.; Banani, A.; Fatemi, H.E.L.; Souho, T.; Erraghay, S.; Bennani, B.; Bennani, B. Detection of Ureaplasma Biovars and Subtyping of Ureaplasma parvum among Women Referring to a University Hospital in Morocco. Infect Dis Obstet Gynecol 2020, 2020, 7286820. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Li, R.H.; Zheng, L.Q.; Shang, X.H. Prevalence and Antimicrobial Susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Female Outpatients, 2009–2013. J. Microbiol. Immunol. Infect. 2016, 49, 359–362. [Google Scholar] [CrossRef]
- Molla, G.; Desalegn, A.; Tigu, F. Prevalence of Gonorrhea and Associated Knowledge, Attitude and Risky Behaviors and Preventive Practices Among High School Students: A Cross-Sectional Study. J. Community Health 2021, 46, 358–366. [Google Scholar] [CrossRef]
- Bashmaq, S.M.; Ahmadi, A.; Mohsenpour, B.; Rahmani, K.; Arasteh, M.; Alizadeh, N.S.; Babahajian, A.; Advay, S.; Abbaszadeh, A. Prevalence of HIV, HBV, HCV, HPV and Syphilis among Female Sex Workers in Kurdistan, West of Iran. Casp. J. Intern. Med. 2024, 15, 38–45. [Google Scholar] [CrossRef]
- Nijhuis, R.H.T.; Duinsbergen, R.G.; Pol, A.; Godschalk, P.C.R. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis Including Relevant Resistance-Associated Mutations in a Single Center in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 591–595. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control. Chlamydia. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Klavs, I.; Milavec, M.; Berlot, L.; Kustec, T.; Grgič-Vitek, M.; Lavtar, D.; Zaletel, M.; Golle, A.; Duh, D.; Čretnik, T.Ž. Prevalence of Sexually Transmitted Infections with Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis: Findings from the National Survey of Sexual Lifestyles, Attitudes and Health, Slovenia, 2016 to 2017. Eurosurveillance 2022, 27, 2100284. [Google Scholar] [CrossRef] [PubMed]
- Hedley, P.L.; Hoffmann, S.; Lausten-Thomsen, U.; Voldstedlund, M.; Bjerre, K.D.; Hviid, A.; Krebs, L.; Jensen, J.S.; Christiansen, M. A Nationwide Observational Study of Chlamydia trachomatis Infections in Denmark during the COVID-19 Pandemic. Acta Derm. Venereol. 2022, 102, 2324. [Google Scholar] [CrossRef] [PubMed]
- Desdorf, R.; Andersen, N.M.; Chen, M. Mycoplasma genitalium Prevalence and Macrolide Resistance-Associated Mutations and Coinfection with Chlamydia trachomatis in Southern Jutland, Denmark. APMIS 2021, 129, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.A.; Saddi, V.A.; Carneiro, M.A.; Figueiredo-Alves, R.R.; da Silva Barros, N.K.; de Almeida Carvalho, K.P.; do Nascimento Tavares, S.B.; de Araújo Teles, S.; D’Alessandro, W.B.; Rabelo-Santos, S.H. Human Papillomavirus and Chlamydia trachomatis Infections in Adolescents and Young Women: Prevalence and Risk Factors. Diagn. Cytopathol. 2020, 48, 736–744. [Google Scholar] [CrossRef]
- DGS Direção Geral de Saúde. Diário Da República, 2.a Série: Despacho No 1150-2021; Direção Geral de Saúde: Lisboa, Portugal, 2021; pp. 137–188. [Google Scholar]
- Gnanadurai, R.; Fifer, H. Mycoplasma genitalium: A Review. Microbiology 2020, 166, 21–29. [Google Scholar] [CrossRef]
- Uysal, H.; Koksal, M.O.; Sarsar, K.; Ilktac, M.; Isik, Z.; Akgun Karapinar, D.B.; Demirci, M.; Ongen, B.; Buyukoren, A.; Kadioglu, A.; et al. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium among Patients with Urogenital Symptoms in Istanbul. Healthcare 2023, 11, 930. [Google Scholar] [CrossRef]
- Yusuf, E.; Mertens, K.; Van Lisdonk, N.; Houwen, C.; Thai, K.T.D. Epidemiology of Mycoplasma genitalium and Trichomonas vaginalis in the Primary Health Care Setting in the Netherlands. Epidemiol. Infect. 2023, 151, e79. [Google Scholar] [CrossRef]
- Perry, M.D.; Jones, S.; Bertram, A.; de Salazar, A.; Barrientos-Durán, A.; Schiettekatte, G.; Lewinski, M.; Arcenas, R.; Hansra, A.; Njoya, M.; et al. The Prevalence of Mycoplasma genitalium (MG) and Trichomonas vaginalis (TV) at Testing Centers in Belgium, Germany, Spain, and the UK Using the Cobas TV/MG Molecular Assay. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 43–52. [Google Scholar] [CrossRef]
- Minetti, C.; Rocha, M.; Duque, L.M.; Meireles, P.; Correia, C.; Cordeiro, D.; João, I.; Manita, C.; Soeiro, S.; Santos, J.A.; et al. Orogenital and Anal Infection by Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Other Sexually Transmitted Infections in Men Who Have Sex with Men in Lisbon. Int. J. STD AIDS 2024, 35, 379–388. [Google Scholar] [CrossRef]
- Silva, J.; Cerqueira, F.; Teixeira, A.L.; Bicho, M.C.; Campainha, R.; Amorim, J.; Medeiros, R. Genital Mycoplasmas and Ureaplasmas in Cervicovaginal Self-Collected Samples of Reproductive-Age Women: Prevalence and Risk Factors. Int. J. STD AIDS 2018, 29, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Spiller, O.B.; Rees, C.L.; Morris, D.J.; Davies, R.L.; Jones, L.C. Mycoplasma genitalium Prevalence in Welsh Sexual Health Patients: Low Antimicrobial Resistance Markers and No Association of Symptoms to Bacterial Load. Microb. Pathog. 2020, 139, 103872. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control. Syphilis. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- ECDC European Centre for Disease Prevention and Control. Gonorrhoea. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Herrero, M.; Broner, S.; Cruells, A.; Esteve, S.; Ferré, L.; Mendioroz, J.; Jané, M.; Ciruela, P.; Benítez, M.Á.; Bosch, J.; et al. Epidemiology and Antimicrobial Resistance Profile of Neisseria gonorrhoeae in Catalonia, Spain, 2016–2019. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 883–893. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control; WHO Office for Europe. HIV/AIDS Surveillance in Europe—2022 Data; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2023. [Google Scholar]
- Razavi-Shearer, D.; Gamkrelidze, I.; Pan, C.; Jia, J.; Berg, T.; Gray, R.; Lim, Y.-S.; Chen, C.-J.; Ocama, P.; Desalegn, H.; et al. Global Prevalence, Cascade of Care, and Prophylaxis Coverage of Hepatitis B in 2022: A Modelling Study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Blach, S.; Manzengo Mingiedi, C.; Gonzalez, M.A.; Sabry Alaama, A.; Mozalevskis, A.; Séguy, N.; Rewari, B.B.; Chan, P.-L.; Le, L.; et al. Global Reporting of Progress towards Elimination of Hepatitis B and Hepatitis C. Lancet Gastroenterol. Hepatol. 2023, 8, 332–342. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- ECDC European Centre for Disease Prevention and Control. Hepatitis B. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Lakoh, S.; García-Tardón, N.; Adekanmbi, O.; van der Valk, M.; Smith, S.J.; Grobusch, M.P. Prevalence of Viral Hepatitis B and C in Sierra Leone—Current Knowledge and Knowledge Gaps: A Narrative Review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1106–1113. [Google Scholar] [CrossRef]
- ECDC European Centre for Disease Prevention and Control. Hepatitis C. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Kenyon, C.; Herrmann, B.; Hughes, G.; de Vries, H.J.C. Management of Asymptomatic Sexually Transmitted Infections in Europe: Towards a Differentiated, Evidence-Based Approach. Lancet Reg. Health Eur. 2023, 34, 100743. [Google Scholar] [CrossRef]
- Silva, J.; Cerqueira, F.; Teixeira, A.L.; Campainha, R.; Amorim, J.; Medeiros, R. Prevalence of Neisseria gonorrhoeae and Trichomonas vaginalis in Portuguese Women of Childbearing Age. J. Obstet. Gynaecol. 2021, 41, 254–258. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Camino, A.F.; Sharma, J.; Kissinger, P.J.; Muzny, C.A. Epidemiology, Natural History, Diagnosis, and Treatment of Trichomonas vaginalis in Men. Clin. Infect. Dis. 2021, 73, 1119–1124. [Google Scholar] [CrossRef]
- Kebbi-Beghdadi, C.; Aeby, S.; Baud, D.; Greub, G. Evaluation of a Multiplex Real-Time PCR Assay for Detecting Chlamydia trachomatis in Vaginal Samples. Diagnostics 2022, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Kasprzykowska, U.; Sobieszczańska, B.; Duda-Madej, A.; Secewicz, A.; Nowicka, J.; Gościniak, G. A Twelve–Year Retrospective Analysis of Prevalence and Antimicrobial Susceptibility Patterns of Ureaplasma spp. and Mycoplasma hominis in the Province of Lower Silesia in Poland. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yang, J.S. Prevalence and Antimicrobial Susceptibility of Mycoplasma hominis and Ureaplasma Species in Nonpregnant Female Patients in South Korea Indicate an Increasing Trend of Pristinamycin-Resistant Isolates. Antimicrob. Agents Chemother. 2020, 64, e01065-20. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.P.; Darós, A.C.; Darós, A.C.M.; De Castro, T.M.M.L.; De Vasconcelos Carneiro, M.; Fidelis, C.R.; Vilioni, M.V.; Da Costa Matsunaga, M.E.; Sidou, J.M.O.; Chaves, M.A.L.D.; et al. Cervical Cytology of Samples with Ureaplasma urealyticum, Ureaplasma parvum, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma hominis, and Neisseria gonorrhoeae Detected by Multiplex PCR. BioMed Res. Int. 2020, 2020, 7045217. [Google Scholar] [CrossRef]
- Cai, S.; Pan, J.; Duan, D.; Yu, C.; Yang, Z.; Zou, J. Prevalence of Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae in Gynecological Outpatients, Taizhou, China. J. Clin. Lab. Anal. 2020, 34, e23072. [Google Scholar] [CrossRef]
- Esen, B.; Gozalan, A.; Sevindi, D.F.; Demirbas, A.; Onde, U.; Erkayıran, U.; Karakoc, A.E.; Hasçiçek, A.M.; Ergün, Y.; Adiloglu, A.K. Ureaplasma urealyticum: Presence among Sexually Transmitted Diseases. Jpn. J. Infect. Dis. 2017, 70, 75–79. [Google Scholar] [CrossRef]
- Abutaleb, A.; Kottilil, S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol. Clin. N. Am. 2020, 49, 191–199. [Google Scholar] [CrossRef]
- Rosendal, E.; von Schreeb, S.; Gomes, A.; Lino, S.; Grau-Pujol, B.; Magalhães, S.; Ricoca Peixoto, V.; Roque, C.; Moreno, J.; Maltez, F.; et al. Ongoing Outbreak of Hepatitis A Associated with Sexual Transmission among Men Who Have Sex with Men, Portugal, October 2023 to April 2024. Eurosurveillance 2024, 29, 2400272. [Google Scholar] [CrossRef]
- ECDC European Centre for Disease Prevention and Control. Hepatitis A. Annual Epidemiological Report for 2022; ECDC European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Vilibic-Cavlek, T.; Zidovec-Lepej, S.; Ferenc, T.; Savic, V.; Nemeth-Blazic, T.; Vujica Ferenc, M.; Bogdanic, M.; Vilibic, M.; Simunov, B.; Janev-Holcer, N.; et al. Seroprevalence Trends and Molecular Epidemiology of Viral Hepatitis in Croatia. Life 2023, 13, 224. [Google Scholar] [CrossRef]
- Mossong, J.; Putz, L.; Patiny, S.; Schneider, F. Seroepidemiology of Hepatitis A and Hepatitis B Virus in Luxembourg. Epidemiol. Infect. 2006, 134, 808–813. [Google Scholar] [CrossRef]
- da Costa e Silva, G.R.; Martins, T.L.S.; de Almeida Silva, C.; Caetano, K.A.A.; dos Santos Carneiro, M.A.; e Silva, B.V.D.; Pacheco, L.R.; Villar, L.M.; de Paula, V.S.; Martins, R.M.B.; et al. Hepatitis A and E among Immigrants and Refugees in Central Brazil. Rev. Saude Publica 2022, 56, 29. [Google Scholar] [CrossRef] [PubMed]
- Wind, C.M.; Schim van der Loeff, M.F.; Unemo, M.; Schuurman, R.; van Dam, A.P.; de Vries, H.J.C. Time to Clearance of Chlamydia trachomatis RNA and DNA after Treatment in Patients Coinfected with Neisseria gonorrhoeae—A Prospective Cohort Study. BMC Infect. Dis. 2016, 16, 554. [Google Scholar] [CrossRef] [PubMed]
- Dalby, J.; Stoner, B. Sexually Transmitted Infections: Updates From the 2021 CDC Guidelines. Am. Fam. Phys. 2022, 105, 514–520. [Google Scholar]
- Geisler, W.M.; Morrison, S.G.; Doemland, M.L.; Iqbal, S.M.; Su, J.; Mancevski, A.; Hook, E.W.; Morrison, R.P. Immunoglobulin-Specific Responses to Chlamydia Elementary Bodies in Individuals with and at Risk for Genital Chlamydial Infection. J. Infect. Dis. 2012, 206, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, Z.W.; Hoenderboom, B.M.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.H.T.M.; Götz, H.M.; van der Sande, M.A.B.; de Vries, H.J.C.; den Hartog, J.E.; Morré, S.A.; van Benthem, B.H.B. Reproductive Tract Complication Risks Following Chlamydia trachomatis Infections: A Long-Term Prospective Cohort Study from 2008 to 2022. Lancet Reg. Health Eur. 2024, 45, 101027. [Google Scholar] [CrossRef]
- Rondeau, P.; Valin, N.; Decré, D.; Girard, P.M.; Lacombe, K.; Surgers, L. Chlamydia trachomatis Screening in Urine among Asymptomatic Men Attending an STI Clinic in Paris: A Cross-Sectional Study. BMC Infect. Dis. 2019, 19, 31. [Google Scholar] [CrossRef]

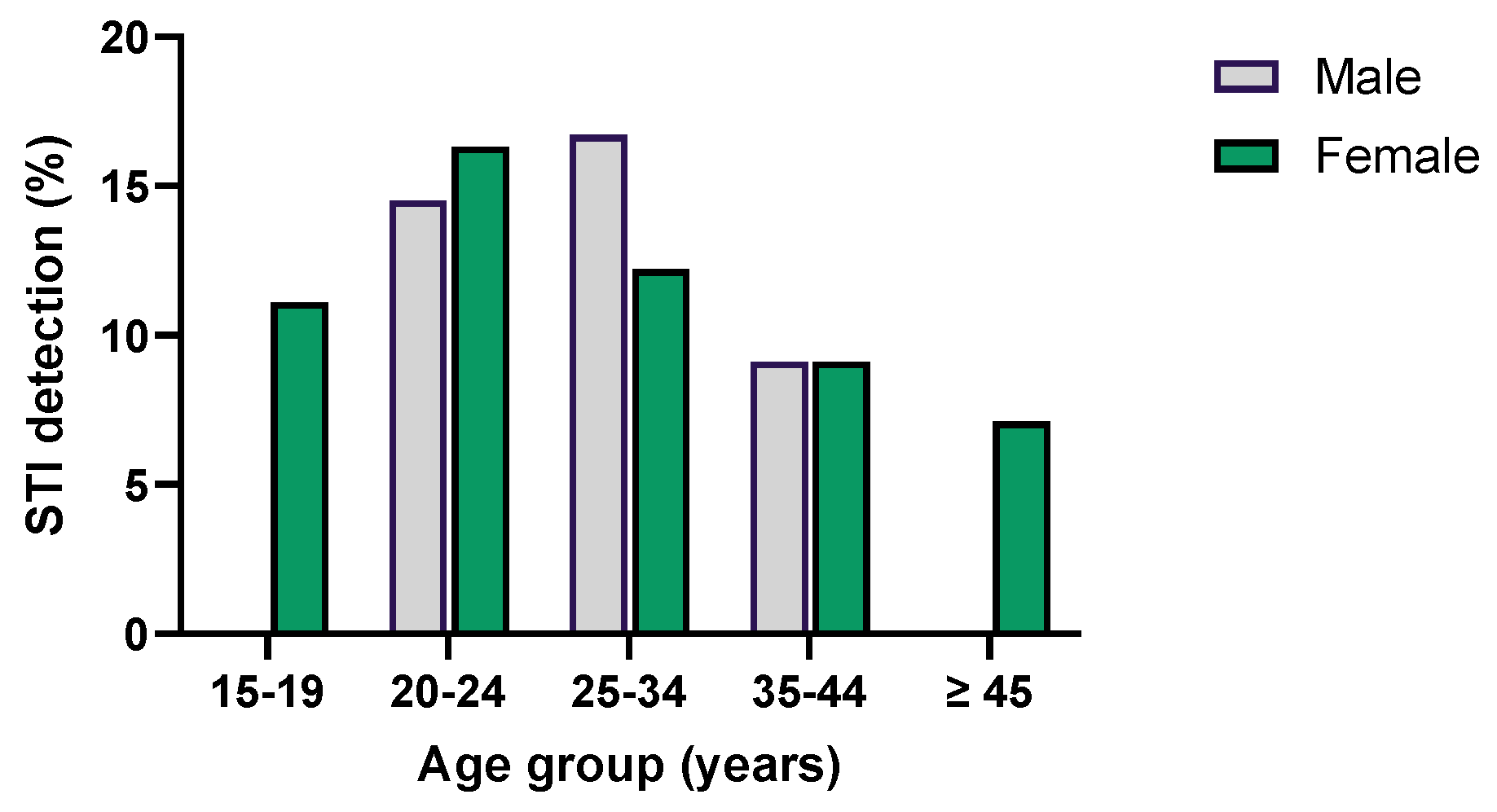





| Microorganism | Total | GENDER | AGE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 15–19 | 20–24 | 25–34 | 35–44 | ≥45 | ||||||||||
| Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | Tested | Detected (%) | |
| C. trachomatis | 475 | 40 (8.4%) | 176 | 9 (5.1%) | 299 | 31 (10.4%) | 31 | 1 (3.2%) | 243 | 26 (10.7%) | 140 | 11 (7.9%) | 44 | 2 (4.5%) | 17 | 0 (0%) |
| M. genitalium | 475 | 11 (2.3%) | 176 | 6 (3.4%) | 299 | 5 (1.7%) | 31 | 0 (0%) | 243 | 4 (1.6%) | 140 | 6 (4.3%) | 44 | 0 (0%) | 17 | 1 (5.9%) |
| T. pallidum | 345 | 7 (2.0%) | 152 | 6 (3.9%) | 193 | 1 (0.5%) | 27 | 0 (0%) | 184 | 4 (2.1%) | 100 | 3 (2.9%) | 27 | 0 (0%) | 7 | 0 (0%) |
| N. gonorrhoeae | 475 | 5 (1.1%) | 176 | 2 (1.1%) | 299 | 3 (1.0%) | 31 | 1 (3.2%) | 243 | 4 (1.6%) | 140 | 0 (0%) | 44 | 0 (0%) | 17 | 0 (0%) |
| HIV | 342 | 2 (0.6%) | 150 | 2 (1.3%) | 192 | 0 (0%) | 27 | 0 (0%) | 183 | 0 (0%) | 97 | 1 (1.0%) | 28 | 1 (3.6%) | 7 | 0 (0%) |
| HBV | 336 | 2 (0.6%) | 143 | 2 (1.4%) | 193 | 0 (0%) | 27 | 0 (0%) | 176 | 0 (0%) | 98 | 2 (2.0%) | 28 | 0 (0%) | 7 | 0 (0%) |
| HCV | 343 | 1 (0.3%) | 150 | 0 (0%) | 193 | 1 (0.5%) | 27 | 0 (0%) | 184 | 0 (0%) | 98 | 0 (0%) | 27 | 1 (3.7%) | 7 | 0 (0%) |
| T. vaginalis | 225 | 0 (0%) | 60 | 0 (0%) | 154 | 0 (0%) | 6 | 0 (0%) | 103 | 0 (0%) | 74 | 0 (0%) | 28 | 0 (0%) | 14 | 0 (0%) |
| U. parvum | 114 | 41 (36.0%) | 41 | 5 (12.2%) | 73 | 36 (49.3%) | 3 | 1 (33.3%) | 58 | 20 (34.5%) | 37 | 15 (40.5%) | 12 | 4 (33.3%) | 4 | 1 (25.0%) |
| U. urealyticum | 114 | 16 (14.0%) | 41 | 3 (7.3%) | 73 | 13 (17.9%) | 3 | 1 (33.3%) | 58 | 8 (13.8%) | 37 | 6 (16.2%) | 12 | 0 (0%) | 4 | 1 (25.0%) |
| M. hominis | 114 | 11 (9.6%) | 41 | 0 (0%) | 73 | 11 (15.1%) | 3 | 0 (0%) | 58 | 5 (8.6%) | 37 | 5 (13.5%) | 12 | 1 (8.3%) | 4 | 0 (0%) |
| HAV * | 295 | * | 130 | * | 165 | * | 26 | * | 165 | * | 80 | * | 20 | * | 4 | * |
| Pathogenic Agent(s) | Commensal Agent(s) | Number of Cases |
|---|---|---|
| C. trachomatis; M. genitalium | - | 1 |
| T. pallidum; M. genitalium | - | 1 |
| T. pallidum; HIV | - | 1 |
| C. trachomatis | U. parvum | 5 |
| C. trachomatis | U. urealyticum; M. hominis | 2 |
| C. trachomatis | U. parvum; U. urealyticum; M. hominis | 2 |
| C. trachomatis | U. parvum; M. hominis | 1 |
| C. trachomatis | U. urealyticum | 1 |
| M. genitalium | U. parvum; U. urealyticum; M. hominis | 1 |
| HCV | U. parvum | 1 |
| U. parvum; M. hominis | 3 | |
| U. parvum; U. urealyticum; M. hominis | 2 | |
| U. parvum; U. urealyticum | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.M.; Martins, A.H.; Veiga, D.; Lavaredas, C.; Queirós, A.; Matos, A.M. Screening for STIs: Results of a Health-Promotion Programme in a Portuguese University. Microorganisms 2024, 12, 2479. https://doi.org/10.3390/microorganisms12122479
Oliveira JM, Martins AH, Veiga D, Lavaredas C, Queirós A, Matos AM. Screening for STIs: Results of a Health-Promotion Programme in a Portuguese University. Microorganisms. 2024; 12(12):2479. https://doi.org/10.3390/microorganisms12122479
Chicago/Turabian StyleOliveira, Joana M., Ana Helena Martins, Daniela Veiga, Célia Lavaredas, António Queirós, and Ana Miguel Matos. 2024. "Screening for STIs: Results of a Health-Promotion Programme in a Portuguese University" Microorganisms 12, no. 12: 2479. https://doi.org/10.3390/microorganisms12122479
APA StyleOliveira, J. M., Martins, A. H., Veiga, D., Lavaredas, C., Queirós, A., & Matos, A. M. (2024). Screening for STIs: Results of a Health-Promotion Programme in a Portuguese University. Microorganisms, 12(12), 2479. https://doi.org/10.3390/microorganisms12122479






