The Role of Fecal Microbiota Transplantation in the Allogeneic Stem Cell Transplant Setting
Abstract
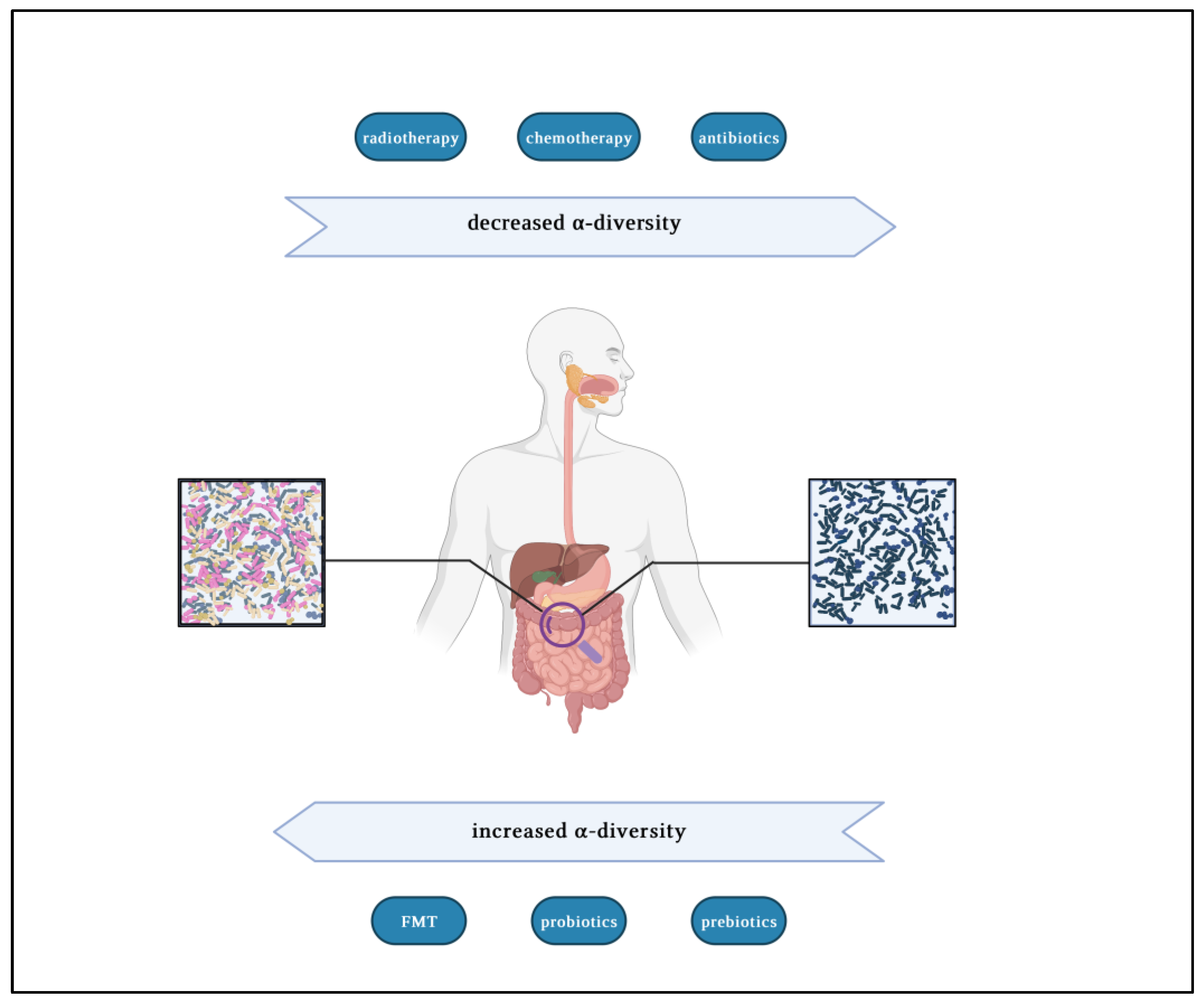
:1. Literature Review
2. Introduction
3. Microbiota Changes during Allogeneic Stem Cell Transplantation
3.1. Antibiotics Affect the Gut Microbiota
3.2. Conditioning Affects the Gut Microbiota
3.3. Diet Affects Microbiota
4. Restoration of Microbiota
4.1. Prebiotics and Probiotics
4.2. Fecal Microbiota Transplantation
5. Microbiota Changes Associated with GvHD
Fecal Microbiota Transplantation for GvHD
6. Fecal Microbiota Transplantation for Bacterial Infection Treatment or Prophylaxis
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Fernandez, L.B.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Chi, M.; Jiang, T.; He, X.; Peng, H.; Li, Y.; Zhang, J.; Wang, L.; Nian, Q.; Ma, K.; Liu, C. Role of Gut Microbiota and Oxidative Stress in the Progression of Transplant-Related Complications following Hematopoietic Stem Cell Transplantation. Oxidative Med. Cell. Longev. 2023, 2023, 3532756. [Google Scholar] [CrossRef]
- Zeiser, R.; Warnatz, K.; Rosshart, S.; Sagar; Tanriver, Y. GVHD, IBD, and primary immunodeficiencies: The gut as a target of immunopathology resulting from impaired immunity. Eur. J. Immunol. 2022, 52, 1406–1418. [Google Scholar] [CrossRef]
- Köhler, N.; Zeiser, R. Intestinal Microbiota Influence Immune Tolerance Post Allogeneic Hematopoietic Cell Transplantation and Intestinal GVHD. Front. Immunol. 2019, 9, 3179. [Google Scholar] [CrossRef]
- Gao, B.; Xiang, X. Interleukin-22 from bench to bedside: A promising drug for epithelial repair. Cell. Mol. Immunol. 2019, 16, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F. IL-22, a new beacon in gastrointestinal aGVHD. Blood 2023, 141, 1369–1370. [Google Scholar] [CrossRef]
- Henig, I.; Yehudai-Ofir, D.; Zuckerman, T. The clinical role of the gut microbiome and fecal microbiota transplantation in allogeneic stem cell transplantation. Haematologica 2021, 106, 933–946. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, C.R.; Sudakaran, S.; Callander, N.S. Clinical effects and applications of the gut microbiome in hematologic malignancies. Cancer 2021, 127, 679–687. [Google Scholar] [CrossRef]
- Chong, P.P.; Koh, A.Y. The gut microbiota in transplant patients. Blood Rev. 2020, 39, 100614. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.-A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Xiong, Q.; Wang, L.; Wang, L.; Li, F.; Hou, C.; Dou, L.; Zhu, B.; Liu, D. The impact of intestinal microbiota in antithymocyte globulin–based myeloablative allogeneic hematopoietic cell transplantation. Cancer 2022, 128, 1402–1410. [Google Scholar] [CrossRef]
- Shallis, R.M.; Terry, C.M.; Lim, S.H. Changes in intestinal microbiota and their effects on allogeneic stem cell transplantation. Am. J. Hematol. 2018, 93, 122–128. [Google Scholar] [CrossRef]
- Hayase, E.; Hayase, T.; Jamal, M.A.; Miyama, T.; Chang, C.-C.; Ortega, M.R.; Ahmed, S.S.; Karmouch, J.L.; Sanchez, C.A.; Brown, A.N.; et al. Mucus-degrading Bacteroides link carbapenems to aggravated graft-versus-host disease. Cell 2022, 185, 3705–3719.e14. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef] [PubMed]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss of Diversity Is Associated with Use of Systemic Antibiotics and More Pronounced in Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef]
- Weber, D.; Oefner, P.J.; Dettmer, K.; Hiergeist, A.; Koestler, J.; Gessner, A.; Weber, M.; Stämmler, F.; Hahn, J.; Wolff, D.; et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 1087–1092. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lim, J.-Y.; Ryu, D.-B.; Kim, T.W.; Park, S.S.; Jeon, Y.-W.; Yoon, J.-H.; Cho, B.-S.; Eom, K.-S.; Kim, Y.-J.; et al. Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 1933–1943. [Google Scholar] [CrossRef]
- Montassier, E.; Batard, E.; Massart, S.; Gastinne, T.; Carton, T.; Caillon, J.; Le Fresne, S.; Caroff, N.; Hardouin, J.B.; Moreau, P.; et al. 16S rRNA Gene Pyrosequencing Reveals Shift in Patient Faecal Microbiota During High-Dose Chemotherapy as Conditioning Regimen for Bone Marrow Transplantation. Microb. Ecol. 2014, 67, 690–699. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Hohmann, E.; Jenq, R.R.; Chen, Y.-B. Fecal Microbiota Transplantation: Restoring the Injured Microbiome after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, e17–e22. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Chiusolo, P.; Metafuni, E.; Sterbini, F.P.; Giammarco, S.; Masucci, L.; Leone, G.; Sica, S. Gut Microbiome Changes after Stem Cell Transplantation. Blood 2015, 126, 1953. [Google Scholar] [CrossRef]
- Kouidhi, S.; Souai, N.; Zidi, O.; Mosbah, A.; Lakhal, A.; Ben Othmane, T.; Belloumi, D.; Ben Ayed, F.; Asimakis, E.; Stathopoulou, P.; et al. High Throughput Analysis Reveals Changes in Gut Microbiota and Specific Fecal Metabolomic Signature in Hematopoietic Stem Cell Transplant Patients. Microorganisms 2021, 9, 1845. [Google Scholar] [CrossRef]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 13254–13259. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.-X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef]
- Shono, Y.; Brink, M.R.M.v.D. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef]
- Beckman, M.F.; Morton, D.S.; Mougeot, F.B.; Mougeot, J.-L.C. Allogenic stem cell transplant-associated acute graft versus host disease: A computational drug discovery text mining approach using oral and gut microbiome signatures. Support. Care Cancer 2021, 29, 1765–1779. [Google Scholar] [CrossRef]
- Beckerson, J.; Szydlo, R.M.; Hickson, M.; Mactier, C.E.; Innes, A.J.; Gabriel, I.H.; Palanicawandar, R.; Kanfer, E.J.; Macdonald, D.H.; Milojkovic, D.; et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin. Nutr. 2019, 38, 738–744. [Google Scholar] [CrossRef]
- Khuat, L.T.; Le, C.T.; Pai, C.-C.S.; Shields-Cutler, R.R.; Holtan, S.G.; Rashidi, A.; Parker, S.L.; Knights, D.; Luna, J.I.; Dunai, C.; et al. Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation. Sci. Transl. Med. 2020, 12, eaay7713. [Google Scholar] [CrossRef]
- Andermann, T.M.; Rezvani, A.; Bhatt, A.S. Microbiota Manipulation With Prebiotics and Probiotics in Patients Undergoing Stem Cell Transplantation. Curr. Hematol. Malign. Rep. 2016, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Eilers, J.; Berger, A.M.; Petersen, M.C. Development, testing, and application of the oral assessment guide. Oncol. Nurs. Forum 1988, 15, 325–330. [Google Scholar] [PubMed]
- Yoshifuji, K.; Inamoto, K.; Kiridoshi, Y.; Takeshita, K.; Sasajima, S.; Shiraishi, Y.; Yamashita, Y.; Nisaka, Y.; Ogura, Y.; Takeuchi, R.; et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020, 4, 4607–4617. [Google Scholar] [CrossRef]
- Muratore, E.; Leardini, D.; Baccelli, F.; Venturelli, F.; Prete, A.; Masetti, R. Nutritional modulation of the gut microbiome in allogeneic hematopoietic stem cell transplantation recipients. Front. Nutr. 2022, 9, 993668. [Google Scholar] [CrossRef] [PubMed]
- Giammarco, S.; Chiusolo, P.; Di Giovanni, A.; Metafuni, E.; Sica, S.; Iavarone, F.; Marini, F.; Castagnola, M. A Pilot Study on the Efficacy of Lactobacillus Brevis CD2 Lozenges in Preventing Oral Mucositis by High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2016, 16, S79. [Google Scholar] [CrossRef]
- Gorshein, E.; Wei, C.; Ambrosy, S.; Budney, S.; Vivas, J.; Shenkerman, A.; Manago, J.; McGrath, M.K.; Tyno, A.; Lin, Y.; et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin. Transplant. 2017, 31, 5. [Google Scholar] [CrossRef]
- López, P.; González-Rodríguez, I.; Gueimonde, M.; Margolles, A.; Suárez, A. Immune Response to Bifidobacterium bifidum Strains Support Treg/Th17 Plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef] [PubMed]
- Yazdandoust, E.; Hajifathali, A.; Roshandel, E.; Zarif, M.N.; Pourfathollah, A.A.; Parkhideh, S.; Mehdizadeh, M.; Amini-Kafiabad, S. Gut microbiota intervention by pre and probiotics can induce regulatory T cells and reduce the risk of severe acute GVHD following allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2023, 78, 101836. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Kazadi, D.; Halaweish, H.; Khan, M.H.; Hoeschen, A.; Cao, Q.; Luo, X.; et al. Randomized Double-Blind Phase II Trial of Fecal Microbiota Transplantation Versus Placebo in Allogeneic Hematopoietic Cell Transplantation and AML. J. Clin. Oncol. 2023, JCO2202366. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef]
- Shouval, R.; Youngster, I.; Geva, M.; Eshel, A.; Danylesko, I.; Shimoni, A.; Beider, K.; A Fein, J.; Sharon, I.; Koren, O.; et al. Repeated Courses of Orally Administered Fecal Microbiota Transplantation for the Treatment of Steroid Resistant and Steroid Dependent Intestinal Acute Graft Vs. Host Disease: A Pilot Study (NCT 03214289). Blood 2018, 132, 2121. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Davids, M.; Haverkate, N.J.E.; de Groot, P.F.; Donker, M.L.; Meijer, E.; Heubel-Moenen, F.C.J.I.; Nur, E.; Zeerleder, S.S.; Nieuwdorp, M.; et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020, 12, eaaz8926. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Peled, J.U.; Li, S.; Mahabamunuge, J.; Dagher, Z.; Slingerland, A.E.; Del Rio, C.; Valles, B.; Kempner, M.E.; Smith, M.; et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018, 2, 745–753. [Google Scholar] [CrossRef]
- Dougé, A.; Ravinet, A.; Corriger, A.; Cabrespine, A.; Wasiak, M.; Pereira, B.; Sokol, H.; Nguyen, S.; Bay, J.-O. Faecal microbiota transplantation to prevent complications after allogeneic stem cell transplantation for haematological malignancies: A study protocol for a randomised controlled phase-II trial (the FMT-allo study). BMJ Open 2023, 13, e068480. [Google Scholar] [CrossRef]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.-R.; Sun, Y.; Rossi, C.; et al. Gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef]
- Sangiorgi, B.; Panepucci, R.A. Modulation of Immunoregulatory Properties of Mesenchymal Stromal Cells by Toll-Like Receptors: Potential Applications on GVHD. Stem Cells Int. 2016, 2016, 9434250. [Google Scholar] [CrossRef] [PubMed]
- Payen, M.; Nicolis, I.; Robin, M.; Michonneau, D.; Delannoye, J.; Mayeur, C.; Kapel, N.; Berçot, B.; Butel, M.-J.; Le Goff, J.; et al. Functional and phylogenetic alterations in gut microbiome are linked to graft-versus-host disease severity. Blood Adv. 2020, 4, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Nitti, R.; Mancini, N.; Pasciuta, R.; Lorentino, F.; Lupo-Stanghellini, M.T.; Barbanti, M.C.; Clementi, N.; Giglio, F.; Clerici, D.; et al. Microbiome markers are early predictors of acute GVHD in allogeneic hematopoietic stem cell transplant recipients. Blood 2021, 137, 1556–1559. [Google Scholar] [CrossRef]
- Qi, L.; Huang, X.; He, C.; Ji, D.; Li, F. Steroid-resistant intestinal aGVHD and refractory CMV and EBV infections complicated by haplo-HSCT were successfully rescued by FMT and CTL infusion. J. Int. Med Res. 2021, 49, 3000605211063292. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.-Z. IL-17A ≠ Th17 in GvHD. Cell. Mol. Immunol. 2018, 15, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Schluter, J.; Gomes, A.L.C.; Littmann, E.R.; Pickard, A.J.; Taylor, B.P.; Giardina, P.A.; Weber, D.; Dai, A.; Docampo, M.D.; et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood 2020, 136, 130–136. [Google Scholar] [CrossRef]
- Biliński, J.; Jasiński, M.; Basak, G.W. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines 2022, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Doki, N.; Suyama, M.; Sasajima, S.; Ota, J.; Igarashi, A.; Mimura, I.; Morita, H.; Fujioka, Y.; Sugiyama, D.; Nishikawa, H.; et al. Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2017, 96, 1517–1523. [Google Scholar] [CrossRef]
- Han, L.; Zhao, K.; Li, Y.; Han, H.; Zhou, L.; Ma, P.; Fan, Z.; Sun, H.; Jin, H.; Jiang, Z.; et al. A gut microbiota score predicting acute graft-versus-host disease following myeloablative allogeneic hematopoietic stem cell transplantation. Am. J. Transplant. 2020, 20, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Hase, K. Commensal microbiota regulates T cell fate decision in the gut. Semin. Immunopathol. 2015, 37, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3 + regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Kaysen, A.; Heintz-Buschart, A.; Muller, E.E.; Narayanasamy, S.; Wampach, L.; Laczny, C.C.; Graf, N.; Simon, A.; Franke, K.; Bittenbring, J.; et al. Integrated meta-omic analyses of the gastrointestinal tract microbiome in patients undergoing allogeneic hematopoietic stem cell transplantation. Transl. Res. 2017, 186, 79–94.e1. [Google Scholar] [CrossRef] [PubMed]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2010, 163, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal Microbiota, Microbial Translocation, and Systemic Inflammation in Chronic HIV Infection. J. Infect. Dis. 2014, 211, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Frank, D.N.; Horch, M.; Chau, S.; Ir, D.; A Horch, E.; Tretina, K.; van Besien, K.; A Lozupone, C.; Nguyen, V.H. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transplant. 2017, 52, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Paczesny, S. Various Forms of Tissue Damage and Danger Signals Following Hematopoietic Stem-Cell Transplantation. Front. Immunol. 2015, 6, 14. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, T.; Yeoh, Y.K.; Cheng, F.W.T.; Liu, Q.; Tang, W.; Cheung, K.C.Y.; Yang, K.; Cheung, C.P.; Mo, C.C.; et al. Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat. Commun. 2021, 12, 65. [Google Scholar] [CrossRef]
- Biagi, E.; Zama, D.; Nastasi, C.; Consolandi, C.; Fiori, J.; Rampelli, S.; Turroni, S.; Centanni, M.; Severgnini, M.; Peano, C.; et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015, 50, 992–998. [Google Scholar] [CrossRef]
- Su, F.; Luo, Y.; Yu, J.; Shi, J.; Zhao, Y.; Yan, M.; Huang, H.; Tan, Y. Tandem fecal microbiota transplantation cycles in an allogeneic hematopoietic stem cell transplant recipient targeting carbapenem-resistant Enterobacteriaceae colonization: A case report and literature review. Eur. J. Med Res. 2021, 26, 37. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Antin, J.H. Therapeutic options for steroid-refractory acute and chronic GVHD: An evolving landscape. Expert Rev. Hematol. 2020, 13, 519–532. [Google Scholar] [CrossRef]
- Severyn, C.J.; Brewster, R.; Andermann, T.M. Microbiota modification in hematology: Still at the bench or ready for the bedside? Hematology 2019, 2019, 303–314. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, X.; Zhao, Y.; Wu, X.; Chen, F.; Ma, X.; Zhang, F.; Wu, D. Treating Steroid Refractory Intestinal Acute Graft-vs.-Host Disease With Fecal Microbiota Transplantation: A Pilot Study. Front. Immunol. 2018, 9, 2195. [Google Scholar] [CrossRef] [PubMed]
- Kaito, S.; Toya, T.; Yoshifuji, K.; Kurosawa, S.; Inamoto, K.; Takeshita, K.; Suda, W.; Kakihana, K.; Honda, K.; Hattori, M.; et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018, 2, 3097–3101. [Google Scholar] [CrossRef]
- Mao, D.B.; Jiang, Q.B.; Sun, Y.B.; Mao, Y.B.; Guo, L.B.; Zhang, Y.B.; Man, M.B.; Ouyang, G.; Sheng, L. Treatment of intestinal graft-versus-host disease with unrelated donor fecal microbiota transplantation capsules. Medicine 2020, 99, e22129. [Google Scholar] [CrossRef] [PubMed]
- Biernat, M.M.; Urbaniak-Kujda, D.; Dybko, J.; Kapelko-Słowik, K.; Prajs, I.; Wróbel, T. Fecal microbiota transplantation in the treatment of intestinal steroid-resistant graft-versus-host disease: Two case reports and a review of the literature. J. Int. Med Res. 2020, 48, 300060520925693. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft- versus -host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef]
- Goeser, F.; Sifft, B.; Stein-Thoeringer, C.; Farowski, F.; Strassburg, C.P.; Brossart, P.; Higgins, P.G.; Scheid, C.; Wolf, D.; Holderried, T.A.W.; et al. Fecal microbiota transfer for refractory intestinal graft-versus-host disease—Experience from two German tertiary centers. Eur. J. Haematol. 2021, 107, 229–245. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Qi, J.; Ma, X.; Qi, X.; Wu, D.; Xu, Y. Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Exp. Hematol. Oncol. 2022, 11, 96. [Google Scholar] [CrossRef]
- Malard, F.; Vekhoff, A.; Lapusan, S.; Isnard, F.; D’incan-Corda, E.; Rey, J.; Saillard, C.; Thomas, X.; Ducastelle-Lepretre, S.; Paubelle, E.; et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat. Commun. 2021, 12, 3084. [Google Scholar] [CrossRef]
- Bansal, R.; Park, H.; Taborda, C.C.; Gordillo, C.; Mapara, M.Y.; Assal, A.; Uhlemann, A.-C.; Reshef, R. Antibiotic Exposure, Not Alloreactivity, Is the Major Driver of Microbiome Changes in Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2022, 28, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Lindner, S.; Gomes, A.L.C.; Devlin, S.M.; Shah, G.L.; Sung, A.D.; Sauter, C.S.; Landau, H.J.; Dahi, P.B.; Perales, M.-A.; et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: A multicenter observational study. Blood 2021, 137, 1527–1537. [Google Scholar] [CrossRef]
- E Ilett, E.; Helleberg, M.; Reekie, J.; Murray, D.D.; Wulff, S.M.; Khurana, M.P.; Mocroft, A.; Daugaard, G.; Perch, M.; Rasmussen, A.; et al. Incidence Rates and Risk Factors of Clostridioides difficile Infection in Solid Organ and Hematopoietic Stem Cell Transplant Recipients. Open Forum Infect. Dis. 2019, 6, ofz086. [Google Scholar] [CrossRef] [PubMed]
- Chopra, T.; Chandrasekar, P.; Salimnia, H.; Heilbrun, L.K.; Smith, D.; Alangaden, G.J. Recent epidemiology of Clostridium difficile infection during hematopoietic stem cell transplantation. Clin. Transplant. 2011, 25, E82–E87. [Google Scholar] [CrossRef]
- Alonso, C.D.; Dufresne, S.F.; Hanna, D.B.; Labbé, A.-C.; Treadway, S.B.; Neofytos, D.; Bélanger, S.; Huff, C.A.; Laverdière, M.; Marr, K.A. Clostridium difficile Infection after Adult Autologous Stem Cell Transplantation: A Multicenter Study of Epidemiology and Risk Factors. Biol. Blood Marrow Transplant. 2013, 19, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Luna, A.J.; Carlson, T.J.; Garey, K.W. Gut microbiota changes associated with Clostridioides difficile infection and its various treatment strategies. Gut Microbes 2023, 15, 2223345. [Google Scholar] [CrossRef]
- Tannock, G.W.; Munro, K.; Taylor, C.; Lawley, B.; Young, W.; Byrne, B.; Emery, J.; Louie, T. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 2010, 156, 3354–3359. [Google Scholar] [CrossRef]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.-K. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. 2), S1–S21. [Google Scholar] [CrossRef]
- Webb, B.; Brunner, A.; Ford, C.; Gazdik, M.; Petersen, F.; Hoda, D. Fecal microbiota transplantation for recurrent Clostridium difficileinfection in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2016, 18, 628–633. [Google Scholar] [CrossRef]
- Bluestone, H.; Kronman, M.P.; Suskind, D.L. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infections in Pediatric Hematopoietic Stem Cell Transplant Recipients. J. Pediatr. Infect. Dis. Soc. 2018, 7, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Falconer, S.B.; Tkachenko, E.; Wang, M.; Systrom, H.; Mahabamunuge, J.; Relman, D.A.; Hohmann, E.L.; Bhatt, A.S. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS ONE 2017, 12, e0182585. [Google Scholar] [CrossRef]
- Bar-Yoseph, H.; Carasso, S.; Shklar, S.; Korytny, A.; Dar, R.E.; Daoud, H.; Nassar, R.; Maharshak, N.; Hussein, K.; Geffen, Y.; et al. Oral Capsulized Fecal Microbiota Transplantation for Eradication of Carbapenemase-producing Enterobacteriaceae Colonization With a Metagenomic Perspective. Clin. Infect. Dis. 2021, 73, e166–e175. [Google Scholar] [CrossRef]
- Innes, A.J.; Mullish, B.H.; Ghani, R.; Szydlo, R.M.; Apperley, J.F.; Olavarria, E.; Palanicawandar, R.; Kanfer, E.J.; Milojkovic, D.; McDonald, J.A.K.; et al. Fecal Microbiota Transplant Mitigates Adverse Outcomes Seen in Patients Colonized With Multidrug-Resistant Organisms Undergoing Allogeneic Hematopoietic Cell Transplantation. Front. Cell. Infect. Microbiol. 2021, 11, 684659. [Google Scholar] [CrossRef] [PubMed]
- Samet, A.; Śledzińska, A.; Krawczyk, B.; Hellmann, A.; Nowicki, S.; Kur, J.; Nowicki, B. Leukemia and risk of recurrent Escherichia coli bacteremia: Genotyping implicates E. coli translocation from the colon to the bloodstream. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1393–1400. [Google Scholar] [CrossRef]
- Cattaneo, C.; on behalf of SEIFEM Group; Di Blasi, R.; Skert, C.; Candoni, A.; Martino, B.; Di Renzo, N.; Delia, M.; Ballanti, S.; Marchesi, F.; et al. Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann. Hematol. 2018, 97, 1717–1726. [Google Scholar] [CrossRef]
- Relman, D.A.; Lipsitch, M. Microbiome as a tool and a target in the effort to address antimicrobial resistance. Proc. Natl. Acad. Sci. 2018, 115, 12902–12910. [Google Scholar] [CrossRef] [PubMed]
- Ghani, R.; Mullish, B.H.; McDonald, J.A.K.; Ghazy, A.; Williams, H.R.T.; Brannigan, E.T.; Mookerjee, S.; Satta, G.; Gilchrist, M.; Duncan, N.; et al. Disease Prevention Not Decolonization: A Model for Fecal Microbiota Transplantation in Patients Colonized With Multidrug-resistant Organisms. Clin. Infect. Dis. 2020, 72, 1444–1447. [Google Scholar] [CrossRef]
- A Otter, J.; Mookerjee, S.; Davies, F.; Bolt, F.; Dyakova, E.; Shersing, Y.; Boonyasiri, A.; Weiße, A.Y.; Gilchrist, M.; Galletly, T.J.; et al. Detecting carbapenemase-producing Enterobacterales (CPE): An evaluation of an enhanced CPE infection control and screening programme in acute care. J. Antimicrob. Chemother. 2020, 75, 2670–2676. [Google Scholar] [CrossRef]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T.; et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682–1688. [Google Scholar] [CrossRef]
- Bilinski, J.; Robak, K.; Peric, Z.; Marchel, H.; Karakulska-Prystupiuk, E.; Halaburda, K.; Rusicka, P.; Swoboda-Kopec, E.; Wroblewska, M.; Wiktor-Jedrzejczak, W.; et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective, Single-Center Study. Biol. Blood Marrow Transplant. 2016, 22, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef] [PubMed]
| References | Population | Antibiotic | Microbiota Changes | Effect |
|---|---|---|---|---|
| Hayase et al. [18] | Mice | Cefepime and Levofloxacin vs. meropenem | ↑ Clostridiales ↓ Bacteroides thetaiotaomicron ↑ Enterococcus | ↓ severe GvHD |
| Shono et al. [19] | T-cell-replete HSCT human and mice | Piperacillin-tazobactam Imipenem-cilastatin Azteronam and Cefepime | ↓ Actinobacteria ↓ Clostridiales ↑ Erisipelotrichia and Enterococcus ↔ Clostridiales | ↑ grade II–IV aGvHD ↑ GvHD-related mortality ↓ GvHD-related mortality |
| Holler et al. [20] | Human HSCT | Ciprofloxacin and broad-spectrum antibiotics | ↑ Enterococci ↓ classical commensal bacteria | ↑ GI-GvHD |
| Weber et al. [21] | Human HSCT | Ciprofloxacin and metronidazole vs. rifaximin | ↔ Enterobacteriaceae | ↓ GI-GvHD ↓ TRM ↑ OS |
| References | Population | Microbiota Changes | Effect |
|---|---|---|---|
| Stein-Thoeringer et al. [25] | Human and mice HSCT | ↑ Enterococcus genus and Enterococcus faecium species | ↓ OS ↑ GvHD-related mortality |
| Chiusolo et al. [26] | Human HSCT | ↑ Bacteroidetes ↓ Firmicutes | - - |
| Kouidhi [27] | HSCT vs. Healthy subjects | ↑ Proteobacteria and Verrucomicrobia phyla ↓ Faecalibacterium, Alistipes, and Prevotella 9 genera ↑Bacteroides, Escherichia/Shigella, ↑ Klebsiella, Veionella and Akkermansia genera ↑ Actinobacteria phylum ↑ Faecalibacterium, Alistipes, and Prevotella 9 genera | - - ↑ GvHD ↓ GvHD |
| Jian et al. [28] | Radiotherapy | ↓ Lactobacillus and Bifidobacterium ↑ Staphylococcus and Escherichia coli | ↑ radiation enteritis ↓ intestinal barrier function ↑ inflammation |
| Gu et al. [16] | Myeloablative conditioning plus ATG | ↓ Bacteroides genus and ↑ Enterococcus, Klebsiella, and Escherichia genera | ↓ OS for low microbial diversity |
| Publication | Registration Number | Indications | Phase | Number of Patients | Age, Years | Intervention | Adverse Events | Status |
|---|---|---|---|---|---|---|---|---|
| DeFilipp [46] | NCT02733744 | HSCT | Early phase 1 | 13 | 18–65 | FMT as oral capsules | 1 AE 2 GvHD 1 Sepsis 1 CDI | Completed |
| Rashidi [42] | NCT03678493 | AML and HSCT | Phase 2 | 45 AML 74 HSCT | 18 and older | FMT as oral capsules vs. placebo | GvHD 18.4% (FMT arm) vs. 0% (placebo arm) BSI 16.3% (FMT arm) and 12% (placebo arm) | Active not recruiting |
| Dougè [47] | NCT04935684 | Myeloablative HSCT | Phase 2 | 150 | 18 and older | FMT vs. placebo as enema via rectal cannula | Not applicable | Recruiting |
| unpublished | NCT03720392 | Myeloablative or intermediate intensity HSCT | Phase 2 | 8 | 18–80 | FMT as oral capsules vs. placebo | 1 sepsis (FMT arm) | Completed |
| Taur [43] | NCT02269150 | HSCT | Phase 2 | 25 | 18 and older | FMT via enema vs. placebo | Not available | Active not recruiting |
| Registration Number | Indications | Phase | Number of Patients | Age, Years | Intervention | Administration Way | Allocation | Status |
|---|---|---|---|---|---|---|---|---|
| NCT04711967 | SD/SR gut aGvHD | Not applicable | 20 | 18–60 | FMT vs. no treatment | unknown | Randomized | Recruiting |
| NCT04935684 | HSCT | 2 | 150 | 18 and older | FMT vs. no treatment | 250 mL of enema | Randomized | Recruiting |
| NCT04139577 | Grade II–IV aGvHD and high risk naïve aGvHD | 1 | 10 | 18 and older | FMT | 40 oral capsules | Single-arm | Active not recruiting |
| NCT04269850 | Grade III–IV GI aGvHD | 2 | 20 | 5–70 | FMT | Ruxolitinib 10 mg twice a day, MP 0.5 mg/Kg, 2 FMT capsules/Kg | Single-arm | Recruiting |
| NCT03812705 | SD/SR GI aGvHD | 2 | 30 | 14–60 | FMT | 200–300 mL fecal microbiota | Single-arm | Recruiting |
| NCT03819803 | GI aGvHD | 3 | 15 | 18 and older | FMT | 200 mL of enema | Single-arm | Recruiting |
| NCT03148743 [72,78,79] | SR GI aGvHD | Not applicable | 50 | 10–60 | FMT | 200 mL of enema | Single-arm | Recruiting |
| NCT04769895 | SR/Ruxolitinib refractory GI GvHD | 3 | 75 | 18 and older | FMT | Enema | Single-arm | Recruiting |
| NCT04745221 | HSCT | Not applicable | 100 | 18–60 | FMT | Oral capsules | Single-arm | Recruiting |
| Registration Number | Indications | Phase | Number of Patients | Age, Years | Intervention | Administration Way | Allocation | Status |
|---|---|---|---|---|---|---|---|---|
| NCT02269150 [43] | HSCT | 2 | 59 | 18 and older | FMT | FMT via enema vs. placebo | Single-arm | Active not recruiting |
| NCT02461199 | Blood diseases | Not applicable | 50 | 18 and older | FMT | 100 mL of enema | Single-arm | Recruiting |
| NCT04431934 | MDR carriers | Not applicable | 437 | 18 and older | FMT vs. Probiotics | 14–17 FMT oral capsules vs. 2 sachets of probiota | Randomized | Recruiting |
| NCT03167398 [93] | CRE carriers | 2 | 15 | 18 and older | FMT | 30 FMT oral capsules | Single-arm | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metafuni, E.; Di Marino, L.; Giammarco, S.; Bellesi, S.; Limongiello, M.A.; Sorà, F.; Frioni, F.; Maggi, R.; Chiusolo, P.; Sica, S. The Role of Fecal Microbiota Transplantation in the Allogeneic Stem Cell Transplant Setting. Microorganisms 2023, 11, 2182. https://doi.org/10.3390/microorganisms11092182
Metafuni E, Di Marino L, Giammarco S, Bellesi S, Limongiello MA, Sorà F, Frioni F, Maggi R, Chiusolo P, Sica S. The Role of Fecal Microbiota Transplantation in the Allogeneic Stem Cell Transplant Setting. Microorganisms. 2023; 11(9):2182. https://doi.org/10.3390/microorganisms11092182
Chicago/Turabian StyleMetafuni, Elisabetta, Luca Di Marino, Sabrina Giammarco, Silvia Bellesi, Maria Assunta Limongiello, Federica Sorà, Filippo Frioni, Roberto Maggi, Patrizia Chiusolo, and Simona Sica. 2023. "The Role of Fecal Microbiota Transplantation in the Allogeneic Stem Cell Transplant Setting" Microorganisms 11, no. 9: 2182. https://doi.org/10.3390/microorganisms11092182





