Local Phenomena Shape Backyard Soil Metabolite Composition
Abstract
:1. Introduction
2. Results
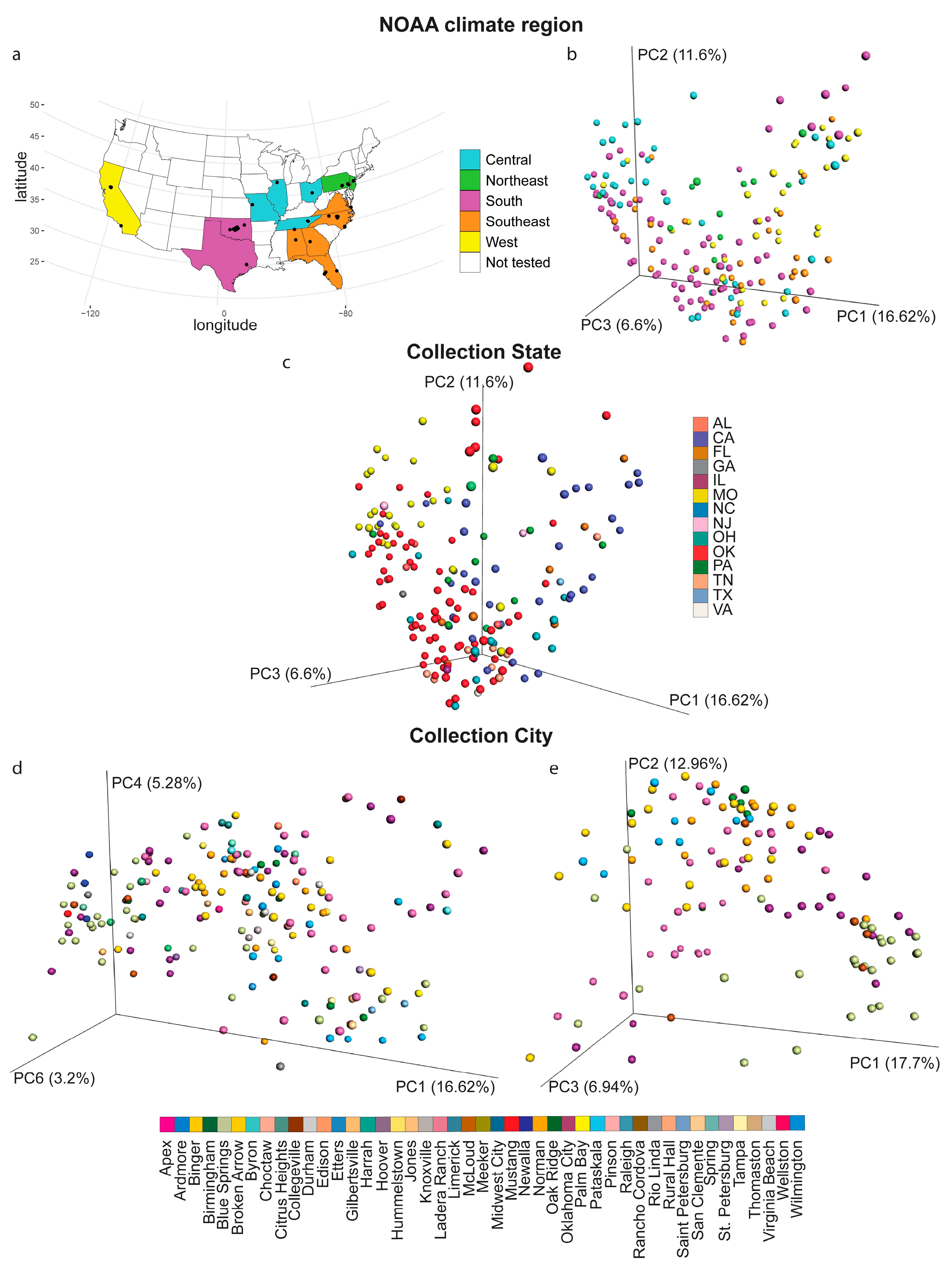
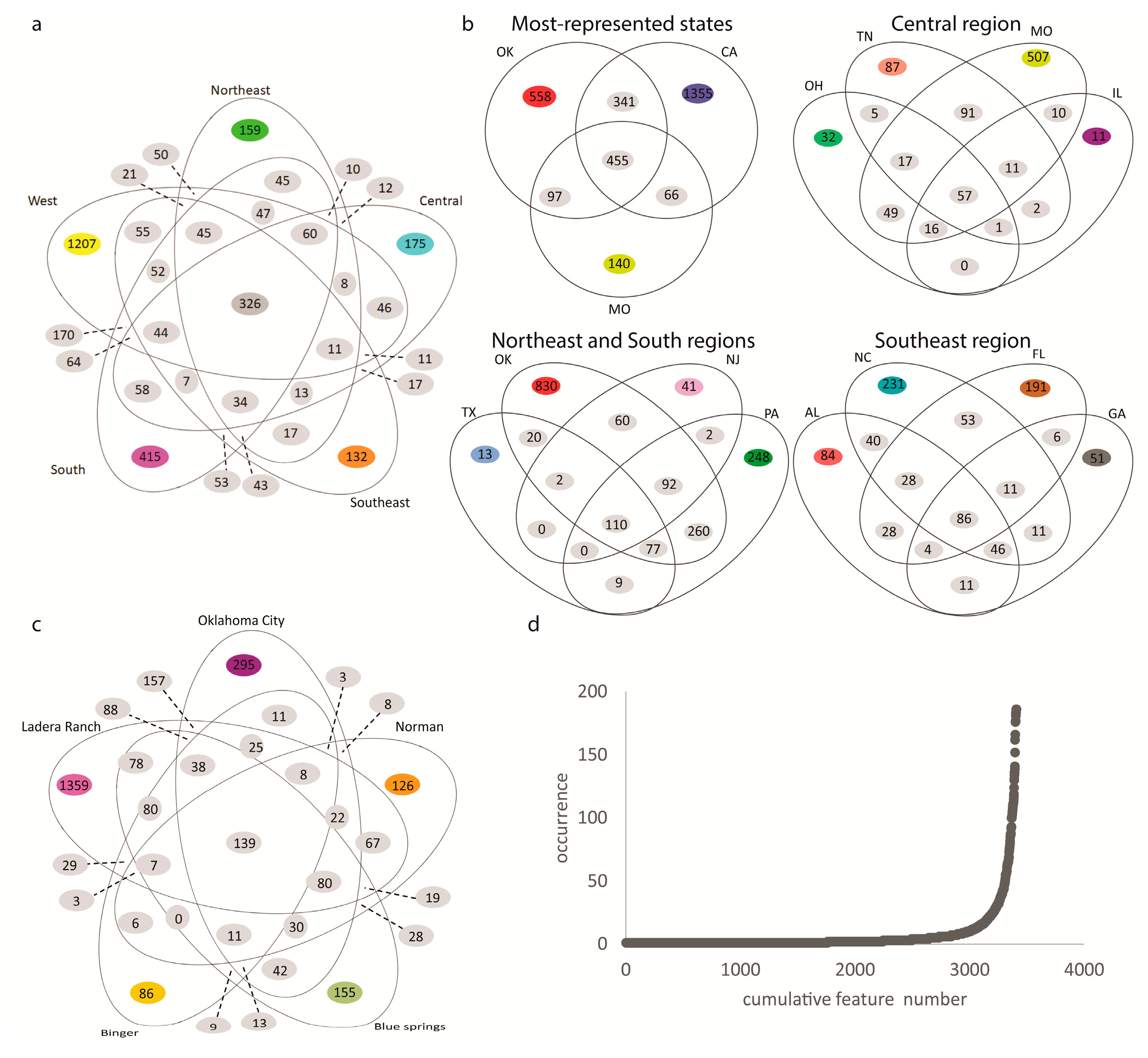
2.1. Impact of Collection City, State and Climate Region on the Overall Soil Metabolite Composition
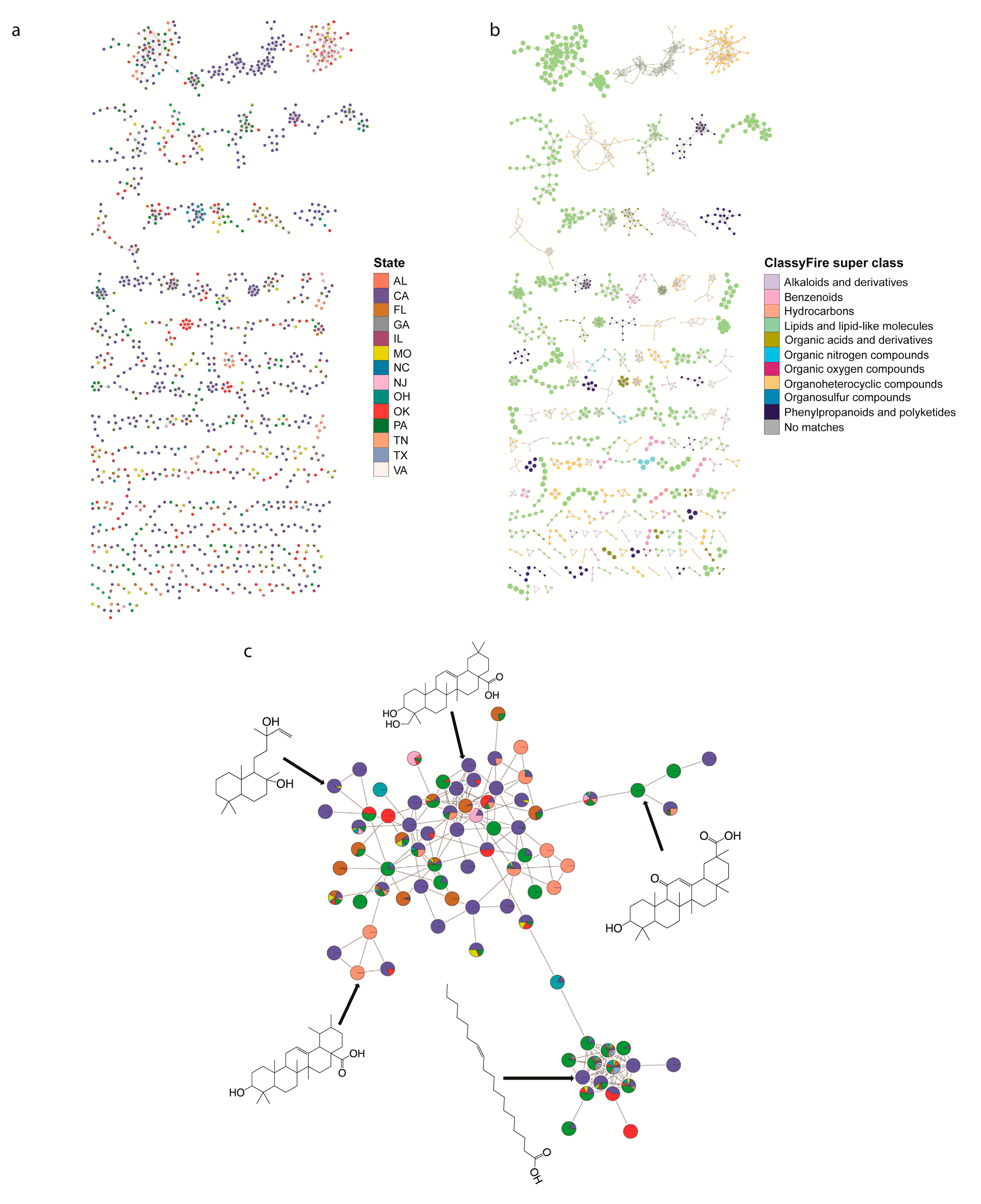
2.2. Specific Chemistries Identified in Backyard Soil Samples
3. Discussion
4. Materials and Methods
4.1. Sample Selection
4.2. Metabolite Extraction
4.3. LC-MS/MS
4.4. Data Analysis
4.5. Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Traxler, M.F.; Kolter, R. Natural products in soil microbe interactions and evolution. Nat. Prod. Rep. 2015, 32, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Swenson, T.L.; Karaoz, U.; Swenson, J.M.; Bowen, B.P.; Northen, T.R. Linking soil biology and chemistry in biological soil crust using isolate exometabolomics. Nat. Commun. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugova, A.; Puschenreiter, M.; Koellensperger, G.; Hann, S. Elucidating rhizosphere processes by mass spectrometry—A review. Anal. Chim. Acta 2017, 956, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Robles, A.J.; King, J.B.; Powell, D.R.; Miller, A.N.; Mooberry, S.L.; Cichewicz, R.H. Crowdsourcing natural products discovery to access uncharted dimensions of fungal metabolite diversity. Angew. Chem. Int. Ed. Engl. 2014, 53, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef] [Green Version]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Amelin, V.G.; Andoralov, A.M. High-performance liquid chromatography–time-of-flight mass spectrometry in the identification and determination of 111 pesticides in food, feed, water, and soil. J. Anal. Chem. 2016, 71, 82–93. [Google Scholar] [CrossRef]
- Kemmerich, M.; Bernardi, G.; Adaime, M.B.; Zanella, R.; Prestes, O.D. A simple and efficient method for imidazolinone herbicides determination in soil by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1412, 82–89. [Google Scholar] [CrossRef]
- Yegemova, S.; Bakaikina, N.V.; Kenessov, B.; Koziel, J.A.; Nauryzbayev, M. Determination of 1-methyl-1H-1,2,4-triazole in soils contaminated by rocket fuel using solid-phase microextraction, isotope dilution and gas chromatography–mass spectrometry. Talanta 2015, 143, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Vila, J.; Yu, M.; Bodnar, W.; Aitken, M.D. Tracing the Biotransformation of Polycyclic Aromatic Hydrocarbons in Contaminated Soil Using Stable Isotope-Assisted Metabolomics. Environ. Sci. Technol. Lett. 2018, 5, 103–109. [Google Scholar] [CrossRef]
- Tetzner, N.F.; Maniero, M.G.; Rodrigues-Silva, C.; Rath, S. On-line solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry as a powerful technique for the determination of sulfonamide residues in soils. J. Chromatogr. A 2016, 1452, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.P.; Giannone, R.J.; Abraham, P.E.; Wullschleger, S.D.; Hettich, R.L. Evaluation of an untargeted nano-liquid chromatography-mass spectrometry approach to expand coverage of low molecular weight dissolved organic matter in Arctic soil. Sci. Rep. 2019, 9, 5810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, S.; Swenson, T.L.; Lau, R.; Rocha, A.M.; Aaring, A.; Hazen, T.C.; Chakraborty, R.; Northen, T.R. Construction of Viable Soil Defined Media Using Quantitative Metabolomics Analysis of Soil Metabolites. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewavitharana, S.S.; Klarer, E.; Reed, A.J.; Leisso, R.; Poirier, B.; Honaas, L.; Rudell, D.R.; Mazzola, M. Temporal Dynamics of the Soil Metabolome and Microbiome During Simulated Anaerobic Soil Disinfestation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Morel, J.L.; Chenu, C.; Lorenz, K. Ecosystem services provided by soils of urban, industrial, traffic, mining, and military areas (SUITMAs). J. Soils Sediments 2015, 15, 1659–1666. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based Molecular Networking in the GNPS Analysis Environment. bioRxiv 2019, 812404. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, P.D.; Almeida, A.; Bak, S. Evolution of Structural Diversity of Triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Stevenson, F. Lipids in soil. J. Am. Oil Chem. Soc. 1966, 43, 203–210. [Google Scholar] [CrossRef]
- Balaria, A.; Johnson, C.E. Compositional characterization of soil organic matter and hot-water-extractable organic matter in organic horizons using a molecular mixing model. J. Soils Sediments 2013, 13, 1032–1042. [Google Scholar] [CrossRef] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podojil, M.; Sedmera, P.; Vokoun, J.; Betina, V.; Barathova, H.; Durackova, Z.; Horakova, K.; Nemec, P. Eurotium (Aspergillus) repens metabolites and their biological activity. Folia Microbiol. 1978, 23, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Mari, M.; Audi-Miro, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Climate change impact on the PAH photodegradation in soils: Characterization and metabolites identification. Environ. Int. 2016, 89–90, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. High diversity of small organic N observed in soil water. Soil Biol. Biochem. 2013, 57, 444–450. [Google Scholar] [CrossRef]
- Behera, N.; Joshi, S.K.; Pati, D.P. Root contribution to total soil metabolism in a tropical forest soil from Orissa, India. For. Ecol. Manag. 1990, 36, 125–134. [Google Scholar] [CrossRef]
- Chamam, A.; Sanguin, H.; Bellvert, F.; Meiffren, G.; Comte, G.; Wisniewski-Dye, F.; Bertrand, C.; Prigent-Combaret, C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry 2013, 87, 65–77. [Google Scholar] [CrossRef]
- Azaizeh, H.A.; Marschner, H.; Römheld, V.; Wittenmayer, L. Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 1995, 5, 321–327. [Google Scholar] [CrossRef]
- Bouslimani, A.; Melnik, A.V.; Xu, Z.; Amir, A.; da Silva, R.R.; Wang, M.; Bandeira, N.; Alexandrov, T.; Knight, R.; Dorrestein, P.C. Lifestyle chemistries from phones for individual profiling. Proc. Natl. Acad. Sci. USA 2016, 113, E7645–E7654. [Google Scholar] [CrossRef] [Green Version]
- McCall, L.-I.; Anderson, V.M.; Fogle, R.S.; Haffner, J.J.; Hossain, E.; Liu, R.; Ly, A.H.; Ma, H.; Nadeem, M.; Yao, S. Analysis of university workplace building surfaces reveals usage-specific chemical signatures. Build. Environ. 2019, 162, 106289. [Google Scholar] [CrossRef]
- McCall, L.-I.; Callewaert, C.; Zhu, Q.; Song, S.J.; Bouslimani, A.; Minich, J.J.; Ernst, M.; Ruiz-Calderon, J.F.; Cavallin, H.; Pereira, H.S.; et al. Home chemical and microbial transitions across urbanization. Nat. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Nothias, L.F.; Quinn, R.A.; Alexandrov, T.; Bandeira, N.; Bouslimani, A.; Castro-Falcon, G.; Chen, L.; Dang, T.; Floros, D.J.; et al. Mass Spectrometry-Based Visualization of Molecules Associated with Human Habitats. Anal. Chem. 2016, 88, 10775–10784. [Google Scholar] [CrossRef] [PubMed]
- Jarmusch, A.K.; Wang, M.; Aceves, C.M.; Advani, R.S.; Aguire, S.; Aksenov, A.A.; Aleti, G.; Aron, A.T.; Bauermeister, A.; Bolleddu, S.; et al. Repository-scale Co- and Re-analysis of Tandem Mass Spectrometry Data. bioRxiv 2019, 750471. [Google Scholar] [CrossRef] [Green Version]
- Patil, C.; Calvayrac, C.; Zhou, Y.; Romdhane, S.; Salvia, M.V.; Cooper, J.F.; Dayan, F.E.; Bertrand, C. Environmental Metabolic Footprinting: A novel application to study the impact of a natural and a synthetic beta-triketone herbicide in soil. Sci. Total Environ. 2016, 566–567, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Postigo, C.; Chirón, S.; Barcelò, D.; Pérez, S. Analysis and fate of 14 relevant wastewater-derived organic pollutants in long-term exposed soil. Anal. Bioanal. Chem. 2019, 411, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Gurevich, A.; Mikheenko, A.; Garg, N.; Nothias, L.F.; Ninomiya, A.; Takada, K.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of peptidic natural products through database search of mass spectra. Nat. Chem. Biol. 2017, 13, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, A.; Mikheenko, A.; Shlemov, A.; Korobeynikov, A.; Mohimani, H.; Pevzner, P.A. Increased diversity of peptidic natural products revealed by modification-tolerant database search of mass spectra. Nat. Microbiol. 2018, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- 7 Draw Maps. Available online: https://socviz.co/maps.html (accessed on 17 February 2020).
| m/z | RT (min) | Annotation | Cosine Score | Shared Seaks | Ppm Error | Class or Usage | Region |
|---|---|---|---|---|---|---|---|
| Human Activity-Derived Chemicals 1 | |||||||
| 121.101 | 6.97 | isophorone | 0.94 | 5 | 1.70 | fertilizer | West |
| 179.070 | 7.89 | 2-propenoic acid, 3-(4-methoxyphenyl)- | 0.98 | 6 | 0.60 | sunscreen | South |
| 192.138 | 4.61 | diethyltoluamide (DEET) | 0.91 | 5 | 2.06 | insect repellent, Pesticide | Central, Northeast, Southeast, West, South |
| 229.086 | 5.92 | oxybenzone | 0.96 | 5 | 0.85 | sunscreen | Central, West, South |
| 251.200 | 6.18 | aleuritic acid | 0.83 | 7 | 0 | shellac | West |
| 278.284 | 5.77 | perhexiline | 0.88 | 7 | 0 | vasodilator | Central, South |
| 282.147 | 7.18 | pendimethalin | 0.93 | 9 | 0 | herbicide | Central, South |
| 300.080 | 4.68 | fenbendazole | 0.92 | 5 | 3 | anthelminthic | Southeast, South |
| 302.177 | 4.94 | indaziflam | 0.97 | 7 | 1 | herbicide | South |
| 305.108 | 6.34 | diazinone | 0.91 | 6 | 0.30 | pesticide | Central |
| 316.075 | 3.43 | oxfendazole | 0.92 | 9 | 0 | anthelmintic | South |
| 327.008 | 5.40 | tris(1-chloro-2-propy) phosphate | 0.91 | 4 | 0.28 | adhesives, flame retardants, paint | Southeast, West, South |
| 342.077 | 5.95 | propiconazole | 0.96 | 7 | 0.89 | fungicide | Southeast, West |
| 351.127 | 7.00 | prodiamine | 0.85 | 8 | 0 | herbicide | Central |
| 412.321 | 6.38 | benzethonium | 0.91 | 9 | 0.22 | pesticide, preservative | West |
| 531.408 | 9.98 | didodecyl 3,3’-thiodipropionate oxide | 0.96 | 10 | 1.72 | antioxidant, stabilizer, food preservative | Southeast, South |
| Plant-Derived Secondary Metabolites 1 | |||||||
| 144.081 | 7.77 | rauwolscine | 0.82 | 5 | 0 | alkaloid | West |
| 163.039 | 8.17 | N-caffeoyl-O-methyltyramine | 0.96 | 6 | 0 | alkaloid | Northeast, Southeast |
| 163.148 | 8.39 | globulol | 0.99 | 7 | 0.56 | sesquiterpenoid | South |
| 201.164 | 6.77 | alpha.-cyperone | 0.83 | 7 | 0 | sesquiterpenoid | West |
| 213.102 | 4.75 | carbanilide | 0.98 | 5 | 0.43 | benzenoid | Central, Northeast, West, South |
| 225.076 | 8.50 | sinapic acid | 0.93 | 9 | 0.88 | hydroxycinnamic acid | Northeast, South |
| 257.081 | 4.65 | isoliquiritin | 0.93 | 7 | 0 | chalcone | South |
| 269.081 | 4.70 | formononetin | 0.86 | 9 | 0 | isoflavonoid | Northeast, West |
| 271.096 | 5.57 | 2’,6’-dihydroxy-4’-methoxychalcone | 0.99 | 11 | 0.68 | chalcone | Southeast, West |
| 279.232 | 6.98 | pinoleic acid | 0.85 | 9 | 0 | Fatty acid | Central, West |
| 279.304 | 7.95 | phytol | 0.94 | 8 | 1 | acyclic diterpene alcohol | Central, Northeast, Southeast, West, South |
| 285.112 | 7.13 | 5,7-dimethoxyflavanone | 0.89 | 6 | 0 | flavonoid | Northeast |
| 301.107 | 5.21 | 5,7-dimethoxy-4′-hydroxyflavanone | 0.92 | 6 | 0 | flavonoid | Southeast |
| 303.232 | 5.79 | isopimaric acid | 0.82 | 13 | 1 | diterpenoid | Central, Northeast |
| 324.170 | 4.46 | (3S,6Z)-3-methyl-6-[[2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl]methylidene]piperazine-2,5-dione | 0.82 | 9 | 1.22 | alkaloid | West |
| 359.149 | 4.31 | matairesinol | 0.92 | 13 | 0 | lignan | West |
| 393.206 | 6.28 | glabrol | 0.81 | 11 | 0.23 | flavonoid | Northeast |
| 407.185 | 7.01 | 5,7-dihydroxy-3-(4-hydroxyphenyl)-6,8-bis(3-methylbut-2-enyl)chromen-4-one | 0.90 | 9 | 0.22 | flavonoid | West |
| 409.346 | 7.56 | echinocystic acid | 0.86 | 13 | 0 | triterpenoid | West |
| 409.383 | 8.56 | cycloartenol acetate | 0.91 | 13 | 0.52 | triterpenoid | Northeast, South |
| 411.362 | 7.78 | oleanolic acid methyl ester | 0.83 | 11 | 0 | triterpenoid | Central, Northeast, Southeast, West, South |
| 443.389 | 8.30 | uvaol | 0.91 | 14 | 0 | triterpenoid | Central, Northeast, West, South |
| 455.352 | 6.26 | dehydro (11,12) ursolic acid lactone | 0.82 | 12 | 0 | triterpenoid lactone | Northeast, West |
| 457.368 | 7.78 | betulinic acid | 0.81 | 13 | 0 | pentacyclic triterpenoid | Central, Northeast, Southeast, West, South |
| Microbial Metabolites 1 | |||||||
| 395.367 | 8.63 | fucosterol | 0.85 | 11 | 0 | sterol | South |
| 462.312 | 6.80 | echinulin | 0.86 | 16 | 3 | diketopiperazine metabolite found in Aspergillus | West |
| State | Sample Number | City | Sample Number |
|---|---|---|---|
| Central Region | |||
| Missouri (MO) | 25 | Blue Springs | 25 |
| Illinois (IL) | 2 | Bryon | 2 |
| Ohio (OH) | 2 | Pataskala | 2 |
| Tennessee (TN) | 8 | Knoxville Oak Ridge | 2 6 |
| Total | 37 | ||
| Northeast Region | |||
| Pennsylvania (PA) | 10 | Collegeville Etters Gilbertsville Hummelstown Limerick | 2 2 1 3 2 |
| New Jersey (NJ) | 2 | Edison | 2 |
| Total | 12 | ||
| South Region | |||
| Oklahoma (OK) | 74 | Binger Broken Arrow Choctaw Harrah Jones McLoud Meeker Midwest City Mustang Newalla Norman Oklahoma City Wellston | 17 1 2 2 2 5 1 4 2 3 14 20 1 |
| Texas (TX) | 2 | Spring | 2 |
| Total | 76 | ||
| Southwest Region | |||
| North Carolina (NC) | 15 | Apex Durham Raleigh Rural Hall Wilmington | 1 3 1 1 9 |
| Alabama (AL) | 6 | Ardmore Birmingham Hoover Pinson | 3 1 1 1 |
| Florida (FL) | 7 | Palm Bay Saint Petersburg Tampa | 2 3 2 |
| Georgia (GA) | 2 | Thomaston | 2 |
| Virginia (VA) | 1 | Virginia Beach | 1 |
| Total | 31 | ||
| West Region | |||
| California (CA) | 32 | Citrus Heights Ladera Ranch Rancho Cordova Rio Linda San Clemente | 1 27 1 2 1 |
| Total | 32 | ||
| Time | Flow (mL/min) | %B |
|---|---|---|
| 0.00 | 0.500 | 5.0 |
| 1.00 | 0.500 | 5.0 |
| 9.00 | 0.500 | 100.0 |
| 11.00 | 0.500 | 100.0 |
| 11.500 | 0.500 | 5.0 |
| 12.500 | 0.500 | 5.0 |
| Properties of Full MS/dd-MS2 | |
|---|---|
| General | |
| Runtime | 0 to 12.5 min |
| Polarity | Positive |
| Default Charge | 1 |
| Inclusion | - |
| Exclusion | On (see Table S2 for full exclusion list: ions present at 1E5 or higher in extraction blanks were excluded) |
| Full MS | |
| Resolution | 70,000 |
| AGC target | 1 × 106 |
| Scan range | 70 to 1050 m/z |
| Maximum IT | 246 ms |
| dd-MS2 | |
| Resolution | 17,500 |
| AGC target | 2 × 105 |
| Maximum IT | 54 ms |
| Loop count | 5 |
| TopN | 5 |
| Isolation window | 1.0 m/z |
| Fixed mass | - |
| (N)CE/stepped | NCE: 20, 40, 60 |
| dd Settings | |
| Minimum AGC | 8.00e3 |
| Peptide match | Preferred |
| Exclude isotopes | on |
| Dynamic exclusion | 10.0 s |
| ESI Ion Source | |
| ID | HESI |
| Sheath gas flow rate | 35 L/min |
| Auxiliary gas flow rate | 10 L/min |
| Sweep gas flow rate | 0 L/min |
| Spray voltage | 3.80 kV |
| S-lens RF level | 50 V |
| Capillary temperature | 320 °C |
| Auxiliary gas temperature | 350 °C |
| Procedure | Parameter | |
|---|---|---|
| Mass Detection | MS level 1: Noise level | 2E5 |
| MS level 2: Noise level | 0.0 | |
| Mass detector | Centroid | |
| ADAP Chromatogram Builder [37] | Min group size # of scans | 5 |
| Group intensity threshold | 2E5 | |
| Min highest intensity | 5E5 | |
| m/z tolerance | 0.003 m/z (or 10 ppm) | |
| Chromatogram Deconvolution | Algorithm | Baseline cut-off |
| Min peak height | 5.0E5 | |
| Peak duration range (min) | 0.02–2.2 | |
| Baseline level | 2E5 | |
| m/z center calculation | MEDIAN | |
| m/z range for MS2 scan pairing (Da) | 0.01 | |
| RT range for MS2 Scan Pairing (min) | 0.1 | |
| Isotopic Peaks Grouper | m/z tolerance | 0.001 m/z (or 10 ppm) |
| Retention time tolerance (absolute: min) | 0.1 | |
| Monotonic shape | No | |
| Maximum charge | 3 | |
| Representative isotope | Lowest m/z | |
| Join Aligner | m/z tolerance | 0.001 m/z (or 10 ppm) |
| Weight for m/z | 1 | |
| Retention time tolerance (absolute: min) | 0.2 | |
| Weight for RT | 0.1 | |
| Require same charge state | Yes | |
| Feature List Row Filter | Retention time (min) | 0.25–12.00 |
| Keep only peaks with MS2 scan (GNPS) | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Lesani, M.; Forrest, I.; Lan, Y.; Dean, D.A.; Gibaut, Q.M.R.; Guo, Y.; Hossain, E.; Olvera, M.; Panlilio, H.; et al. Local Phenomena Shape Backyard Soil Metabolite Composition. Metabolites 2020, 10, 86. https://doi.org/10.3390/metabo10030086
Nguyen TD, Lesani M, Forrest I, Lan Y, Dean DA, Gibaut QMR, Guo Y, Hossain E, Olvera M, Panlilio H, et al. Local Phenomena Shape Backyard Soil Metabolite Composition. Metabolites. 2020; 10(3):86. https://doi.org/10.3390/metabo10030086
Chicago/Turabian StyleNguyen, Tra D., Mahbobeh Lesani, Ines Forrest, Yunpeng Lan, Danya A. Dean, Quentin M. R. Gibaut, Yanting Guo, Ekram Hossain, Marcela Olvera, Hannah Panlilio, and et al. 2020. "Local Phenomena Shape Backyard Soil Metabolite Composition" Metabolites 10, no. 3: 86. https://doi.org/10.3390/metabo10030086





