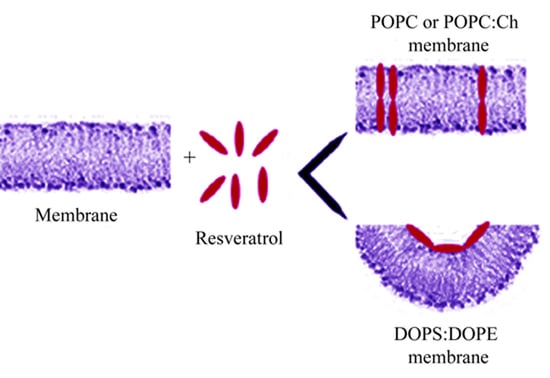
Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Experimental
2.2.1. Preparation of Resveratrol Solution
2.2.2. Preparation of PLMs
2.2.3. Determination of Conductance of Channel-Like Events
2.2.4. Determination of PLM Capacitance
3. Results
3.1. Membrane Stability
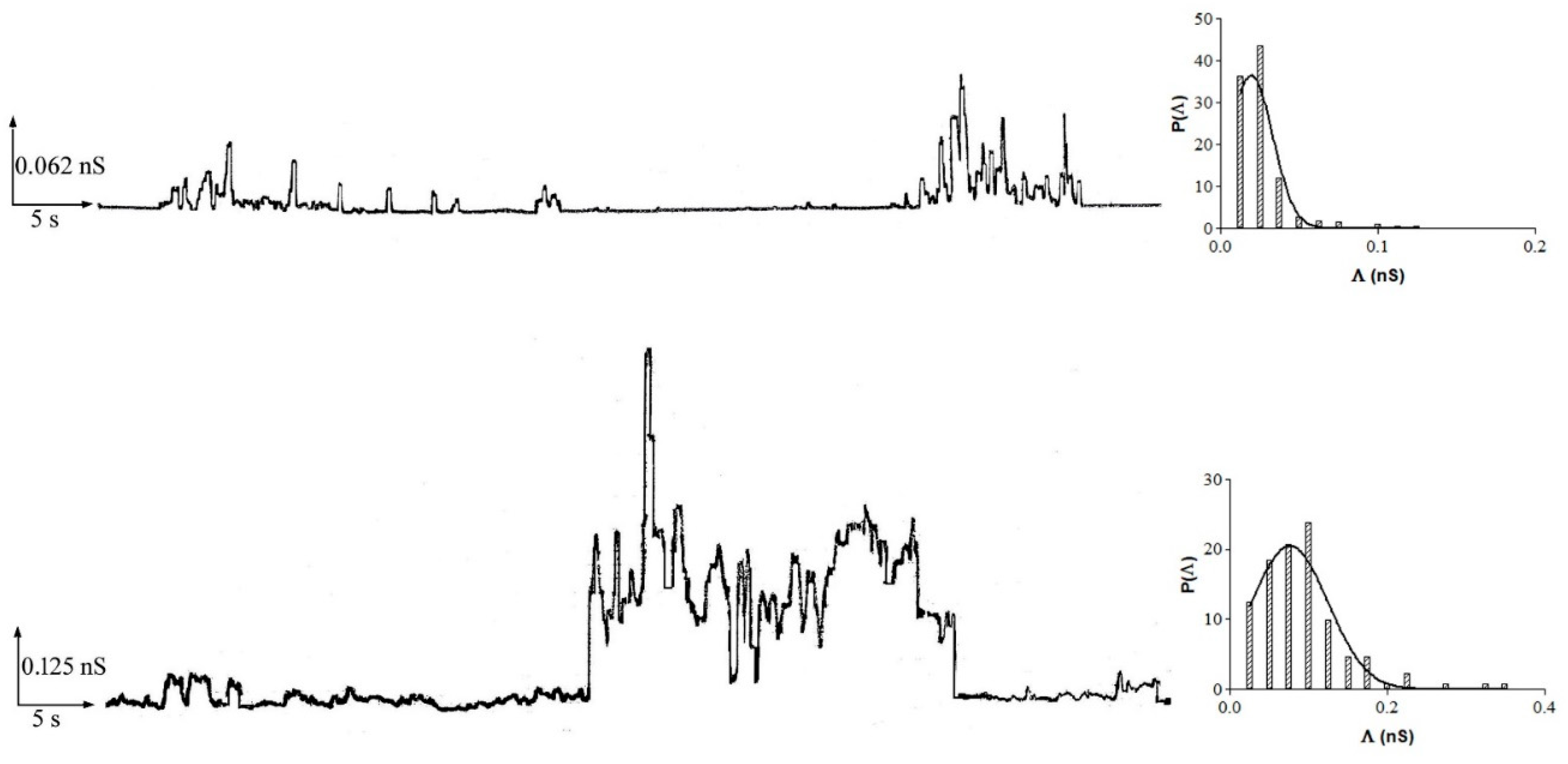
3.2. Resveratrol Interaction with POPC PLMs
3.3. Resveratrol Interaction with POPC:Ch PLM
3.4. Resveratrol Interaction with DOPS:DOPE PLMs
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brittes, J.; Lúcio, M.; Nunes, C.; Lima, J.L.; Reis, S. Effects of Resveratrol on Membrane Biophysical Properties: Relevance for Its Pharmacological Effects. Chem. Phys. Lipids 2010, 163, 747–754. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Perin, C.P.; Cassader, M. Anti-inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers a Randomized, Double-Blind, Placebo-Controlled, Cross-over Trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple Molecular Targets of Resveratrol: Anti-Carcinogenic Mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Baxter, R.A. Anti-aging Properties of Resveratrol: Review and Report of a Potent New Antioxidant Skin Care Formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid. Based Complement. Alternat. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a Potential Therapeutic Candidate for the Treatment and Management of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef]
- Granzotto, A.; Zatta, P. Resveratrol and Alzheimer’s Disease: Message in a Bottle on Red Wine and Cognition. Front. Aging Neurosci. 2014, 6, 95. [Google Scholar] [CrossRef]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate Juice Decreases Amyloid Load and Improves Behavior in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Villalonga-Barber, C.; Csonka, R.; Alexi, X.; Leonis, G.; Dellis, D.; Hamelink, E.; Belda, O.; Steele, B.R.; Micha-Screttas, M.; et al. Biological and Computational Evaluation of Resveratrol Inhibitors against Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2016, 31, 67–77. [Google Scholar] [CrossRef]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent Anti-Amyloidogenic and Fibril-Destabilizing Effects of Polyphenols in Vitro: Implications for the Prevention and Thera-Peutics of Alzheimer’s Disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of Resveratrol and Other Grape-Derived Polyphenols in Alzheimer’s Disease Prevention and Treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective Effects of Resveratrol in Alzheimer Disease Pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective Action of Resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol Increases the Expression and Activity of the Low Density Lipoprotein Receptor in Hepatocytes by the Proteolytic Activation of the Sterol Regulatory Element-Binding Proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hussaini, S.M.; Reiss, A.B. Resveratrol in Cholesterol Metabolism and Atherosclerosis. J. Med. Food 2012, 15, 763–773. [Google Scholar] [CrossRef]
- Galfi, P.; Jakus, J.; Molnar, T.; Neogrady, S.; Csordas, A. Divergent Effects of Resveratrol, a Polyphenolic Phytostilbene, on Free Radical Levels and Type of Cell Death Induced by the Histone Deacetylase Inhibitors Butyrate and Trichostatin A. J. Steroid Biochem. Mol. Biol. 2005, 94, 39–47. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of Human LDL Oxidation by Resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Jensen, M.D.; Sheng, W.; Simonyi, A.; Johnson, G.S.; Sun, A.Y.; Sun, G.Y. Involvement of Oxidative Pathways in Cytokine-Induced Secretory Phospholipase A2-IIA in Astrocytes. Neurochem. Int. 2009, 55, 362–368. [Google Scholar] [CrossRef]
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases a2 and Inflammatory Responses in the Central Nervous System. Neuromol. Med. 2010, 12, 133–148. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting Arachidonic Acid Pathway by Natural Products for Cancer Prevention and Therapy. Semin. Cancer Biol. 2016, 40, 48–81. [Google Scholar] [CrossRef]
- Fei, Q.; Kent, D.; Botello-Smith, W.M.; Nur, F.; Nur, S.; Alsamarah, A.; Chatterjee, P.; Lambros, M.; Luo, Y. Molecular Mechanism of Resveratrol’s Lipid Membrane Protection. Sci. Rep. 2018, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Fabris, S.; Momo, F.; Ravagnan, G.; Stevanato, R. Antioxidant Properties of Resveratrol and Piceid on Lipid Peroxidation in Micelles and Monolamellar Liposomes. Biophys. Chem. 2008, 135, 76–83. [Google Scholar] [CrossRef]
- Ghellinck, d.A.; Shen, C.; Fragneto, G.; Klösgen, B. Probing the Position of Resveratrol in Lipid Bilayers: A Neutron Reflectivity Study. Colloids Surf. B Biointerfaces 2015, 134, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Suga, K.; Hayashi, K.; Okamoto, Y.; Umakoshi, H. Multi-Level Characterization of the Membrane Properties of Resveratrol-Incorporated Liposomes. J. Phys. Chem. B 2017, 121, 4091–4098. [Google Scholar] [CrossRef]
- Cardia, M.C.; Caddeo, C.; Lai, F.; Fadda, A.M.; Sinico, C.; Luhmer, M. H NMR Study of the Interaction of Trans-Resveratrol with Soybean Phosphatidylcholine Liposomes. Sci. Rep. 2019, 9, 17736. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef] [PubMed]
- Balanc, B.; Ota, A.; Djordjevic, V.; Sentjurc, M.; Nedovic, V.; Bugarski, B.; Poklar, U.N. Resveratrol-Loaded Liposomes: Interaction of Resveratrol with Phospholipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1615–1626. [Google Scholar] [CrossRef]
- Neves, A.R.; Nunes, C.; Reis, S. Resveratrol Induces Ordered Domains Formation in Biomembranes: Implication for Its Pleiotropic Action. Biochim. Biophys. Acta 2016, 1858, 12–18. [Google Scholar] [CrossRef]
- Neves, A.R.; Nunes, C.; Reis, S. New Insights on the Biophysical Interaction of Resveratrol with Biomembrane Models: Relevance for Its Biological Effects. J. Phys. Chem. B 2015, 119, 11664–11672. [Google Scholar] [CrossRef]
- Martinović, N.; Abramovič, H.; Poklar, U.N. Inhibition of Copper-Induced Lipid Peroxidation by Sinapic Acid and Its Derivatives in Correlation to Their Effect on the Membrane Structural Properties. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.; Loureiro, A.; Pereira, M. The Biophysical Interaction of Ferulic Acid with Liposomes as Biological Membrane Model: The Effect of the Lipid Bilayer Composition. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Gutiérrez, M.E.; García, A.F.; Madariaga, A.M.; Sagrista, M.L.; Casadó, F.J.; Mora, M. Interaction of Tocopherols and Phenolic Compounds with Membrane Lipid Components: Evaluation of Their Antioxidant Activity in a Liposomal Model System. Life Sci. 2003, 72, 2337–2360. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of Liposomal Membrane Fluidity by Flavonoids and Isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Nagayama, M.; Tanaka, T.; Furusawa, M.; Kashimata, M.; Takeuchi, H. Membrane-Rigidifying Effects of Anti-cancer Dietary Factors. Biofactors 2002, 16, 45–56. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Dose-Dependent Interaction of Trans-Resveratrol with Biomembranes: Effects on Antioxidant Property. J. Med. Chem. 2013, 56, 970–981. [Google Scholar] [CrossRef]
- Shahane, G.; Ding, W.; Palaiokostas, M.; Orsi, M. Physical Properties of Model Biological Lipid Bilayers: Insights from All-Atom Molecular Dynamics Simulations. J. Mol. Model. 2019, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Murugova, T.N.; Balgavý, P. Molecular Volumes of DOPC and DOPS in Mixed Bilayers of Multilamellar Vesicles. Phys. Chem. Chem. Phys. 2014, 16, 18211–18216. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.P.; Fuller, N.L. Structural Dimensions and Their Changes in a Reentrant Hexagonal-Lamellar Transition of Phospholipids. Biophys. J. 1994, 66, 2127–2138. [Google Scholar] [CrossRef]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.; Ottova, A.-L. Membrane Biophysics: As Viewed from Experimental Bilayer Lipid Membranes; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Tien, H.; Ottova, A.-L. The Lipid Bilayer Concept and Its Experimental Realization: From Soap Bubbles, Kitchen Sink, to Bilayer Lipid Membranes. J. Membr. Sci. 2001, 189, 83–117. [Google Scholar] [CrossRef]
- Müller, P.; Rudin, D.; Tien, T.; Weacott, W. Reconstitution of Cell Membrane Structure in Vitro and Its Trasformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Mountz, J.; Martinosi, A. Protein-Lipid Interaction in Bilayer Lipid Membranes (BLM). In Enzyme of Biological Membranes; NY Plenum: New York, NY, USA, 1977; pp. 139–170. [Google Scholar]
- Micelli, S.; Gallucci, E.; Meleleo, D.; Stipani, V.; Picciarelli, V. Mitochondrial Porin Incorporation into Black Lipid Membranes: Ionic and Gating Contribution to the Total Current. Bioelectrochemistry 2002, 57, 97–106. [Google Scholar] [CrossRef]
- Faroux, J.M.; Ureta, M.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. An Overview of Peroxidation Reactions Using Liposomes as Model Systems and Analytical Methods as Monitoring Tools. Colloids Surf. B Biointerfaces 2020, 195, 111254. [Google Scholar] [CrossRef]
- Koller, D.; Lohner, K. The Role of Spontaneous Lipid Curvature in the Interaction of Interfacially Active Peptides with Membranes. Biochim. Biophys. Acta 2014, 1838, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; de Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Durdagi, S.; Siapi, E.; Villalonga-Barber, C.; Alexi, X.; Steele, B.R.; Micha, M.-S.; Alexis, M.N.; Tsantili-Kakoulidou, A.; Mavromoustakos, T. Comparison of Thermal Effects of Stilbenoid Analogs in Lipid Bilayers Using Differential Scanning Calorimetry and Molecular Dynamics: Correlation of Thermal Effects and Topographical Position with Antioxidant Activity. Eur. Biophys. J. 2011, 40, 865–875. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Yang, P.; Liu, X.; Li, Y.; Gu, Z. ROS Scavenging Biopolymers for Anti-Inflammatory Diseases: Classification and Formulation. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Rand, R.P.; Fuller, N.L.; Gruner, S.M.; Parsegian, V.A. Membrane Curvature, Lipid Segregation, and Structural Transitions for Phospholipids under Dual-Solvent Stress. Biochemistry 1990, 29, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sodt, A.J.; Pastor, R.W. Molecular Modeling of Lipid Membrane Curvature Induction by a Peptide: More Than Simply Shape. Biophys. J. 2014, 106, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Nathoo, S.; Vogel, H.J.; Prenner, E.J. Induction of Non-lamellar Lipid Phases by Antimicrobial Peptides: A Potential Link to Mode of Action. Chem. Phys. Lipids 2010, 163, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, E.; Meleleo, D.; Micelli, S.; Picciarelli, V. Magainin 2 Channel Formation in Planar Lipid Membranes: The Role of Lipid Polar Groups and Ergosterol. Eur. Biophys. J. 2003, 32, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Mitani, Y.; Akada, K.Y.; Murase, O.; Yoneyama, S.; Zasloff, M.; Miyajima, K. Mechanism of Synergism between Antimicrobial Peptides Magainin 2 and PGLa. Biochemistry 1998, 37, 15144–15153. [Google Scholar] [CrossRef]
- Yesylevskyy, S.; Rivel, T.; Ramseyer, C. Curvature Increases Permeability of the Plasma Membrane for Ions, Water and the Anti-cancer Drugs Cisplatin and Gemcitabine. Sci. Rep. 2019, 9, 17214. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.; Lee, D.K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Basso, L.G.; Rodrigues, R.Z.; Naal, R.M.; Costa-Filho, A.J. Effects of the Antimalarial Drug Primaquine on the Dynamic Structure of Lipid Model Membranes. Biochim. Biophys. Acta 2011, 1808, 55–64. [Google Scholar] [CrossRef]





| [Res] = 10 µM | [Res] = 20 µM | |||
|---|---|---|---|---|
| Vs mV | Λc ± SE nS | F ± SD | Λc ± SE nS | F ± SD |
| 120 | 0.012 ± 0.003 | 12.28 ± 1.08 | ||
| 100 | 0.022 ± 0.002 | 13.90 ± 1.48 | ||
| 80 | 0.019 ± 0.006 | 14.47 ± 0.74 | ||
| 60 | 0.029 ± 0.004 | 9.88 ± 0.52 | 0.034 ± 0.001 | 12.20 ± 0.55 |
| 40 | 0.046 ± 0.003 | 13.03 ± 0.52 | ||
| −40 | 0.054 ± 0.003 | 12.50 ± 1.01 | ||
| −60 | 0.030 ± 0.003 | 16.97 ± 0.98 | 0.034 ± 0.001 | 9.71 ± 0.51 |
| −80 | 0.016 ± 0.008 | 30.28 ± 4.16 | ||
| −100 | 0.020 ± 0.001 | 21.93 ± 1.35 | ||
| −120 | 0.016 ± 0.0006 | 18.78 ± 2.21 | ||
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| T1 | 0.11 ± 0.002 | 0.10 ± 0.003 |
| T2 | 0.28 ± 0.02 | 0.29 ± 0.01 |
| T3 | 0.30 ± 0.02 | 0.31 ± 0.02 |
| [Res] = 10 µM | [Res] = 20 µM | |||
|---|---|---|---|---|
| Vs mV | Λc ± SE nS | F ± SD | Λc ± SE nS | F ± SD |
| 120 | 0.020 ± 0.001 | 11.10 ± 0.49 | ||
| 100 | 0.020 ± 0.001 | 9.63 ± 0.62 | ||
| 80 | 0.019 ± 0.002 | 6.80 ± 0.47 | ||
| 40 | 0.065 ± 0.003 | 9.87 ± 0.86 | ||
| −40 | 0.050 ± 0.003 | 6.95 ± 0.70 | ||
| −80 | 0.020 ± 0.002 | 10.78 ± 0.79 | ||
| −100 | 0.021 ± 0.0006 | 12.74 ± 0.60 | ||
| −120 | 0.016 ± 0.0004 | 7.20 ± 1.07 | ||
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.30 ± 0.02 | 0.30 ± 0.02 |
| T1 | 0.11 ± 0.002 | 0.12 ± 0.002 |
| T2 | 0.29 ± 0.01 | 0.28 ± 0.01 |
| T3 | 0.30 ± 0.02 | 0.31 ± 0.01 |
| Time | C ± SE µF/cm2 [Res] = 10 µM | C ± SE µF/cm2 [Res] = 20 µM |
|---|---|---|
| T0 | 0.26 ± 0.01 | 0.28 ± 0.01 |
| T1 | 0.10 ± 0.003 | 0.11 ± 0.002 |
| T2 | 0.10 ± 0.002 | 0.11 ± 0.002 |
| T3 | 0.10 ± 0.003 | 0.12 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleleo, D. Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes 2021, 11, 132. https://doi.org/10.3390/membranes11020132
Meleleo D. Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes. 2021; 11(2):132. https://doi.org/10.3390/membranes11020132
Chicago/Turabian StyleMeleleo, Daniela. 2021. "Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers" Membranes 11, no. 2: 132. https://doi.org/10.3390/membranes11020132
APA StyleMeleleo, D. (2021). Study of Resveratrol’s Interaction with Planar Lipid Models: Insights into Its Location in Lipid Bilayers. Membranes, 11(2), 132. https://doi.org/10.3390/membranes11020132