Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review
Abstract
:1. Introduction
- Bone exposure or bone detection by probing through a fistula in the oral cavity for at least 8 weeks.
- Simultaneous or former therapy with antiresorptive or antiangiogenic drugs.
- No history of radiotherapy or metastatic malignancies in the jaw area [3].
2. Materials and Methods
2.1. Focused Questions
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Research
2.5. Quality Assessment of Included Studies
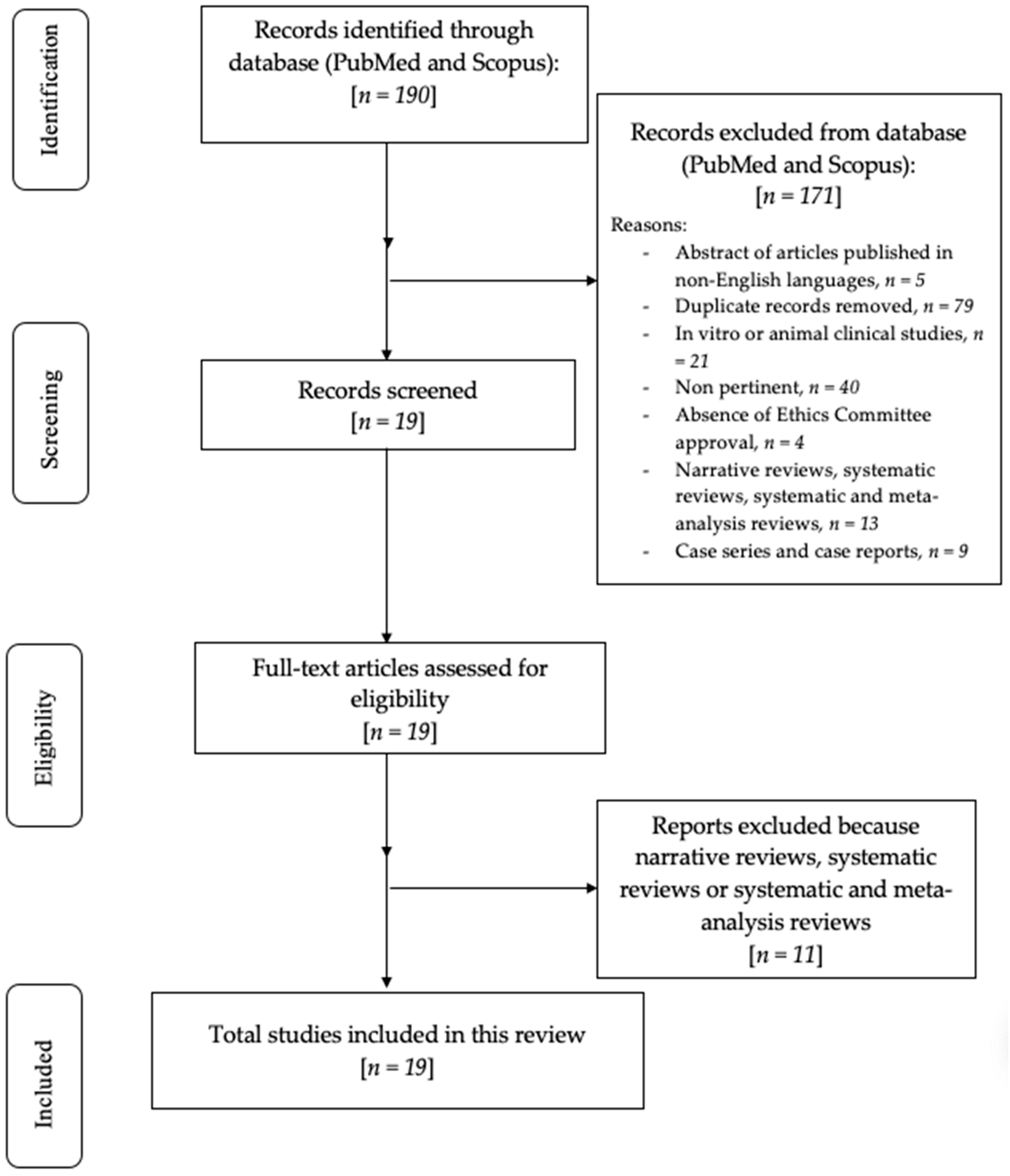
3. Results
Risk of Bias
4. Discussion
4.1. MRONJ Prevention Strategies
4.2. Treatment Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewiecki, E.M. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011, 7, 791–814. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American association of oral and maxillofacial surgeons’ position paper on medication-related osteonecrosis of the jaw—2022 update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradi, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- López-Cedrún, J.L.; Sanromán, J.F.; García, A.; Peñarrocha, M.; Feijoo, J.F.; Limeres, J.; Diz, P. Oral bisphosphonate-related osteonecrosis of the jaws in dental implant patients: A case series. Br. J. Oral Maxillofac. Surg. 2013, 51, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Freitas, M.; Limeres, J. Prevention of medication-related osteonecrosis of the jaws secondary to tooth extractions. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 2, e250–e259. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, X.; Liu, Z.; Qiu, Y.; Niu, Y. Pathogenesis and multidisciplinary management of medication-related osteonecrosis of the jaw. Int. J. Oral Sci. 2020, 12, 30. [Google Scholar] [CrossRef]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 489–496. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Gallesio, M.; Mozzati, M. Autologous platelet concentrates for bisphosphonate-related osteonecrosis of the jaw treatment and prevention. A systematic review of the literature. Eur. J. Cancer 2015, 51, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, D.; Piccart, F.; Ockerman, A.; Coropciuc, R.; Politis, C.; Jacobs, R. Adjuvant therapies for MRONJ: A systematic review. Bone 2020, 141, 115676. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.M.; Chang, H.P.; Wang, J.C. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung J. Med. Sci. 2020, 36, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 584–594. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tool. NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 March 2023).
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.B.; Pereira, E.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. [Google Scholar] [CrossRef]
- Razavi, P.; Jafari, A.; Vescovi, P.; Fekrazad, R. Efficacy of Adjunctive Photobiomodulation in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 777–791. [Google Scholar] [CrossRef]
- Cano-Durán, J.A.; Peña-Cardelles, J.F.; Ortega-Concepción, D.; Paredes-Rodríguez, V.M.; García-Riart, M.; López-Quiles, J. The role of Leucocyte-rich and platelet-rich fibrin (L-PRF) in the treatment of the medication-related osteonecrosis of the jaws (MRONJ). J. Clin. Exp. Dent. 2017, 9, e1051–e1059. [Google Scholar] [CrossRef]
- De Santis, D.; Gelpi, F.; Luciano, U.; Zarantonello, M.; Poscolere, A.; Modena, N.; Faccioni, P.; Causarano, G.; Finotti, M.; Zotti, F.; et al. New trends in adjunctive treatment and diagnosis in medication-related osteonecrosis of the jaw: A 10-year review. J. Biol. Regul. Homeost. Agents 2020, 34, 37–48. [Google Scholar] [PubMed]
- Mijiritsky, E.; Assaf, H.D.; Kolerman, R.; Mangani, L.; Ivanova, V.; Zlatev, S. Autologous Platelet Concentrates (APCs) for Hard Tissue Regeneration in Oral Implantology, Sinus Floor Elevation, Peri-Implantitis, Socket Preservation, and Medication-Related Osteonecrosis of the Jaw (MRONJ): A Literature Review. Biology 2022, 11, 1254. [Google Scholar] [CrossRef]
- Rusilas, H.; Balčiūnaitė, A.; Žilinskas, J. Autologous platelet concentrates in treatment of medication related osteonecrosis of the jaw. Stomatologija 2020, 22, 23–27. [Google Scholar] [PubMed]
- Lopez-Jornet, P.; Sanchez Perez, A.; Amaral Mendes, R.; Tobias, A. Medication-related osteonecrosis of the jaw: Is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Hao, L.; Tian, Z.; Li, S.; Yan, K.; Xue, Y. Osteonecrosis of the jaw induced by bisphosphonates therapy in bone metastases patient: Case report and literature review. Oral Oncol. 2022, 128, 105852. [Google Scholar] [CrossRef] [PubMed]
- de Souza Tolentino, E.; de Castro, T.F.; Michellon, F.C.; Passoni, A.C.C.; Ortega, L.J.A.; Iwaki, L.C.V.; da Silva, M.C. Adjuvant therapies in the management of medication-related osteonecrosis of the jaws: Systematic review. Head Neck 2019, 41, 4209–4228. [Google Scholar] [CrossRef]
- Goker, F.; Grecchi, E.; Grecchi, F.; Francetti, L.; Del Fabbro, M. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2662–2673. [Google Scholar]
- Momesso, G.A.C.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Laser surgery in management of medication-related osteonecrosis of the jaws: A meta-analysis. Oral Maxillofac. Surg. 2020, 24, 133–144. [Google Scholar] [CrossRef]
- Li, F.L.; Wu, C.B.; Sun, H.J.; Zhou, Q. Effectiveness of laser-assisted treatments for medication-related osteonecrosis of the jaw: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 256–267. [Google Scholar] [CrossRef]
- Mauceri, R.; Panzarella, V.; Pizzo, G.; Oteri, G.; Cervino, G.; Mazzola, G.; Di Fede, O.; Campisi, G. Platelet-Rich Plasma (PRP) in dental extraction of patients at risk of bisphosphonate-related osteonecrosis of the jaws: A two-year longitudinal study. Appl. Sci. 2020, 10, 4487. [Google Scholar] [CrossRef]
- Asaka, T.; Ohga, N.; Yamazaki, Y.; Sato, J.; Satoh, C.; Kitagawa, Y. Platelet-rich fibrin may reduce the risk of delayed recovery in tooth-extracted patients undergoing oral bisphosphonate therapy: A trial study. Clin. Oral Investig. 2017, 21, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Parise, G.K.; Costa, B.N.; Nogueira, M.L.; Sassi, L.M.; Schussel, J.L. Efficacy of fibrin-rich platelets and leukocytes (L-PRF) in tissue repair in surgical oral procedures in patients using zoledronic acid-case-control study. Oral Maxillofac. Surg. 2022, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Gianfreda, F.; Raffone, C.; Antonacci, D.; Pistilli, V.; Bollero, P. The Role of Platelet-Rich Fibrin (PRF) in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ). BioMed Res. Int. 2021, 2021, 4948139. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef]
- Vescovi, P.; Meleti, M.; Merigo, E.; Manfredi, M.; Fornaini, C. Tooth Extractions in High-Risk Patients Under Bisphosphonate Therapy and Previously Affected with Osteonecrosis of the Jaws: Surgical Protocol Supported by Low-Level Laser Therapy. J. Craniofac. Surg. 2015, 26, 696–699. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.W.; Kim, S.J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef]
- Sahin, O.; Tatar, B.; Ekmekcioğlu, C.; Aliyev, T.; Odabaşi, O. Prevention of medication related osteonecrosis of the jaw afterdentoalveolar surgery: An institution’s experience. J. Clin. Exp. Dent. 2020, 12, e771–e776. [Google Scholar] [CrossRef]
- Merigo, E.; Cella, L.; Oppici, A.; Cristina Arbasi, M.; Clini, F.; Fontana, M.; Fornaini, C. Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws. J. Lasers Med. Sci. 2018, 9, 92–100. [Google Scholar] [CrossRef]
- Tartaroti, N.C.; Marques, M.M.; Naclério-Homem, M.D.G.; Migliorati, C.A.; Zindel Deboni, M.C. Antimicrobial photodynamic and photobiomodulation adjuvant therapies for prevention and treatment of medication-related osteonecrosis of the jaws: Case series and long-term follow-up. Photodiagn. Photodyn. Ther. 2020, 29, 101651. [Google Scholar] [CrossRef] [PubMed]
- Özalp, Ö.; Yıldırımyan, N.; Öztürk, C.; Kocabalkan, B.; Şimşek Kaya, G.; Sindel, A.; Altay, M.A. Promising results of surgical management of advanced medication related osteonecrosis of the jaws using adjunctive leukocyte and platelet rich fibrin. BMC Oral Health 2021, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.; Martins, M.D.; Lascala, C.A.; Curi, M.M.; Migliorati, C.A.; Tenis, C.A.; Marques, M.M. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: A preliminary study. Oral Oncol. 2012, 48, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Valente, N.A.; Chatelain, S.; Alfonsi, F.; Mortellaro, C.; Barone, A. Medication-Related Osteonecrosis of the Jaw: The Use of Leukocyte-Platelet-Rich Fibrin as an Adjunct in the Treatment. J. Craniofac. Surg. 2019, 30, 1095–1101. [Google Scholar] [CrossRef]
- Mauceri, R.; Panzarella, V.; Maniscalco, L.; Bedogni, A.; Licata, M.E.; Albanese, A.; Toia, F.; Cumbo, E.M.G.; Mazzola, G.; Di Fede, O.; et al. Conservative Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Er, Cr: YSGG Laser and Platelet-Rich Plasma: A Longitudinal Study. BioMed Res. Int. 2018, 2018, 3982540. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.; Akan, E.; Tatar, B.; Ekmekcioğlu, C.; Ünal, N.; Odabaşı, O. Combined approach to treatment of advanced stages of medication-related osteonecrosis of the jaw patients. Braz. J. Otorhinolaryngol. 2022, 88, 613–620. [Google Scholar] [CrossRef]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Pedrazzi, G.; Fornaini, C.; Bonanini, M.; Ferri, T.; Nammour, S. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): A retrospective analysis of 101 treated sites with long-term follow-up. Photomed. Laser Surg. 2012, 30, 5–13. [Google Scholar] [CrossRef]
- Nica, D.F.; Riviș, M.; Roi, C.I.; Todea, C.D.; Duma, V.F.; Sinescu, C. Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ). Medicina 2021, 57, 145. [Google Scholar] [CrossRef]
- Longo, F.; Guida, A.; Aversa, C.; Pavone, E.; Di Costanzo, G.; Ramaglia, L.; Ionna, F. Platelet rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw: Personal experience and review of the literature. Int. J. Dent. 2014, 2014, 298945. [Google Scholar] [CrossRef] [PubMed]
- Borumandi, F.; Aghaloo, T.; Cascarini, L.; Gaggl, A.; Fasanmade, K. Anti-resorptive Drugs and their Impact on Maxillofacial Bone among Cancer Patients. Anticancer Agents Med. Chem. 2015, 15, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Evangelos, T.; Goloubeva, O. Long-term follow- up of multiple myeloma (MM) patients (pts) with osteonecro- sis of the jaw (ONJ). ASH Annu. Meet. Abstr. 2007, 110, 3519. [Google Scholar]
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Lo Muzio, L.; SIPMO ONJ Board; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. BioMed Res. Int. 2018, 2018, 2684924. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Gallesio, G.; Arata, V.; Pol, R.; Scoletta, M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: A report of 32 cases. Oral Oncol. 2012, 48, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.; Sharma, M.; Dube, A.; Gupta, P.K. Topical antimicrobial photodynamic therapy improves angiogenesis in wounds of diabetic mice. Lasers Med. Sci. 2015, 30, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Nisi, M.; Karapetsa, D.; Gennai, S.; Ramaglia, L.; Graziani, F.; Gabriele, M. Conservative surgical treatment of medication related osteonecrosis of the jaw (MRONJ) lesions in patients affected by osteoporosis exposed to oral bisphosphonates: 24 months follow-up. J. Craniomaxillofac. Surg. 2018, 46, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Rückschloß, T.; Müller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Craniomaxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Rupel, K.; Ottaviani, G.; Gobbo, M.; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Biasotto, M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014, 50, 1049–1057. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Yin, J. Platelet-rich plasma: A promising product for treatment of peripheral nerve regeneration after nerve injury. Int. J. Neurosci. 2011, 121, 176–180. [Google Scholar] [CrossRef]
- Anitua, E.; Begoña, L.; Orive, G. Treatment of hemimandibular paresthesia in a patient with bisphosphonate-related osteonecrosis of the jaw (BRONJ) by combining surgical resection and PRGF-Endoret. Br. J. Oral Maxillofac. Surg. 2013, 51, e272–e274. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M.; Orive, G. PRGF exerts a cytoprotective role in zoledronic acid-treated oral cells. Clin. Oral Investig. 2016, 20, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Latifyan, S.; Genot, M.T.; Klastersky, J. Bisphosphonate-related osteonecrosis of the jaw: A review of the potential efficacy of low-level laser therapy. Support. Care Cancer 2016, 24, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, I.R.; Park, B.S.; Kim, Y.D.; Chung, I.K.; Song, J.M.; Shin, S.H. Effect of low-level laser therapy on oral keratinocytes exposed to bisphosphonate. Lasers Med. Sci. 2015, 30, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Cortese, A.; Casarella, A.; Howard, C.M.; Claudio, P.P. Epi-Mucosa Fixation and Autologous Platelet-Rich Fibrin Treatment in Medication-Related Osteonecrosis of the Jaw. Dent. J. 2021, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, M.; Kim, T.; Kim, S.; Williams, D.; Esmaeili, M.; Hong, C.; Shin, K.H.; Kang, M.K.; Park, N.H.; et al. Indigenous microbiota protects development of medication-related osteonecrosis induced by periapical disease in mice. Int. J. Oral Sci. 2022, 14, 16. [Google Scholar] [CrossRef]
- Williams, D.W.; Vuong, H.E.; Kim, S.; Lenon, A.; Ho, K.; Hsiao, E.Y.; Sung, E.C.; Kim, R.H. Indigenous Microbiota Protects against Inflammation-Induced Osteonecrosis. J. Dent. Res. 2020, 99, 676–684. [Google Scholar] [CrossRef]
- Shanbhag, S.; Kampleitner, C.; Mohamed-Ahmed, S.; Yassin Mohammed, A.; Dongre, H.; Costea, D.E.; Tangl, S.; Hassan Mohamad, N.; Stavropoulos, A.; Bolstad, A.I.; et al. Ectopic Bone Tissue Engineering in Mice Using Human Gingiva or Bone Marrow-Derived Stromal/Progenitor Cells in Scaffold-Hydrogel Constructs. Front. Bioeng. Biotechnol. 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Behnezhad, M.; Asgharzadeh, M.; Zeinalzadeh, E.; Kafil, H.S. Antibacterial Properties of Aloe vera on Intracanal Medicaments against Enterococcus faecalis Biofilm at Different Stages of Development. Int. J. Dent. 2020, 2020, 8855277. [Google Scholar] [CrossRef]
- Di Fede, O.; Del Gaizo, C.; Panzarella, V.; La Mantia, G.; Tozzo, P.; Di Grigoli, A.; Lo Casto, A.; Mauceri, R.; Campisi, G. Ozone Infiltration for Osteonecrosis of the Jaw Therapy: A Case Series. J. Clin. Med. 2022, 11, 5307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Yu, J.; Ding, R.; Pei, D.; Zhang, Y.; He, G.; Cheng, Y.; Li, A. Injectable hydrogels with high drug loading through B-N coordination and ROS-triggered drug release for efficient treatment of chronic periodontitis in diabetic rats. Biomaterials 2022, 282, 121387. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
| Random Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | |
|---|---|---|---|---|---|
| Mauceri et al., 2020 [33] |  |  |  |  |  |
| Asaka et al., 2017 [34] |  |  |  |  |  |
| Parise et al., 2022 [35] |  |  |  |  |  |
| Miranda et al., 2021 [36] |  |  |  |  |  |
| Giudice et al., 2018 [37] |  |  |  |  |  |
| Tenore et al., 2020 [38] |  |  |  |  |  |
| Vescovi et al., 2015 [39] |  |  |  |  |  |
| Park et al., 2017 [40] |  |  |  |  |  |
| Sahin et al., 2020 [41] |  |  |  |  |  |
| Merigo et al., 2018 [42] |  |  |  |  |  |
| Tartaroti et al., 2020 [43] |  |  |  |  |  |
| Ozalp et al., 2021 [44] |  |  |  |  |  |
| Martins et al., 2012 [45] |  |  |  |  |  |
| Valente et al., 2019 [46] |  |  |  |  |  |
| Mauceri et al., 2018 [47] |  |  |  |  |  |
| Sahin et al., 2021 [48] |  |  |  |  |  |
| Vescovi et al., 2012 [49] |  |  |  |  |  |
| Nica et al., 2021 [50] |  |  |  |  |  |
| Longo et al., 2014 [51] |  |  |  |  |  |
| Authors and Study Design | N° of Patients | % Women | Mean Age (Years), Mean (SD or Range) | Treatment Tested |
|---|---|---|---|---|
| Mauceri et al., 2020 Controlled intervention study [33] | Trial Group: 20 Control Group: 905 | Trial Group: 80% Control Group: NR | Trial Group: 72.35 (±7.19) Control Group: NR | Platelet-rich plasma (PRP) |
| Asaka et al., 2017 Controlled intervention study [34] | Trial Group: 29 Control Group: 73 | Trial Group: 89.6% Control Group: 91.7% | Trial Group: 73 (24–87) Control Group: 68 (33–88) | Platelet-rich fibrin (PRF) |
| Parise et al., 2022 Randomized controlled trial [35] | Group 1: 7 Group 2: 8 Group 3: 5 | Group 1: 71.4% Group 2: 62.5% Group 3: 0.4% | Group 1: 59.42 (41–77) Group 2: 58.38 (41–73) Group 3: 71 (57–91) | L-PRF |
| Miranda et al., 2021 Retrospective controlled clinical trial [36] | Trial Group: 11 Control Group: 26 | Trial group: 100% Control group: 96.15% | Trial group: 74.81 (SD: 8.88) Control group: 70.69 (SD: 8.03) | PRF |
| Giudice et al., 2018 Randomized controlled trial [37] | Trial Group: 24 Control Group: 23 | Trial Group: 41.6% Control Group: 60.8% | Trial Group: 75.5 (±5.6) Control Group: 73.9 (±7.4) | PRF |
| Tenore et al., 2020 Retrospective controlled clinical study [38] | Trial Group: 13 Control Group: 8 Control Group:13 | Trial Group: 61.5% Control Group: 100% Control Group: 76.9% | 58.09 (45–92) | L-PRF + photobiomodulation therapy (PBMT) |
| Vescovi et al., 2015 Controlled clinical trial [39] | 36 | 24/36 (66.67%) | 68.5 (48–85) | Nd:YAG laser PBMT |
| Park et al., 2017 Randomized controlled trial [40] | Group L-PRF: 25 Group L-PRF + BMP-2: 30 | Group L-PRF: 88% Group L-PRF + BMP-2: 96.7% | Group L-PRF: 75.24 (59–97) Group L-PRF + BMP-2: 75.2 (60–85) | PRF + bone morphogenetic protein-2 (BMP-2) |
| Sahin et al., 2020 Observational study [41] | 44 | 32/44 (72.7%) | 66.3 | L-PRF + Nd:YAG laser PBMT |
| Merigo et al., 2018 Observational study [42] | 21 | 16/21 (76.1%) | 72.6 (60–85) | Er:YAG laser + PRP |
| Tartaroti et al., 2020 Prospective cohort study [43] | 17 | 15/17 (88.2%) | 73.37 (±9.97) | Antimicrobial photodynamic therapy (aPDT) + PBMT |
| Ozalp et al., 2021 Retrospective study [44] | 13 | 7/13 (53.8%) | 72.4 (54–84) | L-PRF |
| Martins et al., 2012 Retrospective study [45] | 22 | 16/22 (72.7%) | 58.1 (42–90) | PRP + PBMT |
| Valente et al., 2019 Retrospective study [46] | 15 | 9/15 (60%) | 64 (56–71) | L-PRF |
| Mauceri et al., 2018 Longitudinal cohort study [47] | 10 | 7/10 (70%) | 75.2 ± 5.94 | Er,Cr:YSGG laser + PRP |
| Sahin et al., 2022 Retrospective cohort study [48] | 21 | 14/21 (66.67%) | 68.04 (49–85) | L-PRF + Nd:YAG laser |
| Vescovi et al., 2012 Retrospective study [49] | 128 | 95/128 (74.2%) | NR | Antibiotic (G1) Antibiotic + LLLT (G2) Surgery (G3) Surgery + LLLT (G4) |
| Nica et al., 2021 Prospective observational study [50] | 241 | 184/241 (76.34%) | 67.7 (46–79) | PMBT (diode laser) + PRF |
| Longo et al., 2014 Retrospective observational study [51] | 72 | 60/72 (83.3%) | 59 (37–81) | PRP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; Ghizzoni, M.; Pellegrini, M.; Pulicari, F.; Spadari, F. Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review. Medicina 2023, 59, 972. https://doi.org/10.3390/medicina59050972
Scribante A, Ghizzoni M, Pellegrini M, Pulicari F, Spadari F. Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review. Medicina. 2023; 59(5):972. https://doi.org/10.3390/medicina59050972
Chicago/Turabian StyleScribante, Andrea, Martina Ghizzoni, Matteo Pellegrini, Federica Pulicari, and Francesco Spadari. 2023. "Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review" Medicina 59, no. 5: 972. https://doi.org/10.3390/medicina59050972








