Quality of Life and Persistence of Symptoms in Outpatients after Recovery from COVID-19
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindert, J.; Jakubauskiene, M.; Bilsen, J. The COVID-19 disaster and mental health-assessing, responding and recovering. Eur. J. Public Health 2021, 31, 31–35. Available online: https://academic.oup.com/eurpub/article/31/Supplement_4/iv31/6423463 (accessed on 11 July 2022). [CrossRef]
- WHO. Weekly Epidemiological Update on COVID-19—6 July 2022. 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-july-2022 (accessed on 11 July 2022).
- Merad, M.; Martin, J.C. Author correction: Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 448. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32488203 (accessed on 28 June 2022). [CrossRef]
- Jain, U. Effect of COVID-19 on the Organs. Cureus 2020, 12, e9540. Available online: https://pubmed.ncbi.nlm.nih.gov/32905500/ (accessed on 28 June 2022). [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. Available online: https://pubmed.ncbi.nlm.nih.gov/32644129/ (accessed on 11 July 2022). [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. Available online: https://pubmed.ncbi.nlm.nih.gov/33892403/ (accessed on 29 June 2022). [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. Available online: https://pubmed.ncbi.nlm.nih.gov/33428867/ (accessed on 29 June 2022). [CrossRef] [PubMed]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2021, 174, 576–578. Available online: https://pubmed.ncbi.nlm.nih.gov/33175566/ (accessed on 29 June 2022). [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. Available online: https://pubmed.ncbi.nlm.nih.gov/32729939/ (accessed on 29 June 2022). [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. Available online: https://pubmed.ncbi.nlm.nih.gov/34627560/ (accessed on 29 June 2022).
- Desgranges, F.; Tadini, E.; Munting, A.; Regina, J.; Filippidis, P.; Viala, B.; Karachalias, E.; Suttels, V.; Haefliger, D.; Kampouri, E.; et al. Post-COVID-19 syndrome in outpatients: A cohort study. J. Gen. Intern. Med. 2022, 37, 1943–1952. Available online: https://link.springer.com/article/10.1007/s11606-021-07242-1 (accessed on 29 July 2022). [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open 2021, 4, e210830. Available online: https://pubmed.ncbi.nlm.nih.gov/33606031/ (accessed on 29 June 2022). [CrossRef]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The stanford hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. Available online: https://pubmed.ncbi.nlm.nih.gov/32475821/ (accessed on 29 June 2022). [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. Available online: https://pubmed.ncbi.nlm.nih.gov/18049194/ (accessed on 29 June 2022). [CrossRef]
- Evans, R.A.; Leavy, O.C.; Richardson, M.; Elneima, O.; McCauley, H.J.C.; Shikotra, A.; Singapuri, A.; Sereno, M.; Saunders, R.M.; Harris, V.C.; et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, 761–775. Available online: https://pubmed.ncbi.nlm.nih.gov/35472304/ (accessed on 11 July 2022).
- Levis, B.; Benedetti, A.; Thombs, B.D.; DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ 2019, 365, l1476. Available online: https://pubmed.ncbi.nlm.nih.gov/30967483/ (accessed on 15 November 2022). [CrossRef]
- Plummer, F.; Manea, L.; Trepel, D.; McMillan, D. Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen. Hosp. Psychiatry 2016, 39, 24–31. Available online: https://pubmed.ncbi.nlm.nih.gov/26719105/ (accessed on 15 November 2022). [CrossRef]
- Vista de Evaluación del Estado de Salud con la Encuesta SF-36: Resultados Preliminares en México|Salud Pública de México. Available online: https://www.saludpublica.mx/index.php/spm/article/view/6138/7233 (accessed on 11 July 2022).
- The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1593914/ (accessed on 29 June 2022).
- Barile, J.P.; Horner-Johnson, W.; Krahn, G.; Zack, M.; Miranda, D.; DeMichele, K.; Ford, D.; Thompson, W.W. Measurement characteristics for two health-related quality of life measures in older adults: The SF-36 and the CDC healthy days items. Disabil. Health J. 2016, 9, 567–574. Available online: https://pubmed.ncbi.nlm.nih.gov/27259343/ (accessed on 11 July 2022). [CrossRef]
- Del Core, M.A.; Ahn, J.; Wukich, D.K.; Liu, G.T.; Lalli, T.; VanPelt, M.D.; Raspovic, K.M. Gender differences on SF-36 patient-reported outcomes of diabetic foot disease. Int. J. Low Extrem. Wounds 2018, 17, 87–93. Available online: https://pubmed.ncbi.nlm.nih.gov/29929411/ (accessed on 11 July 2022). [CrossRef]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in orthopaedics: A brief guide. J. Bone Joint Surg. Am. 2015, 97, 1628–1634. Available online: https://pubmed.ncbi.nlm.nih.gov/26446970/ (accessed on 11 July 2022). [CrossRef]
- Ware, J. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; Health Institute New England Medical Center: Boston, MA, USA, 1994. [Google Scholar]
- VassarStats. Available online: http://vassarstats.net/ (accessed on 10 August 2022).
- Farivar, S.S.; Cunningham, W.E.; Hays, R.D. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual. Life Outcomes 2007, 5, 54–58. Available online: https://pubmed.ncbi.nlm.nih.gov/17825096/ (accessed on 29 June 2022). [CrossRef] [PubMed]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. Available online: https://pubmed.ncbi.nlm.nih.gov/11491194/ (accessed on 29 June 2022). [CrossRef]
- Anastasio, F.; Barbuto, S.; Scarnecchia, E.; Cosma, P.; Fugagnoli, A.; Rossi, G.; Parravicini, M.; Parravicini, P. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir. J. 2021, 58, 2004015. Available online: https://pubmed.ncbi.nlm.nih.gov/33574080/ (accessed on 11 July 2022). [CrossRef]
- Nehme, M.; Braillard, O.; Chappuis, F.; Courvoisier, D.S.; Kaiser, L.; Soccal, P.M.; Reny, J.L.; Assal, F.; Bondolfi, G.; Tardin, A.; et al. One-year persistent symptoms and functional impairment in SARS-CoV-2 positive and negative individuals. J. Intern. Med. 2022, 292, 103–115. Available online: https://pubmed.ncbi.nlm.nih.gov/35555926/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Eberst, G.; Claudé, F.; Laurent, L.; Meurisse, A.; Roux-Claudé, P.; Barnig, C.; Vernerey, D.; Paget-Bailly, S.; Bouiller, K.; Chirouze, C.; et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann. Intensive Care 2022, 12, 23. Available online: https://pubmed.ncbi.nlm.nih.gov/35262794/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Chen, K.Y.; Li, T.; Gong, F.H.; Zhang, J.S.; Li, X.K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front. Psychiatry 2020, 11, 668. Available online: https://pubmed.ncbi.nlm.nih.gov/32733299/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Qu, G.; Zhen, Q.; Wang, W.; Fan, S.; Wu, Q.; Zhang, C.; Li, B.; Liu, G.; Yu, Y.; Li, Y.; et al. Health-related quality of life of COVID-19 patients after discharge: A multicenter follow-up study. J. Clin. Nurs. 2021, 30, 1742–1750. Available online: https://pubmed.ncbi.nlm.nih.gov/33656210/ (accessed on 4 July 2022). [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. Available online: https://pubmed.ncbi.nlm.nih.gov/34373540/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Zhou, F.; Tao, M.; Shang, L.; Liu, Y.; Pan, G.; Jin, Y.; Wang, L.; Hu, S.; Li, J.; Zhang, M.; et al. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front. Med. 2021, 8, 717194. Available online: https://pubmed.ncbi.nlm.nih.gov/34888318/ (accessed on 4 July 2022). [CrossRef]
- Jacobs, L.G.; Paleoudis, E.G.; Bari, D.L.-D.; Nyirenda, T.; Friedman, T.; Gupta, A.; Rasouli, L.; Zetkulic, M.; Balani, B.; Ogedegbe, C.; et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE 2020, 15, e0243882. Available online: https://pubmed.ncbi.nlm.nih.gov/33306721/ (accessed on 4 July 2022). [CrossRef] [PubMed]
- Kim, Y.; Kim, S.-W.; Chang, H.-H.; Kwon, K.T.; Hwang, S.; Bae, S. One year follow-up of COVID-19 related symptoms and patient quality of life: A prospective cohort study. Yonsei Med. J. 2022, 63, 499. Available online: https://pubmed.ncbi.nlm.nih.gov/35619573/ (accessed on 11 July 2022). [CrossRef]
- Temperoni, C.; Grieco, S.; Pasquini, Z.; Canovari, B.; Polenta, A.; Gnudi, U.; Montalti, R.; Barchiesi, F. Clinical characteristics, management and health related quality of life in young to middle age adults with COVID-19. BMC Infect. Dis. 2021, 21, 134. Available online: https://pubmed.ncbi.nlm.nih.gov/33522907/ (accessed on 4 July 2022). [CrossRef]
- Muñoz-Corona, C.; Gutiérrez-Canales, L.G.; Ortiz-Ledesma, C.; Martínez-Navarro, L.J.; Macías, A.E.; Scavo-Montes, D.A.; Guaní-Guerra, E. Quality of life and persistence of COVID-19 symptoms 90 days after hospital discharge. J. Int. Med. Res. 2022, 50, 3000605221110492. Available online: https://pubmed.ncbi.nlm.nih.gov/35822272/ (accessed on 27 July 2022). [CrossRef]
- Monti, G.; Leggieri, C.; Fominskiy, E.; Scandroglio, A.M.; Colombo, S.; Tozzi, M.; Moizo, E.; Mucci, M.; Crivellari, M.; Pieri, M.; et al. Two-months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol. Scand. 2021, 65, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Haitao, T.; Vermunt, J.V.; Abeykoon, J.; Ghamrawi, R.; Gunaratne, M.; Jayachandran, M.; Narang, K.; Parashuram, S.; Suvakov, S.; Garovic, V.D. COVID-19 and sex differences: Mechanisms and biomarkers. Mayo Clin. Proc. 2020, 95, 2189–2203. Available online: https://pubmed.ncbi.nlm.nih.gov/33012349/ (accessed on 8 July 2022). [CrossRef] [PubMed]
- Tizabi, Y.; Getachew, B.; Copeland, R.L.; Aschner, M. Nicotine and the nicotinic cholinergic system in COVID-19. FEBS J. 2020, 287, 3656–3663. Available online: https://pubmed.ncbi.nlm.nih.gov/32790936/ (accessed on 11 July 2022). [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.23033 (accessed on 28 July 2022). [CrossRef] [PubMed]
- Poudel, R.; Daniels, L.B.; DeFilippis, A.P.; Hamburg, N.M.; Khan, Y.; Keith, R.J.; Kumar, R.S.; Strokes, A.C.; Robertson, R.M.; Bhatnagar, A. Smoking is associated with increased risk of cardiovascular events, disease severity, and mortality among patients hospitalized for SARS-CoV-2 infections. PLoS ONE 2022, 17, e0270763. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0270763 (accessed on 28 July 2022). [CrossRef]
- Roy, D.; Tripathy, S.; Kar, S.K.; Sharma, N.; Verma, S.K.; Kaushal, V. Study of knowledge, attitude, anxiety & perceived mental healthcare need in Indian population during COVID-19 pandemic. Asian J. Psychiatry 2020, 51, 102083. Available online: https://pubmed.ncbi.nlm.nih.gov/32283510/ (accessed on 15 November 2022).
- Hossain, M.M.; Tasnim, S.; Sultana, A.; Faizah, F.; Mazumder, H.; Zou, L.; McKyer, E.L.J.; Ahmed, H.U.; Ma, P. Epidemiology of mental health problems in COVID-19: A review. F1000Research 2020, 23, 636. Available online: https://pubmed.ncbi.nlm.nih.gov/33093946/ (accessed on 15 November 2022). [CrossRef] [PubMed]
| Study Group, n = 206 | |
|---|---|
| Age, median (IQR) | 28 (22–45.5) |
| Male, n(%) | 77 (37.4%) |
| Female, n(%) | 129 (62.6%) |
| Smoking, n(%) | 52 (25.2%) |
| Comorbidities, n(%) | 75 (36.4%) |
| Diabetes, n(%) | 10 (4.9%) |
| Hypertension, n(%) | 15 (7.3%) |
| Overweight, n(%) | 29 (14.1%) |
| Cardiopathy, n(%) | 3 (1.5%) |
| Dyslipidemia, n(%) | 5 (2.4%) |
| Depression, n(%) | 19 (9.2%) |
| Asthma, n(%) | 9 (4.4%) |
| Autoimmune disease, n(%) | 4 (1.9%) |
| Home supplemental oxygen, n(%) | 23 (11.2%) |
| Complications/sequelae attributed to COVID-19, n(%) | 16 (7.8%) |
| Inability to walk, n(%) | 12 (5.8%) |
| Neuro/myopathy, n(%) | 1 (0.5%) |
| Myocardial infarction/ heart failure, n(%) | 1 (0.5%) |
| Treatments used during disease, n(%) | |
| Macrolide, n(%) | 72 (35%) |
| Ivermectin, n(%) | 47 (22.8%) |
| Vitamin D, n(%) | 56 (27.2%) |
| Corticosteroid, n(%) | 56 (27.2%) |
| Study Group, n = 206 | ||||
|---|---|---|---|---|
| Persistent Symptoms (n = 151) | Asymptomatic (n = 55) | p-Value * | OR (95 % CI) | |
| Female, n(%) | 105 (69.5) | 24 (43.6) | 0.001 | 2.95 (1.56–5.57) |
| Age in years, median (IQR) | 30 (22–49) | 28 (23–46) | 0.941 | |
| Hypertension, n(%) | 13 (8.6) | 2 (3.6) | 0.253 | 2.50 (0.54–11.4) |
| Smoking, n(%) | 31 (20.5) | 21 (38.2) | 0.012 | 0.42 (0.21–0.82) |
| Overweight, n(%) | 24 (15.9) | 5 (9.1) | 0.262 | 1.89 (0.68–5.23) |
| Diabetes, n(%) | 7 (4.6) | 3 (5.5) | 1.000 | 0.84 (0.21–3.38) |
| Macrolides treatment | 62 (41.1) | 10 (18.2) | 0.003 | 3.13 (1.47–6.67) |
| Vitamin D treatment | 43 (28.5) | 13 (23.6) | 0.596 | 1.29 (0.63–2.63) |
| Corticosteroids | 46 (30.5) | 10 (18.2) | 0.110 | 1.97 (0.91–4.25) |
| Study Group, n = 206 | |||
|---|---|---|---|
| Variable | ≤5 Months, (n = 107) | >5 Months, (n = 99) | p-Value * |
| Days after the onset of COVID-19 symptoms, median (IQR) | 107 (85–130) | 211 (176–269) | <0.001 |
| Male, n(%) | 36 (33.6) | 41 (41.4) | 0.31 |
| Female, n(%) | 71 (66.4) | 58 (58.6) | |
| Smoking, n(%) | 22 (20.6) | 30 (30.3) | 0.112 |
| Comorbidities, n(%) | 35 (32.7) | 40 (40.4) | 0.31 |
| Diabetes, n(%) | 7 (6.5) | 3 (3) | 0.335 |
| Hypertension, n(%) | 8 (7.5) | 7 (7.1) | 1.000 |
| Dyslipidemia, n(%) | 2 (1.9) | 3 (3) | 0.673 |
| Overweight, n(%) | 18 (16.8) | 11 (11.1) | 0.316 |
| Depression, n(%) | 9 (8.4) | 10 (10.1) | 0.811 |
| Asthma, n(%) | 5 (4.7) | 4 (4) | 1.000 |
| Home supplemental oxygen, n(%) | 12(11.2) | 11 (11.1) | 1.000 |
| Study Group, n = 206 | |||
|---|---|---|---|
| Variable | ≤5 Months, (n = 107) | >5 Months, (n = 99) | p-Value * |
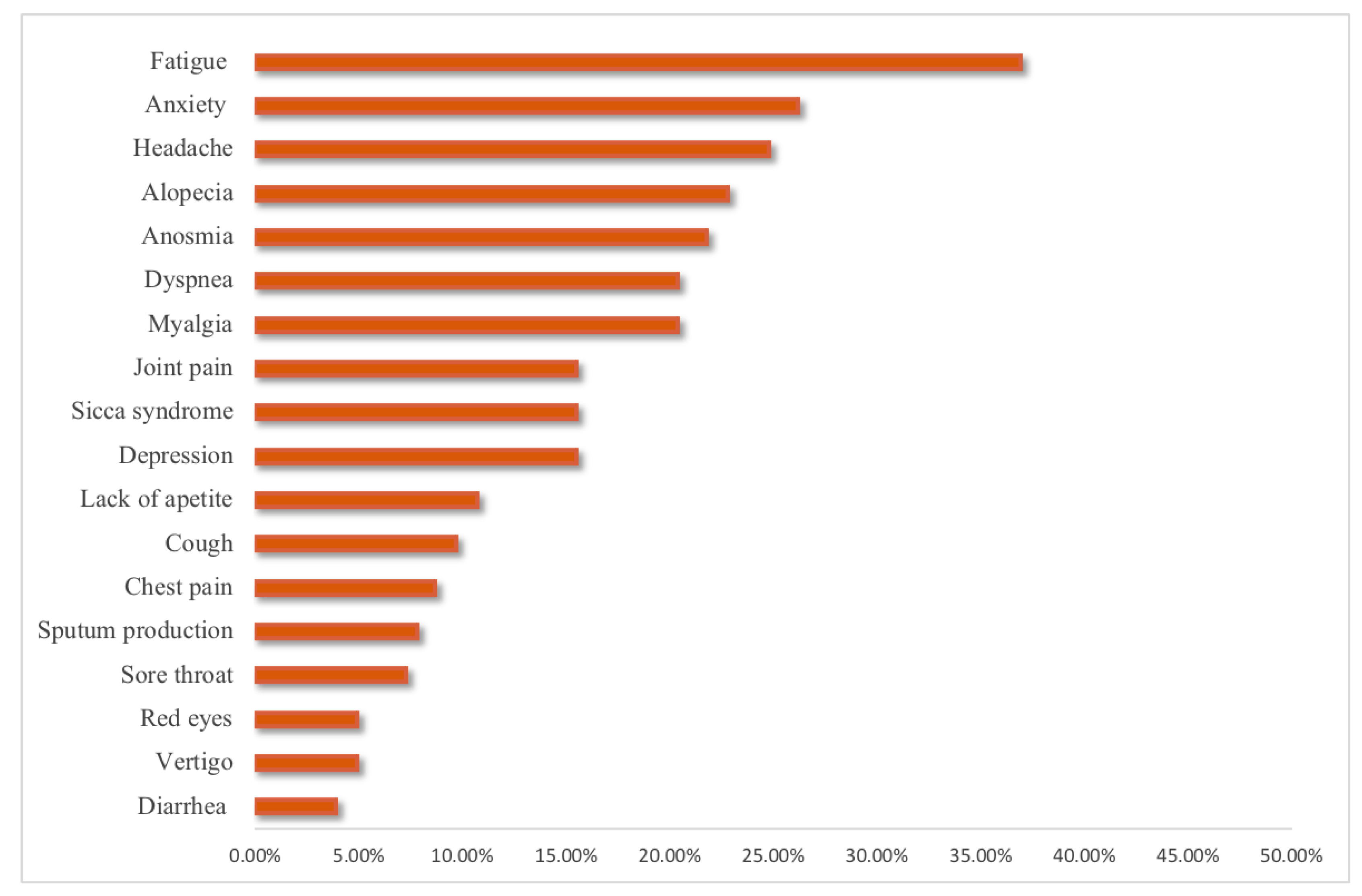
| Persistence of any COVID-19 symptoms, n(%) | 78 (25.7) | 73 (21.8) | 0.510 |
| Fatigue, n(%) | 40 (37.4) | 36 (36.4) | 0.886 |
| Dyspnea, n(%) | 23 (21.5) | 19 (19.2) | 0.731 |
| Joint pain, n(%) | 16 (15) | 16 (16.2) | 0.849 |
| Chest pain, n(%) | 12 (11.2) | 6 (6.1) | 0.223 |
| Cough, n(%) | 9 (8.4) | 11 (11.1) | 0.639 |
| Smell dysfunction, n(%) | 20 (18.7) | 25 (25.3) | 0.312 |
| Headache, n(%) | 24 (22.4) | 27 (27.3) | 0.518 |
| Sicca syndrome, n(%) | 15 (14) | 17 (17.2) | 0.568 |
| Red eyes, n(%) | 6 (5.6) | 4 (4) | 0.750 |
| Sputum production, n(%) | 9 (8.4) | 7 (7.1) | 0.798 |
| Lack of appetite, n(%) | 14 (13.1) | 8 (8.1) | 0.268 |
| Sore throat, n(%) | 6 (5.6) | 9 (9.1) | 0.424 |
| Vertigo, n(%) | 4 (3.7) | 6 (6.1) | 0.526 |
| Myalgia, n(%) | 28 (26.2) | 14 (14.1) | 0.038 |
| Diarrhea, n(%) | 4 (3.7) | 4 (4%) | 1.000 |
| Anxiety, n(%) | 26 (24.3) | 28 (28.3) | 0.530 |
| Depression, n(%) | 13 (12.1) | 19 (19.2) | 0.182 |
| Alopecia, n(%) | 28 (26.2) | 19 (19.2) | 0.249 |
| Study Group, n = 206 | |
|---|---|
| SF-36 Scale | Score, Median (IQR) |
| Physical function | 95 (80–100) |
| Role physical function | 100 (50–100) |
| Body pain | 90 (68–100) |
| General health | 65 (50–80) |
| Mental health | 60 (48–76) |
| Role emotional | 100 (33–100) |
| Vitality | 55 (45–70) |
| Social function | 75 (50–100) |
| Health change | 50 (25–75) |
| Correlated physical component summary | 50.2 (44.6–55.2) |
| Correlated mental component summary | 45.4 (38.7–52.1) |
| Study Group, n = 206 | |||
|---|---|---|---|
| ≤5 Months (n = 107) | >5 Months (n = 99) | p-Value * | |
| Score, Median (IQR) | Score, Median (IQR) | ||
| PF | 95 (80–100) | 86.92 (80–100) | 0.478 |
| RP | 100 (50–100) | 100 (75–100) | 0.299 |
| RE | 65.14 (33–100) | 100 (33–100) | 0.412 |
| VT | 55 (45–70) | 55 (45–70) | 0.477 |
| MH | 64 (50–76) | 60 (48–76) | 0.783 |
| SF | 75 (63–88) | 75 (50–100) | 0.784 |
| BP | 90 (68–100) | 90 (68–100) | 0.473 |
| GH | 65 (50–80) | 60 (50–75) | 0.730 |
| HC | 50 (25–50) | 50 (25–75) | 0.204 |
| PCSc | 50.74 (40.80–55.29) | 50.13 (44.28–55.41) | 0.823 |
| MCSc | 45.13 (39.09–52.16) | 45.69 (38.50–52.16) | 0.900 |
| Outcomes | Variables | Odds Ratio (95 % CI) | p-Value |
|---|---|---|---|
| Poor PCS | Comorbidities | 2.22 (1.13–4.37) | 0.021 |
| Sequalae | 5.93 (1.13–31.02) | 0.035 | |
| Persistent symptoms | 2.83 (1.15–6.91) | 0.023 | |
| Fatigue | 2.18 (1.04–4.57) | 0.038 | |
| Myalgia | 2.42 (1.00–5.87) | 0.050 | |
| Poor MCS | Female | 2.70 (1.41–5.15) | 0.003 |
| Anxiety | 5.54 (1.82–16.87) | 0.003 | |
| Depression | 8.53 (1.08–67.39) | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Canales, L.G.; Muñoz-Corona, C.; Barrera-Chávez, I.; Viloria-Álvarez, C.; Macías, A.E.; Guaní-Guerra, E. Quality of Life and Persistence of Symptoms in Outpatients after Recovery from COVID-19. Medicina 2022, 58, 1795. https://doi.org/10.3390/medicina58121795
Gutiérrez-Canales LG, Muñoz-Corona C, Barrera-Chávez I, Viloria-Álvarez C, Macías AE, Guaní-Guerra E. Quality of Life and Persistence of Symptoms in Outpatients after Recovery from COVID-19. Medicina. 2022; 58(12):1795. https://doi.org/10.3390/medicina58121795
Chicago/Turabian StyleGutiérrez-Canales, Lizeth Guadalupe, Carolina Muñoz-Corona, Isaac Barrera-Chávez, Carlos Viloria-Álvarez, Alejandro E. Macías, and Eduardo Guaní-Guerra. 2022. "Quality of Life and Persistence of Symptoms in Outpatients after Recovery from COVID-19" Medicina 58, no. 12: 1795. https://doi.org/10.3390/medicina58121795
APA StyleGutiérrez-Canales, L. G., Muñoz-Corona, C., Barrera-Chávez, I., Viloria-Álvarez, C., Macías, A. E., & Guaní-Guerra, E. (2022). Quality of Life and Persistence of Symptoms in Outpatients after Recovery from COVID-19. Medicina, 58(12), 1795. https://doi.org/10.3390/medicina58121795







