Rosai–Dorfman Disease: Breast Involvement—Case Report and Literature Review
Abstract
:1. Introduction
2. Objective
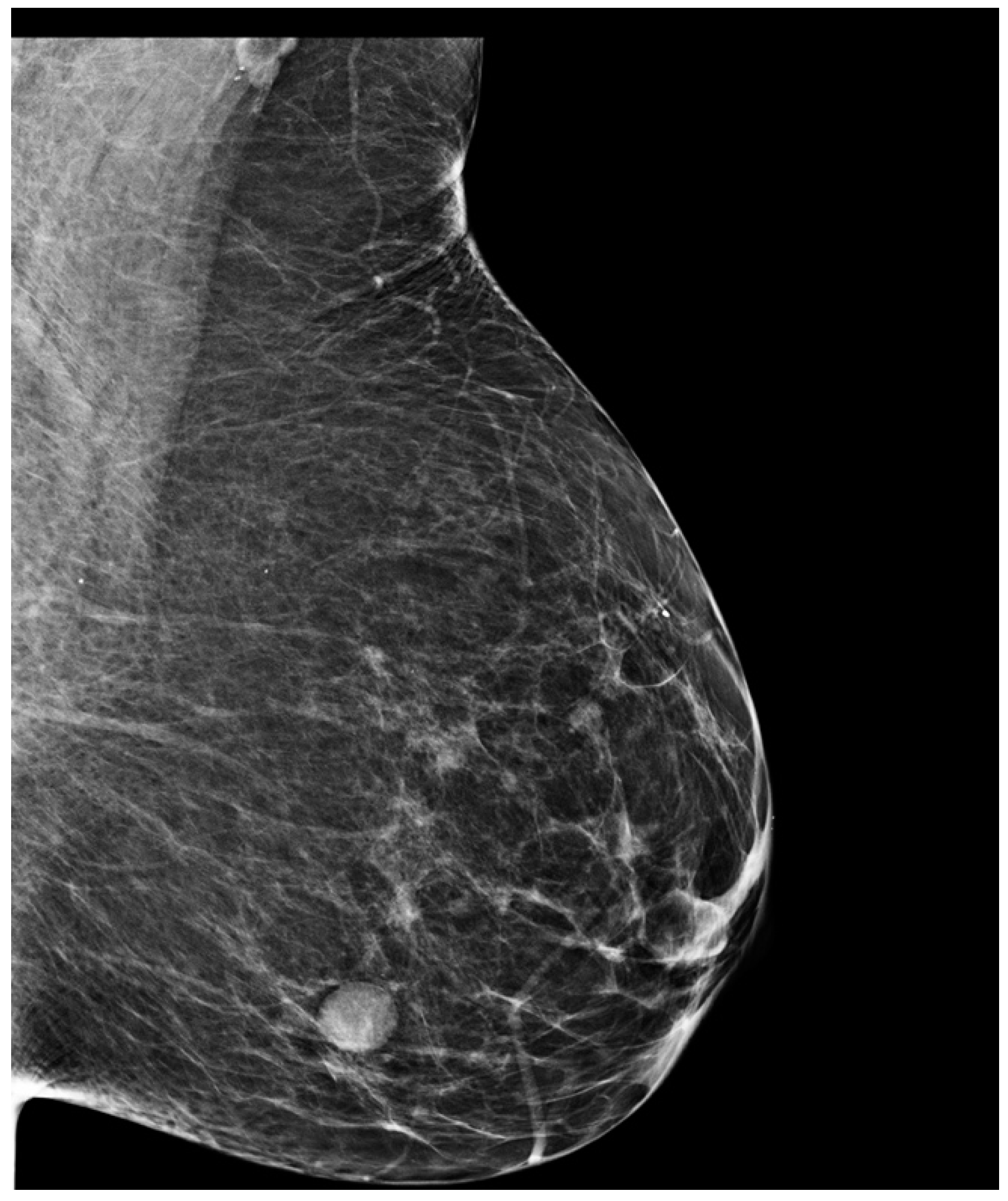
3. Case Presentation
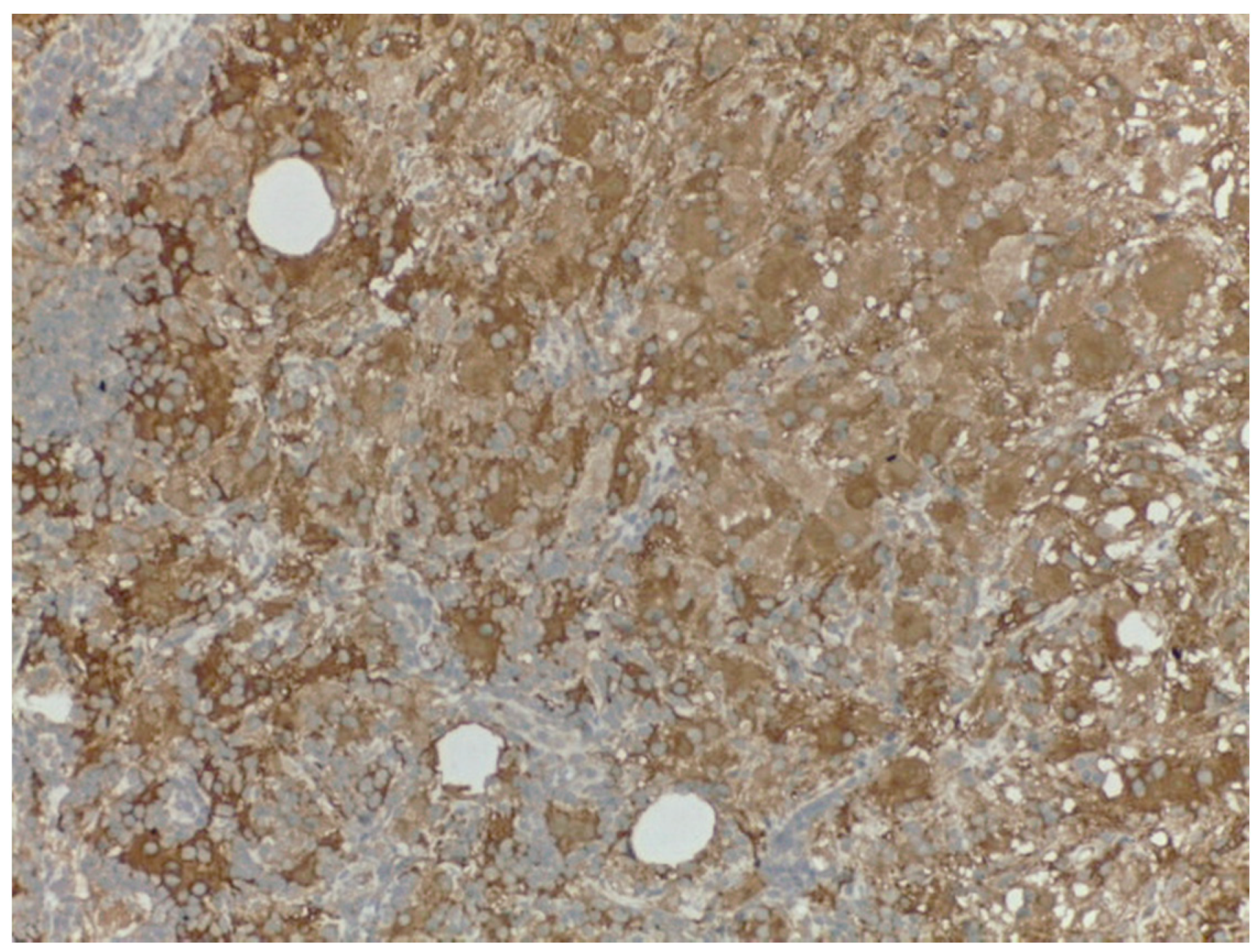
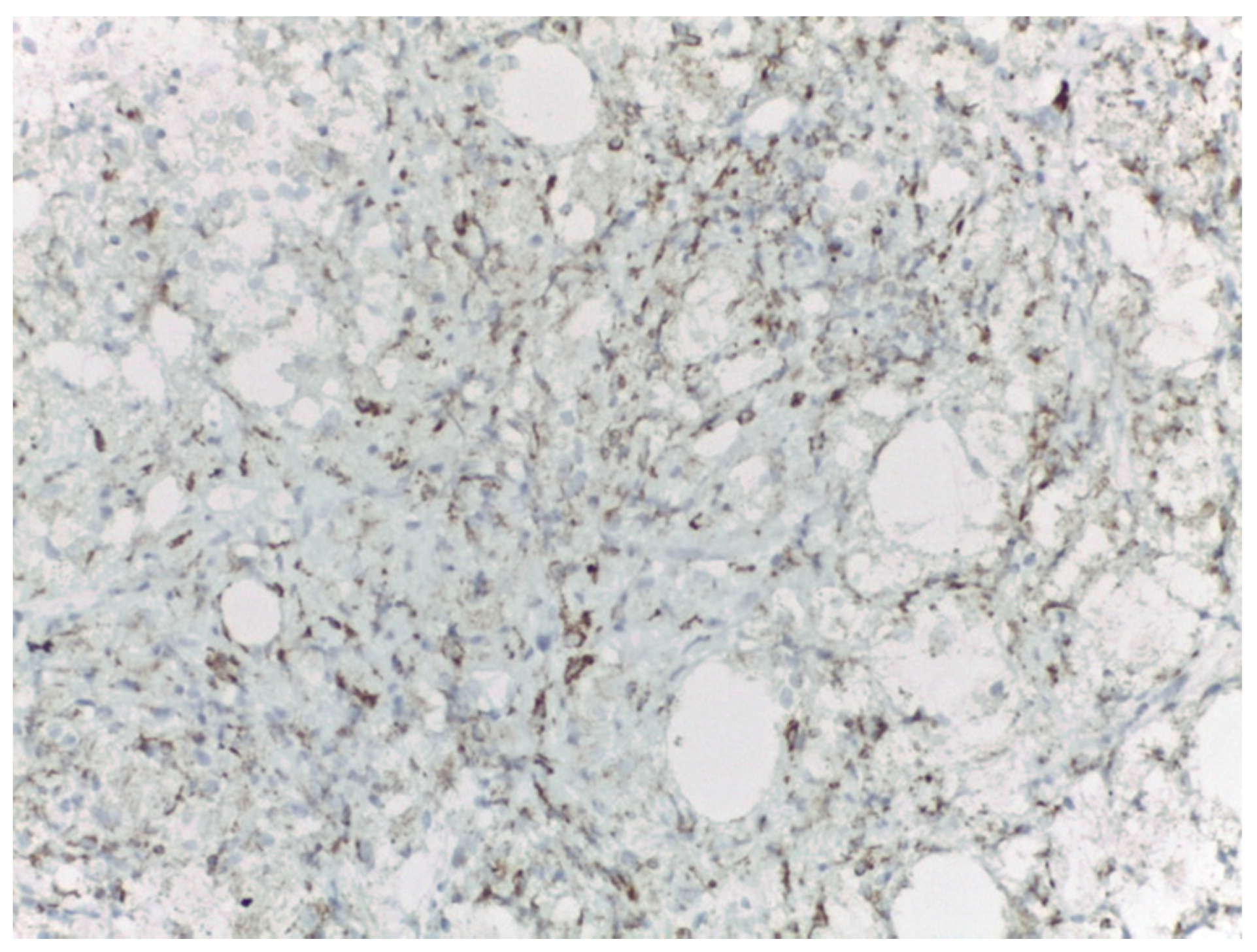
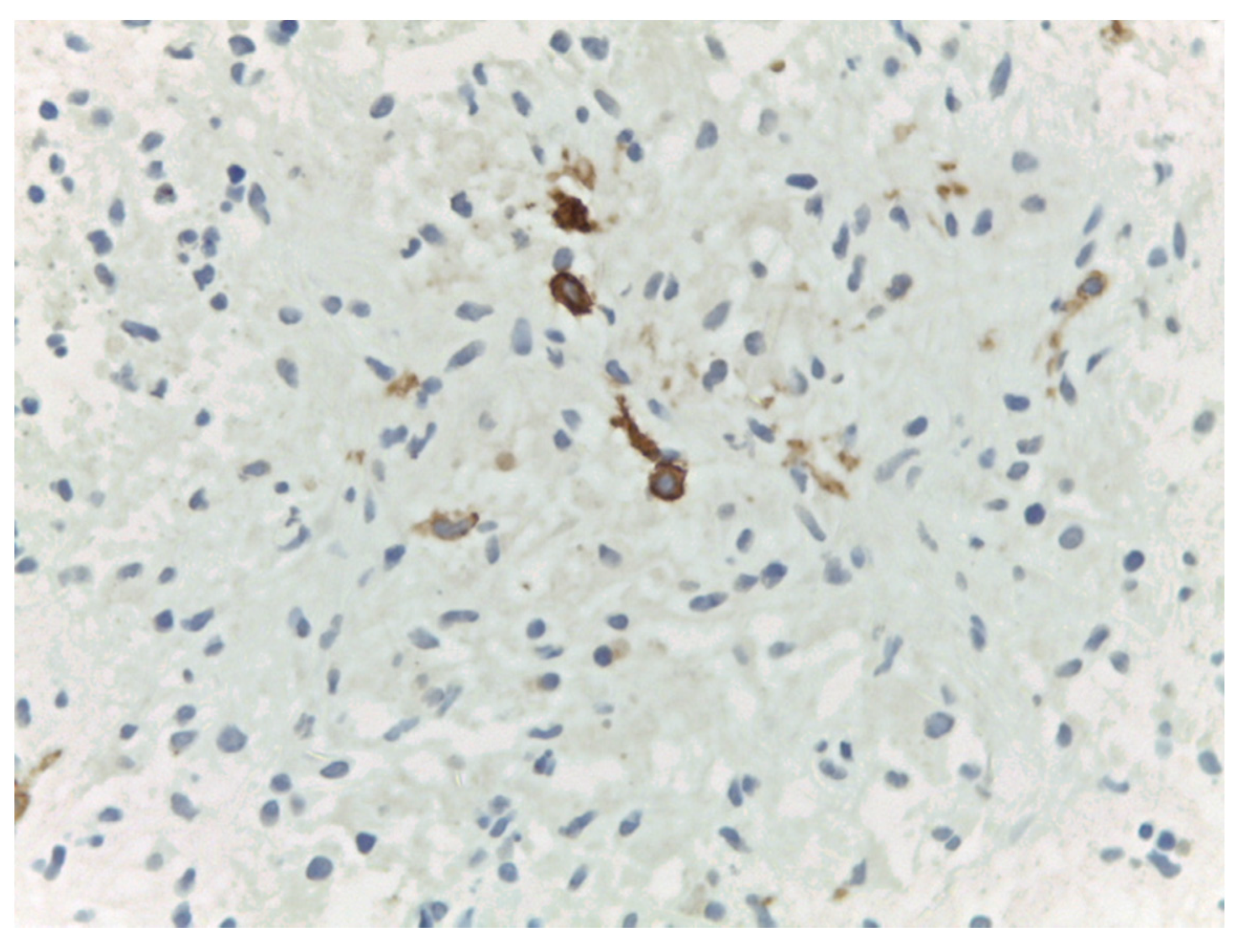
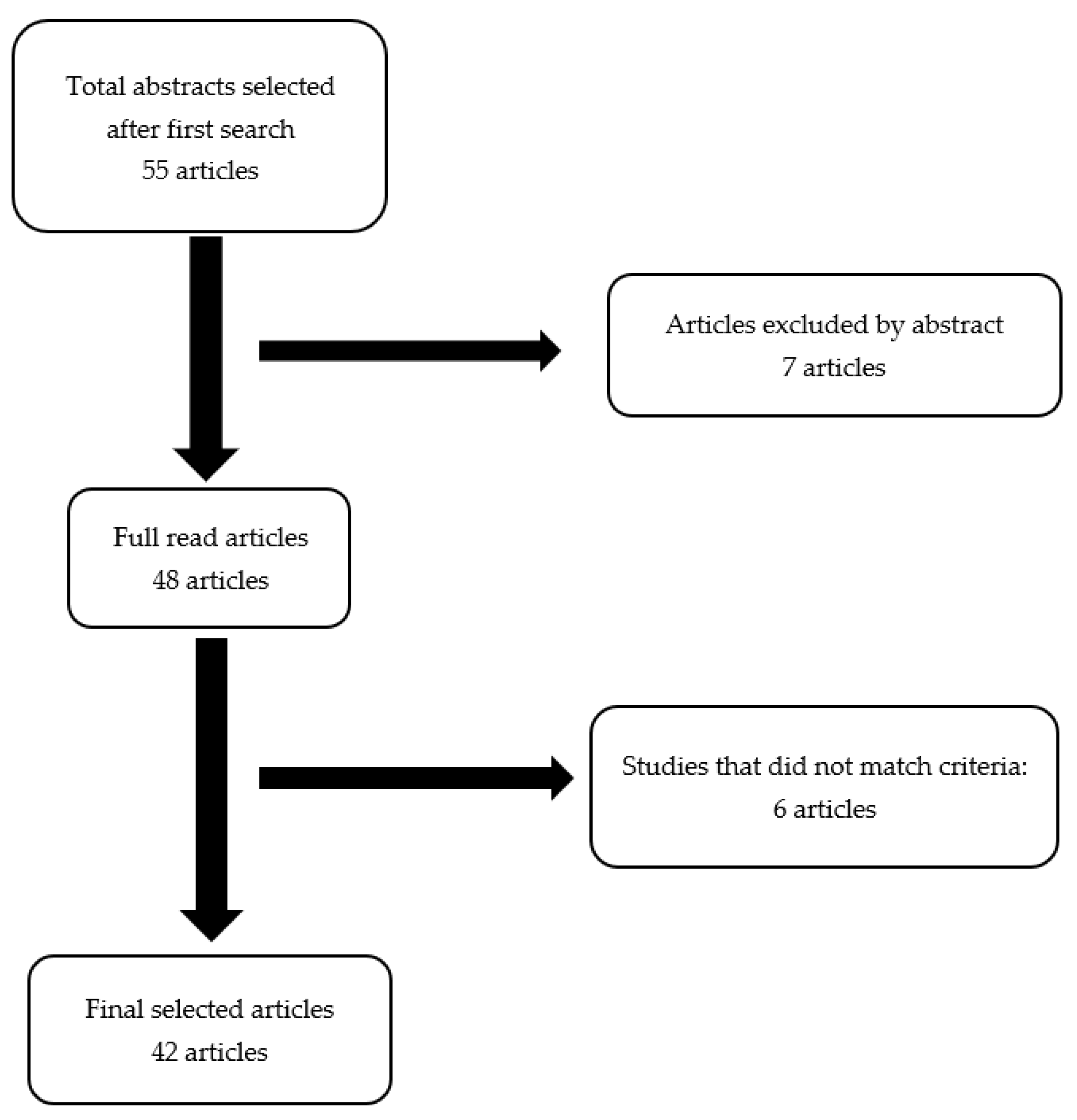
4. Materials and Methods
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Destombes, P. Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali (4 cases). Bull. Soc. Pathol. Exot. 1965, 58, 1169–1175. [Google Scholar]
- Rosai, J.; Dorfman, R.F. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch. Pathol. 1969, 87, 63–70. [Google Scholar]
- Symss, N.; Cugati, G.; Vasudevan, M.; Ramamurthi, R.; Pande, A. Intracranial Rosai Dorfman Disease: Report of three cases and literature review. Asian J. Neurosurg. 2010, 5, 19–30. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3201083/ (accessed on 6 April 2015). [PubMed]
- Rosai, J.; Dorfman, R.F. Sinus histiocytosis lymphadenopathy: A pseudolymphomatous disease: Analysis of 34 cases. Cancer 1972, 30, 1174–1188. [Google Scholar] [CrossRef]
- Emile, J.F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Histiocyte Society. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce-Brand, C.; Schneider, J.W.; Schubert, P. Rosai-Dorfman disease: An overview. J. Clin. Pathol. 2020, 73, 697–705. [Google Scholar] [CrossRef]
- Sharma, M.S.; De Padua, M.; Jha, A.N. Rosai-Dorfman disease mimicking a sphenoid wing meningioma. Neurol. India 2005, 53, 110. [Google Scholar]
- Paulli, M.; Bergamaschi, G.; Tonon, L.; Viglio, A.; Rosso, R.; Fachetti, F.; Geerts, M.L.; Magrini, U.; Cazzola, M. Evidence for a polycloncal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai Dorfman Disease). Br. J. Haematol. 1995, 91, 415–418. [Google Scholar] [CrossRef]
- Delacrétaz, F.; Meugé-Moraw, C.; Anwar, D.; Borisch, B.; Chave, J.P. Sinus histiocytosis with massive lymphadenopathy (Rosai Dorfman disease) in an HIV-positive patient. Virchows Archiv A Pathol. Anat. Histopathol. 1991, 419, 251–254. [Google Scholar] [CrossRef]
- Al-Khateeb, T.H. Cutaneous Rosai-Dorfman disease of the face: A comprehensive literature review and case report. J. Oral Maxillofac. Surg. 2016, 74, 528–540. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Suri, S.; Nijhawan, R.; Kang, M.; Gautam, V. Rosai-Dorfman disease: Unusual cause of diffuse and massive retroperitoneal lymphadenopathy. Br. J. Radiol. 2005, 78, 845–847. [Google Scholar] [CrossRef]
- Abla, O.; Jacobsen, E.; Picarsic, J.; Krenova, Z.; Jaffe, R.; Emile, J.F.; Durham, B.H.; Braier, J.; Charlotte, F.; Donadieu, J.; et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 2018, 131, 2877–2890. [Google Scholar] [CrossRef] [Green Version]
- Parkash, O.; Yousaf, M.S.; Fareed, G. Rosai-Dorfman disease, an uncommon cause of common clinical presentation. J. Pak. Med. Assoc. 2019, 69, 1213–1215. [Google Scholar] [PubMed]
- Mohammadi, O.; Lisigurski, M.Z.; Mehra, D.; Pishdad, R.; Gulec, S. Rosai-Dorfman Disease and Unusual Local Invasive Presentation. Cureus 2020, 12, e7328. [Google Scholar] [CrossRef] [Green Version]
- Pulsoni, A.; Anghel, G.; Falcucci, P.; Matera, R.; Pescarmona, E.; Ribersani, M.; Villiva’, N.; Mandelli, F. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Report of a case and literature review. Am. J. Hematol. 2002, 69, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Foucar, E.; Rosai, J.; Dorfman, R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Review of the entity. Semin. Diagn. Pathol. 1990, 7, 19–73. [Google Scholar] [PubMed]
- Battle, B.; McIntire, P.; Babagbemi, K.; Mema, E. Extranodal multifocal Rosai-Dorfman disease of the breast: A case report. Clin. Imaging 2021, 71, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Delaney, E.E.; Larkin, A.; MacMaster, S.; Sakhdari, A.; DeBenedectis, C.M. Rosai-Dorfman disease of the breast. Cureus 2017, 9, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Li, X.; Li, Y.; Wang, Z.; Cheng, L.; Song, X.; Wu, Y. Rosai-Dorfman disease with features of IgG4-related disease in the breast: Cases report and literature review. Asian Pac. J. Allergy Immunol. 2018, 36, 51–57. [Google Scholar] [PubMed] [Green Version]
- Hoffmann, J.C.; Lin, C.Y.; Bhattacharyya, S.; Weinberg, O.K.; Chisholm, K.M.; Bayerl, M.; Cascio, M.; Venkataraman, G.; Allison, K.; Troxell, M.; et al. Rosai-Dorfman disease of the breast with variable IgG4+ plasma cells: A diagnostic Mimicker of other malignant and reactive entities. Am. J. Surg. Pathol. 2019, 43, 1653–1660. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jiang, X.N.; Lu, J.P.; Zhang, H.; Li, X.Q.; Chen, G. Clinicopathologic analysis of extranodal Rosai-Dorfman disease of breast: A report of 12 cases. Zhonghua Bing Li Xue Za Zhi Chin. J. Pathol. 2016, 45, 556–560. [Google Scholar]
- El-Attrache, B.; Gluck, B.; Heimann, A.; Kapenhas, E. A rarity in breast pathology: First recurrent male case of Rosai-Dorfman disease. Int. J. Surg. Case Rep. 2018, 52, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Sharma, N.; Booth, C.N.; Oshilaja, O.; Downs-Kelly, E.P.; McKenney, J.K.; Sturgis, C.D. Mammary extranodal Rosai-Dorfman disease with and without associated axillary lymphadenopathy: Insights for practitioners of breast pathology. Int. J. Surg. Pathol. 2020, 28, 541–548. [Google Scholar] [CrossRef]
- Shin, G.W.; Park, Y.M.; Heo, Y.J.; Baek, J.W.; Lee, Y.J.; Han, J.Y.; Park, H. Sonographic features of Rosai-Dorfman disease in the breast: A case report. J. Clin. Ultrasound 2020, 48, 108–110. [Google Scholar] [CrossRef] [PubMed]
- De Mello Tucunduva, T.C.; Gaziero, A.; Tostes, V.S.; Chaves, M.C.; Stiepcich, M.M.A.; Torres, U.S.; Chala, L.F.; de Mello, G.G.N. Extranodal Rosai-Dorfman disease manifesting with breast involvement: Imaging and histopathological findings. Breast J. 2019, 25, 1266–1267. [Google Scholar] [CrossRef]
- Jorns, J.M. Extranodal Rosai-Dorfman Disease of the Breast. Breast J. 2017, 23, 105–107. [Google Scholar] [CrossRef]
- Goldbach, A.R.; Hava, S.; Caroline, D.; Zhao, X.; Bains, A.; Pascarella, S. Rosai-Dorfman disease of the breast: A potential marker of systemic disease. Breast J. 2019, 25, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Parkin, C.K.E.; Keevil, C.; Howe, M.; Maxwell, A.J. Rosai-Dorfman disease of the breast. BJR Case Rep. 2015, 1, 20150010. [Google Scholar] [CrossRef]
- Ciurea, A.; Ciortea, C.; Cosarca, M.; Rogojan, L. Breast Involvement in Pure Cutaneous Rosai-Dorfman Disease: Ultrasound and Sonoelastography appearance with a review of the literature. Ultrasound Q. 2016, 32, 183–186. [Google Scholar] [CrossRef]
- Tenny, S.O.; McGinness, M.; Zhang, D.; Damjanov, I.; Fan, F. Rosai-Dorfman Disease Presenting as a Breast Mass and Enlarged Axillary Lymph Node Mimicking Malignancy: A Case Report and Review of the Literature. Breast J. 2011, 17, 516–520. [Google Scholar] [CrossRef]
- Morkowski, J.J.; Nguyen, C.V.; Lin, P.; Farr, M.; Abraham, S.C.; Gilcrease, M.Z.; Moran, C.A.; Wu, Y. Rosai-Dorfman disease confined to the breast. Ann. Diagn. Pathol. 2010, 14, 81–87. [Google Scholar] [CrossRef]
- Vaidya, T.; Mahajan, A.; Rane, S. Multimodality imaging manifestations of Rosai-Dorfman disease. Acta Radiol. Open 2020, 9, 2058460120946719. [Google Scholar] [CrossRef]
- Simmons, N.R.; Xu, M.L.; Tavassoli, F.A.; Geisel, J.; Killelea, B.; Philpotts, L.E. A Rare Presentation of Rosai-Dorfman Disease as a Breast Mass. Breast J. 2016, 22, 581–583. [Google Scholar] [CrossRef]
- Zhou, Q.; Ansari, U.; Keshav, N.; Davis, F.; Cundiff, M. Extranodal manifestation of Rosai-Dorfman disease in the breast tissue. Radiol. Case Rep. 2016, 11, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Green, I.; Dorfman, R.F.; Rosai, J. Breast involvement by extranodal Rosai-Dorfman disease: Report of seven cases. Am. J. Surg. Pathol. 1997, 21, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Yang, W.I.; Park, S.H.; Koo, J.S. Rosai-Dorfman disease in the breast with increased IgG4 expressing plasma cells: A case report. Korean J. Pathol. 2012, 46, 489. [Google Scholar] [CrossRef]
- Da Silva, B.B.; Lopes-Costa, P.V.; Pires, C.G.; Moura, C.S.; Borges, R.S.; da Silva, R.G. Rosai-Dorfman disease of the breast mimicking cancer. Pathol. Res. Pract. 2007, 203, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.B.; Tan, L.H.; Tan, P.H. Rosai-Dorfman disease of the breast: A mimic of breast malignancy. Pathology 2000, 32, 10–15. [Google Scholar] [CrossRef]
- Moyon, Q.; Boussouar, S.; Maksud, P.; Emile, J.F.; Charlotte, F.; Aladjidi, N.; Prévot, G.; Donadieu, J.; Amoura, Z.; Grenier, P.; et al. Lung involvement in Destombes-Rosai-Dorfman disease: Clinical and radiological features and response to the MEK inhibitor cobimetinib. Chest 2020, 157, 323–333. [Google Scholar] [CrossRef]
- Bansal, P.; Chakraborti, S.; Krishnanand, G.; Bansal, R. Rosai-Dorfman disease of the breast in a male: A case report. Acta Cytol. 2010, 54, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, M.; Song, Z.; Xu, B.; Tian, J. 18 F-fluoro-deoxyglucose positron emission tomography/computed tomography scan findings in Rosai-Dorfman disease with IgG4-positive plasma cell infiltration mimicking breast malignancy: A case report and literature review. J. Med. Case Rep. 2012, 6, 411. [Google Scholar] [CrossRef] [Green Version]
- Mantilla, J.G.; Goldberg-Stein, S.; Wang, Y. Extranodal Rosai-Dorfman disease: Clinicopathologic series of 10 patients with radiologic correlation and review of the literature. Am. J. Clin. Pathol. 2016, 145, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Krbanjevic, A.; Brown, H.G. Rosai-Dorfman disease extending to the brain. Clin. Neuropathol. 2021, 40, 195–200. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wang, C.H.; Lin, Y.Y.; Yen, K.Y.; Hsieh, T.C.; Sun, S.S.; Chang, H.W.; Kao, C.H. A mimic of breast lymphoma: Extranodal Rosai-Dorfman disease. Am. J. Med. Sci. 2010, 339, 282–284. [Google Scholar] [CrossRef]
- Mac-Moune Lai, F.; Lam, W.Y.; Chin, C.W.; Ng, W.L. Cutaneous Rosai-Dorfman disease presenting as a suspicious breast mass. J. Cutan. Pathol. 1994, 21, 377–382. [Google Scholar] [CrossRef]
- Wang, J.S.; Hsieh, S.P.; Shih, D.F.; Tseng, H.H. Cutaneous Rosai-Dorfman disease manifestating as recurrent breast tumor: A case report. Zhonghua Yi Xue Za Zhi Chin. Med. J. 1997, 59, 269–273. [Google Scholar]
- Gwin, K.; Cipriani, N.; Zhang, X.; Schmidt, R.; Hyjek, E. Bilateral Breast Involvement by Disseminated Extranodal Rosai-Dorfman Disease. Breast J. 2011, 17, 309–311. [Google Scholar] [CrossRef]
- Noordzij, W.; Weernink, E.E.; van Imhoff, G.W.; Kluin, P.M.; de Haan, L.D. Benign histiocytosis: Rosai-Dorfman disease. Ned. Tijdschr. Geneeskd. 2011, 155, A3176. [Google Scholar]
- Pham, C.B.; Abruzzo, L.V.; Cook, E.; Whitman, G.J.; Stephens, T.W. Rosai-Dorfman disease of the breast. Am. J. Roentgenol. 2005, 185, 971–972. [Google Scholar] [CrossRef]
- Elshikh, M.; Schellingerhout, D.; Rayan, J.; Taher, A.; Elsayes, A.K.; Mujtaba, B.; Garg, N. Disease characteristics, radiologic patterns, comorbid diseases, and ethnic differences in 32 patients with Rosai-Dorfman disease. J. Comput. Assist. Tomogr. 2020, 44, 450–461. [Google Scholar] [CrossRef]
- Hammond, L.A.; Keh, C.; Rowlands, D.C. Rosai-Dorfman disease in the breast. Histopathology 1996, 29, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Kuzmiak, C.M.; Koomen, M.; Lininger, R.; Pisano, E. Rosai-Dorfman disease presenting as a suspicious breast mass. Am. J. Roentgenol. 2003, 180, 1740–1742. [Google Scholar] [CrossRef]
- Baladandapani, P.; Hu, Y.; Kapoor, K.; Merriam, L.; Fisher, P.R. Rosai-Dorfman disease presenting as multiple breast masses in an otherwise asymptomatic male patient. Clin. Radiol. 2012, 67, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Hummel, P.; Waisman, J.; Chhieng, D.; Yan, Z.; Cohen, J.M.; Cangiarella, J. Fine-needle aspiration cytology of Rosai-Dorfman disease of the breast: A case report. Diagn. Cytopathol. 1999, 21, 287–291. [Google Scholar] [CrossRef]
- Dahlgren, M.; Smetherman, D.H.; Wang, J.; Corsetti, R.L. Rosai-Dorfman disease of the breast and parotid gland. J. La. State Med. Soc. Off. Organ La. State Med. Soc. 2008, 160, 35–38. [Google Scholar]
- Dias Perera, A.S.; Keleher, A.J.; Nath, M. Rosai-Dorfman disease presenting as a male breast mass. Am. Surg. 2007, 73, 294–295. [Google Scholar] [CrossRef]
- Picón-Coronel, G.; Palmerín-Bucio, M.E.; Méndez-Pérez, V.; Alvarado-Cabrero, I. Mammary gland Rosai Dorfman disease. A case report and literature review. Gac. Med. Mex. 2010, 146, 212–215. [Google Scholar]
- Pérez-Guillermo, M.; Sola-Perez, J.; Rodriguez-Bermejo, M. Malacoplakia and Rosai-Dorfman disease: Two entities of histiocytic origin infrequently localized in the female breast—The cytologic aspect in aspirates obtained via fine-needle aspiration cytology. Diagn. Cytopathol. 1993, 9, 698–704. [Google Scholar] [CrossRef]
- Iancu, G.; Vasile, D.; Iancu, R.C.; Davitoiu, D.V. “Triple positive” breast cancer—A novel category. Rom. J. Morphol. Embryol. 2017, 58, 21–26. [Google Scholar] [PubMed]
- Rastogi, V.; Sharma, R.; Misra, S.R.; Yadav, L.; Sharma, V. Emperipolesis—A review. J. Clin. Diagn. Res. 2014, 8, ZM01. [Google Scholar] [CrossRef]


| Author | No of pts | Age | Breast Side | Breast Localization | Axillary | Diagnosis | Pathology | Mangement | FUp | Gender | Observations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Battle 2021 [17] | 1 | 49 | RB | Multifocal, UIQ | no | 1,2,3 | 1 | 1 | N/A | F | N/A |
| Delaney 2017 [18] | 1 | 63 | RB | UOQ | no | 1,2,3 | 1,2 | 1 | 1 | F | N/A |
| Liu 2018 [19] | 4 | 46–68 | N/A | N/A | no | 1,2,3 | 1 | 1 | N/A | F | N/A |
| Hoffmann 2019 [20] | 22 | median 54 (range 37–71) | RB, LB | N/A | no | 1,2,3 | 1,2 | 4 cases—1; 18 cases—2; (1 case mastect) | 4 cases—1; 2 cases—2 | 18—F, 4—M | N/A |
| Chen 2016 [21] | 12 | median 37 (range 28–47) | 7—RB, 5—LB | N/A | yes—1 no—11 | 1,2,3 | 2 | N/A | N/A | 12—F | Article in chinese |
| El-Attrache 2018 [22] | 1 | 55 | RB | LQ | no | 1,3 | 1,2 | 2 | 2 | F | Breast recurrence of RDD |
| Shetty 2020 [23] | 4 | median 58 (range 43–69) | N/A | N/A | yes—2 no—2 | 3—1,2 | 1,2 | 2 | 3 cases—1; 2 cases—3 1 case—N/A | F | N/A |
| Shin 2020 [24] | 1 | 54 | RB | OUQ | no | 1,2,3 | 1,2 | 2 | 2 | F | N/A |
| de Mello Tucundova 2017 [25] | 1 | 55 | LB | N/A | no | 1,2,3 | 1,2 | 2 | N/A | F | N/A |
| Jorns 2017 [26] | 1 | N/A | LB | N/A | no | 1,2,3 | 1,2 | 1 | 1 | F | No recurrence |
| Goldbach 2019 [27] | 1 | 44 | RB | UIQ | no | 1,2,3 | 1,2 | 2 | 1 | F | Recurrence at 6 months |
| Parkin 2015 [28] | 1 | 56 | LB | N/A | no | 1,2,3 | 1,2 | 1 | 1 | F | N/A |
| Ciurea 2016 [29] | 1 | 59 | RB | UIQ | no | 1,2,3 | 2 | 1 | 1 | F | Hyperpigmentation of tegument |
| Tenny 2011 [30] | 1 | 64 | RB | N/A | yes | 1,2,3 | 1,2 | 2 | 2 | F | Multiple distant recurrence at 6 months |
| Morkowski 2010 [31] | 3 | median 51 (range 39–62) | 2 LB, 1 RB | 2 UOQ 1 UIQ | no | 1,2,3 | 1,2 | 2 | 1 | F | No recurrence after excision |
| Vaidya 2020 [32] | 1 | 43 | LB | UOQ | no | 1,2,3 | 1,2 | 2 | 1 | F | N/A |
| Simmons 2016 [33] | 1 | 41 | RB | Multifocal | no | 1,2,3 | 1,2 | 2 | 1 | F | No recurrence at 9 months FU |
| Zhou 2016 [34] | 1 | 71 | LB | Multifocal | no | 1,2,3 | 1,2 | 2 | 1 | F | N/A |
| Green 1997 [35] | 7 | median 46 (range 15–84) | 4 RB, 2 LB 1 axillary | 2—bilateral | yes—1 no—6 | 1,2,3 | 1,2 | 6—2 1—1 | N/A | F | N/A |
| Cha 2012 [36] | 1 | 62 | RB | LOQ | no | 1,2,3 | 1,2 | 2 | 1 | F | No recurrence at 10 months FU |
| da Silva 2007 [37] | 1 | 50 | LB | UOQ | no | 1,2,3 | 1,2 | 2 | 1 | F | N/A |
| Ng 2000 [38] | 2 | median 50 (47–58) | RB | UOQ | no | 1,FNA 1,2 | 1,2 | 2 | 1 | F | No recureence at 1 and 6 months FU |
| Moyon 2020 [39] | 1 | 29 | N/A | N/A | no | 1,2,3 | 1,2 | 1 | N/A | F | N/A |
| Bansal 2010 [40] | 1 | 35 | RB | LOQ | no | 1, FNA | 1,2 | 1 | 1 | M | No recurrence at 18 Mo FU |
| Fu 2012 [41] | 1 | 78 | RB | UOQ | no | PET/CT scan | 1,2 | 2 | N/A | F | N/A |
| Mantilla 2016 [42] | 2 | 63 | LB | 1 multicentric | no | 1,2,3 | 1,2 | 1 | 1 | F | At 3 years FU—subcutaneous soft mass |
| Krbanevic 2021 [43] | 1 | 50 | Bilateral | N/A | no | 1,3 | 1 | 1 | N/A | F | N/A |
| Wu YC 2010 [44] | 1 | 33 | RB | UOQ | no | 1,3 | 2 | 2,3 | 1 | F | No recurrence at 2 years FU |
| Mac-Moune Lai 1994 [45] | 1 | 34 | LB | LIQ | no | 1,2 | 2 | 2 | 1 | M | No recurrence at 3 months FU |
| Wang 1997 [46] | 1 | 35 | LB | N/A | no | 1,2,3 | 2 | 2 | 2 | F | Recurrent breast tumor |
| Gwin 2011 [47] | 1 | 68 | Bilateral | N/A | no | 1,2,3 | 1,2 | 1 | N/A | F | N/A |
| Noordzij 2011 [48] | 1 | 75 | RB | Multifocal | no | 1,3 | 1,2 | 1 | N/A | F | Article in Dutch |
| Pham 2005 [49] | 1 | 53 | LB | LIQ | no | 1,2,3 | 1,2 | 1 | N/A | F | N/A |
| Elshikh 2020 [50] | 3 | 60 | LB | N/A | no | 1,2 | 1,2 | 1 | N/A | F | N/A |
| Hammond 1996 [51] | 1 | 67 | RB | UOQ | no | 2,3 | 1 | 2 | 1 | F | No recurrence at 6 Mo FU |
| Kuzmiak 2003 [52] | 1 | 30 | RB | UOQ, Multifocal | no | 1,2,3 | 1,2 | 2 | N/A | F | N/A |
| Baladandapani 2012 [53] | 1 | 59 | LB | Multifocal, UQ | no | 1,2,3 | 1,2 | 2 | N/A | M | N/A |
| Hummel 1999 [54] | 1 | 52 | LB | UIQ | no | 1 | 1,2 | 2 | 1 | F | No recurrence at 11 Mo FU |
| Dahlgren 2008 [55] | 1 | 64 | Bilateral | Bilateral | no | 1,2,3 | 1,2, | 2 | N/A | F | N/A |
| Dias Perera 2007 [56] | 1 | 23 | LB | N/A | no | 1,2 | 2 | 2 | N/A | M | N/A |
| Picon-Coronel 2010 [57] | 1 | 67 | N/A | N/A | no | 1,2 | 1,2 | 2 | N/A | F | Aticle in spanish |
| Perez-Guillermo 1993 [58] | 1 | 71 | Bilateral | UIQ left, LIQ right | no | 1,2 | 1,2 | 2 | N/A | F | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, G.; Gica, N.; Mustata, L.M.; Panaitescu, A.M.; Vasile, D.; Peltecu, G. Rosai–Dorfman Disease: Breast Involvement—Case Report and Literature Review. Medicina 2021, 57, 1167. https://doi.org/10.3390/medicina57111167
Iancu G, Gica N, Mustata LM, Panaitescu AM, Vasile D, Peltecu G. Rosai–Dorfman Disease: Breast Involvement—Case Report and Literature Review. Medicina. 2021; 57(11):1167. https://doi.org/10.3390/medicina57111167
Chicago/Turabian StyleIancu, George, Nicolae Gica, Laura Mihaela Mustata, Anca Maria Panaitescu, Danut Vasile, and Gheorghe Peltecu. 2021. "Rosai–Dorfman Disease: Breast Involvement—Case Report and Literature Review" Medicina 57, no. 11: 1167. https://doi.org/10.3390/medicina57111167







