Parameters of Oxidative and Inflammatory Status in a Three-Month Observation of Patients with Acute Myocardial Infarction Undergoing Coronary Angioplasty—A Preliminary Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Statistical Analyses
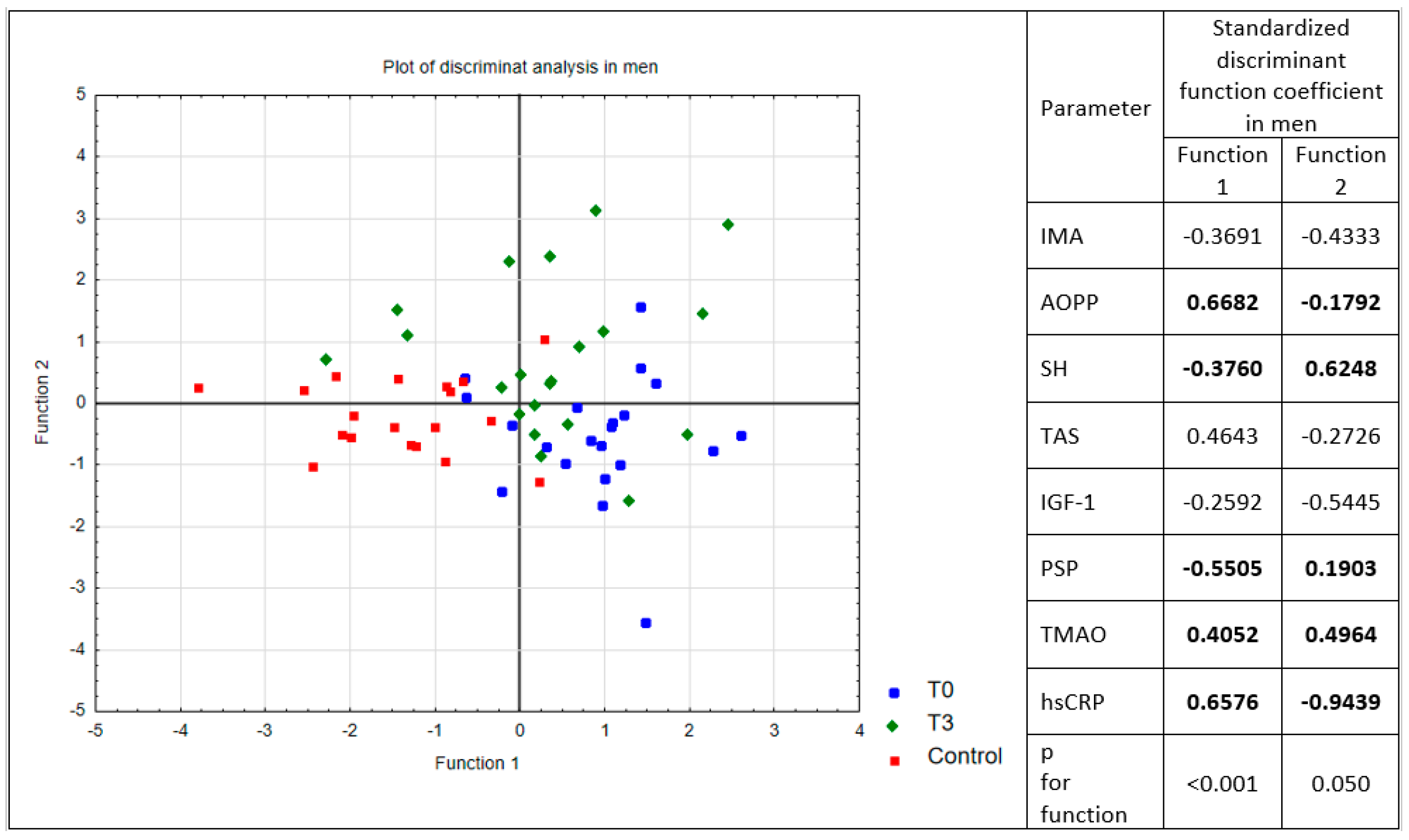
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization: Cardiovascular Diseases (CVDs), Version Current: 17 May 2017. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 5 May 2019).
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, G.K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Montazerifar, F.; Karajibani, M.; Gilani, S.M.; Bolouri, A.; Hashemi, M.; Dashipour, A. Study of prooxidant-antioxidant balance and some risk factors of coronary artery disease. Res. Cardiovasc. Med. 2018, 7, 69–73. [Google Scholar] [CrossRef]
- Kelly, F.; Fussell, J. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic. Biol. Med. 2017, 110, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Żurawska-Płaksej, E.; Grzebyk, E.; Marciniak, D.; Szymańska-Chabowska, A.; Piwowar, A. Oxidatively modified forms of albumin in patients with risk factors of metabolic syndrome. J. Endocrinol. Invest. 2014, 37, 819–827. [Google Scholar]
- Ridker, P. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Adukauskienė, D.; Čiginskienė, A.; Adukauskaitė, A.; Pentiokinienė, D.; Šlapikas, R.; Čeponienė, I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina (Kaunas) 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef]
- Caglar, F.N.T.; Isiksacan, N.; Biyik, I.; Opan, S.; Cebe, H.; Akturk, I.F. Presepsin (sCD14-ST): Could it be a novel marker for the diagnosis of ST elevation myocardial infarction? Arch. Med. Sci. Atheroscler. Dis. 2017, 2, e3–e8. [Google Scholar] [CrossRef]
- Popov, D.; Plyushch, M.; Ovseenko, S.; Abramyan, M.; Podshchekoldina, O.; Yaroustovsky, M. Prognostic value of sCD14-ST (presepsin) in cardiac surgery. Kardiochir. Torakochirurgia Pol. 2015, 12, 30–36. [Google Scholar] [PubMed]
- Wang, Z.; Tang, W.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Heaney, L.M.; Jones, D.J.; Ng, L.L. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin. Chem. 2017, 63, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.Y.; Delafontaine, P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinal. Metab. 2010, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Lyass, A.; Courchesne, P.; Chen, G.; Liu, C.; Yin, X.; Hwang, S.J.; Massaro, J.M.; Larson, M.G.; Levy, D. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 2018, 7, e008108. [Google Scholar] [CrossRef] [PubMed]
- Kobza, J.; Geremek, M. Explaining the decrease in deaths from cardiovascular disease in Poland. The top-down risk assessment approach, from policy to health impact. Postepy Hig. Med. Doswiadczalnej 2016, 70, 295–304. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers 2013, 35, 773–790. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Kardiol. Pol. 2015, 73, 1207–1294. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Kardiol. Pol. 2018, 76, 229–313. [Google Scholar] [CrossRef]
- Winther, J.R.; Thorpe, C. Quantification of thiols and disulfides. Biochim. Biophys. Acta 2014, 1840, 838–846. [Google Scholar] [CrossRef]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. Markers of oxidative protein damage in plasma and urine of type 2 diabetic patients. Br. J. Biomed. Sci. 2009, 66, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. Ischemia-modified albumin level in type 2 diabetes mellitus—Preliminary report. Dis. Markers 2008, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pinnell, A.E.; Northam, B.E. New automated dye-binding method for serum albumin determination with bromcresol purple. Clin. Chem. 1978, 24, 80–86. [Google Scholar] [PubMed]
- Means, G.; End, C.; Kaul, P. Management of percutaneous coronary intervention complications. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Kumar, P.G.; Swami, A.; Dinker, Y. Evaluation of changes in perfusion defect and left ventricular systolic function using Tc-99m tetrofosmin single photon emission computed tomography over 3 month period in patients of acute myocardial infarction undergoing primary angioplasty. Nucl. Med. Rev. Cent. East Eur. 2018, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in cardiovascular aging. Biomed. Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative stress in kidney diseases: The cause or the consequence? Arch. Immunol. Ther. Exp. (Warsz) 2018, 66, 211–220. [Google Scholar] [CrossRef]
- Knapik-Kordecka, M.; Piwowar, A.; Warwas, M. Disturbances of oxidative-antioxidative balance and atherosclerotic risk factors and vascular complications in patients with diabetes type 2. Wiad. Lek. 2007, 60, 329–334. (In Polish) [Google Scholar]
- Skvarilova, M.; Bulava, A.; Stejskal, D.; Adamovska, S.; Bartek, J. Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomed. Pap. 2005, 149, 83–87. [Google Scholar] [CrossRef]
- Knapik-Kordecka, M.; Piwowar, A.; Żurawska-Płaksej, E.; Warwas, M. Ischemia modified albumin—Specific marker in cardiological diagnostics? Wiad. Lek. 2008, 61, 263–268. [Google Scholar] [PubMed]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, A. Targeting the gut to protect the heart. Nat. Rev. Cardiol. 2018, 15, 581. [Google Scholar] [CrossRef]
- Parenica, J.; Jarkovsky, J.; Malaska, J.; Mebazaa, A.; Gottwaldova, J.; Helanova, K.; Litzman, J.; Dastych, M.; Tomandl, J.; Spinar, J.; et al. Infectious complications and immune/inflammatory response in cardiogenic shock patients: A prospective observational study. Shock 2017, 47, 165–174. [Google Scholar] [CrossRef]
- Saito, J.; Hashiba, E.; Mikami, A.; Kudo, T.; Niwa, H.; Hirota, K. Pilot study of changes in presepsin concentrations compared with changes in procalcitonin and C-reactive protein concentration after cardiovascular surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1262–1267. [Google Scholar] [CrossRef]
- Handke, J.; Scholz, A.S.; Gillmann, H.J.; Janssen, H.; Dehne, S.; Arens, C.; Kummer, L.; Uhle, F.; Weigand, M.A.; Motsch, J.; et al. Elevated presepsin is associated with perioperative major adverse coardiovascular and cerebrovascular complications in elevated-risk patients undergoing noncardiac surgery. Anesth. Analg. 2018, 128, 1344–1353. [Google Scholar] [CrossRef]
- Biyik, I.; Caglar, F.N.T.; Isiksacan, N.; Kocamaz, N.; Kasapoglu, P.; Gedikbasi, A.; Akturk, F. Serum presepsin levels are not elevated in patients with controlled hypertension. Int. J. Hypertens. 2018, 8, 8954718. [Google Scholar] [CrossRef]
- Ezzat, V.A.; Duncan, E.R.; Wheatcroft, S.B.; Kearney, M.T. The role of IGF-I and its binding proteins in the development of type 2 diabetes and cardiovascular disease. Diabetes Obes. Metab. 2008, 10, 198–211. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Komamura, K.; Choraku, M.; Hirono, A.; Takamori, N.; Tamura, K.; Akaike, M.; Azuma, H. Impact of serum insulin-like growth factor-1 on early prognosis in acute myocardial infarction. Intern. Med. 2008, 47, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Westermeier, F.; Bustamante, M.; Pavez, M.; García, L.; Chiong, M.; Ocaranza, M.P.; Lavandero, S. Novel players in cardioprotection: Insulin like growth factor-1, angiotensin-(1-7) and angiotensin-(1-9). Pharmacol. Res. 2015, 101, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ukinc, K.; Eminagaoglu, S.; Ersoz, H.O.; Erem, C.; Karahan, C.; Hacihasanoglu, A.B.; Kocak, M. A novel indicator of widespread endothelial damage and ischemia in diabetic patients: Ischemia-modified albumin. Endocrine 2009, 36, 425–432. [Google Scholar] [CrossRef] [PubMed]
| Parameter (Unit) | Patients (n = 30) | Controls (n = 30) | Statistical Significance of Differences [p-Value] |
|---|---|---|---|
| Age (years) | 63 (54–75) | 59.5 (56–63) | 0.065 |
| Male sex (n) | 21 | 19 | 0.58 |
| BMI (kg/m2) | 27.1 (24.4–31.8) | 25.05 (22.7–28.1) | 0.057 |
| SBP (mmHg) | 120 (117–130) | 130 (120–138) | 0.104 |
| DBP (mmHg) | 72 (70–80) | 82 (75–87) | 0.051 |
| WBC (109/L) | 8.68 (7.63–11.06) | 5.65 (4.70–6.20) | <0.001 |
| hsCRP (mg/L) | 3.65 (2.26–9.90) | 1.54 (0.84–2.96) | 0.001 |
| Glucose (mmol/L) | 6.0 (5.4–7.0) | 5.2 (5.0–5.5) | <0.001 |
| Cholesterol (mmol/L) | 5.4 (4.3–6.3) | 5.9 (5.2–6.6) | 0.075 |
| TG (mmol/L) | 1.51 (0.90–2.27) | 1.11 (0.80–1.46) | 0.025 |
| HDL (mmol/L) | 1.16 (0.98–1.34) | 1.47 (1.21–1.65) | 0.002 |
| LDL (mmol/L) | 3.38 (2.42–4.16) | 3.49 (2.92–4.21) | 0.53 |
| Creatinine (µmol/L) | 76.1 (62.8–92.1) | 74.3 (64.6–90.2) | 0.65 |
| Troponin I (µg/L | 7.1 (2.37–28.24) | NA | |
| LV mass (g) | 315.5 (261–417) | NA | |
| EF (%) | 60 (35–65) | NA | |
| Presence of DM (n) | 6 | 0 | 0.031 |
| Smoking habit (n) | 13 | 9 | 0.423 |
| Parameter (Unit) | Patients | Controls | Statistical Significance of Differences [p-Value] | |||
|---|---|---|---|---|---|---|
| T0 | T3 | C | T0 vs. T3 | T0 vs. C | T3 vs. C | |
| IMA (ABSU/g) | 40.5 (34.5–51.8) | 45.8 (39.8–57.3) | 41.1 (38.6–49.1) | 0.33 | 1.00 | 0.94 |
| AOPP (μmol/L) | 226 (106–389) | 274 (180–391) | 147 (130–183) | 0.44 | 0.18 | 0.002 |
| SH groups (mmol/L) | 0.41 (0.35–0.43) | 0.41 (0.23–0.46) | 0.52 (0.45–0.57) | 1.00 | <0.001 | <0.001 |
| TAS (μmol/L) | 17.2 (15.6–17.9) | 16.6 (15.7–17.5) | 16.3 (15.8–16.9) | 1.00 | 0.11 | 0.54 |
| TMAO (μmol/L) | 1.16 (0.92–1.24) | 1.22 (0.78–1.87) | 1.00 (0.84–2.96) | 0.93 | 0.38 | 0.033 |
| IGF-1 (ng/mL) | 129 (84–176) | 148 (103–195) | 184 (145–235) | 1.00 | 0.030 | 0.24 |
| Insulin (μU/mL) | 13.4 (10.6–29.8) | 16.0 (8.5–32.2) | 10.0 (7.2–13.2) | 1.00 | 0.044 | 0.026 |
| hsCRP (mg/L) | 3.65 (2.26–9.90) | 1.31 (0.54–3.57) | 1.54 (0.84–2.96) | <0.001 | 0.005 | 1.00 |
| PSP (mg/L) | 2.39 (2.25–2.83) | 2.55 (2.20–2.79) | 2.89 (2.67–3.44) | 1.00 | 0.008 | 0.020 |
| Parameter (Unit) | T0 | T3 | EF < 50% | EF ≥ 50% | ||||
|---|---|---|---|---|---|---|---|---|
| EF < 50% | EF ≥ 50% | p | EF < 50% | EF ≥ 50% | p | T0 vs. T3 | T0 vs. T3 | |
| n = 10 | n = 20 | n = 10 | n = 20 | n = 10 | n = 20 | |||
| IMA (ABSU/g) | 52.0 (42.5–80.3) | 37.6 (32.0–46.6) | 0.007 | 57.3 (42.5–80.3) | 46.3 (40.0–51.9) | 0.344 | 0.799 | 0.030 |
| AOPP (μmol/L) | 105.3 (78.9–125.6) | 286.6 (181.1–400.5) | 0.015 | 283.2 (141.1–315.1) | 266.6 (181.4–397.0) | 0.982 | 0.017 | 0.708 |
| SH groups (mmol/L) | 0.41 (0.34–0.45) | 0.43 (037–0.46) | 0.552 | 0.42 (0.20–0.50) | 0.39 (0.26–0.46) | 0.982 | 0.508 | 0.433 |
| TAS (μmol/L) | 17.5 (17.0–17.8) | 16.3 (15.3–18.1) | 0.379 | 17.1 (16.4–17.8) | 16.5 (15.3–17.9) | 0.135 | 0.386 | 0.550 |
| TMAO (μmol/L) | 1.13 (0.92–1.38) | 1.22 (0.81–1.27) | 0.758 | 1.74 (1.17–3.10) | 1.03 (0.61–1.71) | 0.031 | 0.093 | 0.970 |
| IGF-1 (ng/mL) | 107.4 (55.9–151.2) | 154.2 (95.2–261.0) | 0.056 | 117.4 (77.6–180.1) | 153.9 (132.2–213.3) | 0.209 | 0.047 | 0.526 |
| Insulin (μU/mL) | 13.4 (11.2–18.3) | 13.9 (10.5–36.4) | 0.644 | 18.7 (8.5–29.8) | 18.0 (9.8–35.1) | 0.947 | 0.169 | 0.970 |
| hsCRP (mg/L) | 11.00 (2.90–29.00) | 3.17 (1.20–7.55) | 0.045 | 1.68 (0.59–2.19) | 1.18 (0.52–3.63) 2.38 (2.30–2.79) | 0.741 | 0.012 | 0.015 |
| PSP (mg/L) | 2.41 (1.89–3.43) | 2.38 (2.30–2.79) | 0.982 | 2.21 (1.95–2.54) | 2.59 (2.15–2.77) | 0.159 | 0.284 | 0.794 |
| Variables | r and p-Values |
|---|---|
| T0 | |
| IMA vs. IGF-1 | r = −0.376, p = 0.041 |
| IMA vs. hsCRP | r = 0.466, p = 0.009 |
| TAS vs. AOPP | r = −0.373, p = 0.042 |
| TAS vs. SH groups | r = 0.409, p = 0.025 |
| TAS vs. IGF | r = −0.375, p = 0.041 |
| TMAO vs. hsCRP | r = 0.371, p = 0.044 |
| TMAO vs. PSP | r = 0.381, p = 0.038 |
| TMAO vs. insulin | r = 0.374, p = 0.042 |
| T3 | |
| TMAO vs. glucose | r = 0.374, p = 0.042 |
| C | |
| PSP vs. hsCRP | r = 0.356, p = 0.039 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurawska-Płaksej, E.; Płaczkowska, S.; Pawlik-Sobecka, L.; Czapor-Irzabek, H.; Stachurska, A.; Mysiak, A.; Sebzda, T.; Gburek, J.; Piwowar, A. Parameters of Oxidative and Inflammatory Status in a Three-Month Observation of Patients with Acute Myocardial Infarction Undergoing Coronary Angioplasty—A Preliminary Study. Medicina 2019, 55, 585. https://doi.org/10.3390/medicina55090585
Żurawska-Płaksej E, Płaczkowska S, Pawlik-Sobecka L, Czapor-Irzabek H, Stachurska A, Mysiak A, Sebzda T, Gburek J, Piwowar A. Parameters of Oxidative and Inflammatory Status in a Three-Month Observation of Patients with Acute Myocardial Infarction Undergoing Coronary Angioplasty—A Preliminary Study. Medicina. 2019; 55(9):585. https://doi.org/10.3390/medicina55090585
Chicago/Turabian StyleŻurawska-Płaksej, Ewa, Sylwia Płaczkowska, Lilla Pawlik-Sobecka, Hanna Czapor-Irzabek, Aneta Stachurska, Andrzej Mysiak, Tadeusz Sebzda, Jakub Gburek, and Agnieszka Piwowar. 2019. "Parameters of Oxidative and Inflammatory Status in a Three-Month Observation of Patients with Acute Myocardial Infarction Undergoing Coronary Angioplasty—A Preliminary Study" Medicina 55, no. 9: 585. https://doi.org/10.3390/medicina55090585
APA StyleŻurawska-Płaksej, E., Płaczkowska, S., Pawlik-Sobecka, L., Czapor-Irzabek, H., Stachurska, A., Mysiak, A., Sebzda, T., Gburek, J., & Piwowar, A. (2019). Parameters of Oxidative and Inflammatory Status in a Three-Month Observation of Patients with Acute Myocardial Infarction Undergoing Coronary Angioplasty—A Preliminary Study. Medicina, 55(9), 585. https://doi.org/10.3390/medicina55090585





