Nanostructured Thin Films Obtained from Fischer Aminocarbene Complexes
Abstract
:1. Introduction
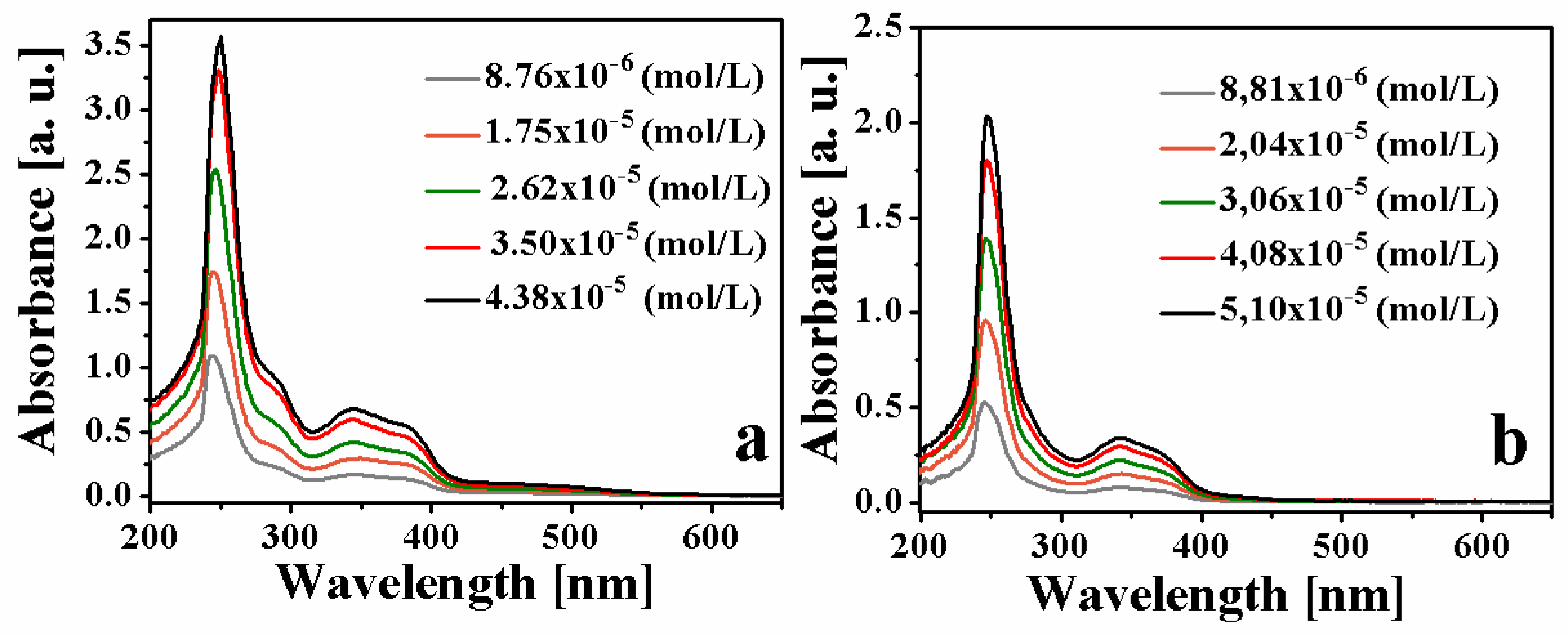
2. Results and Discussion
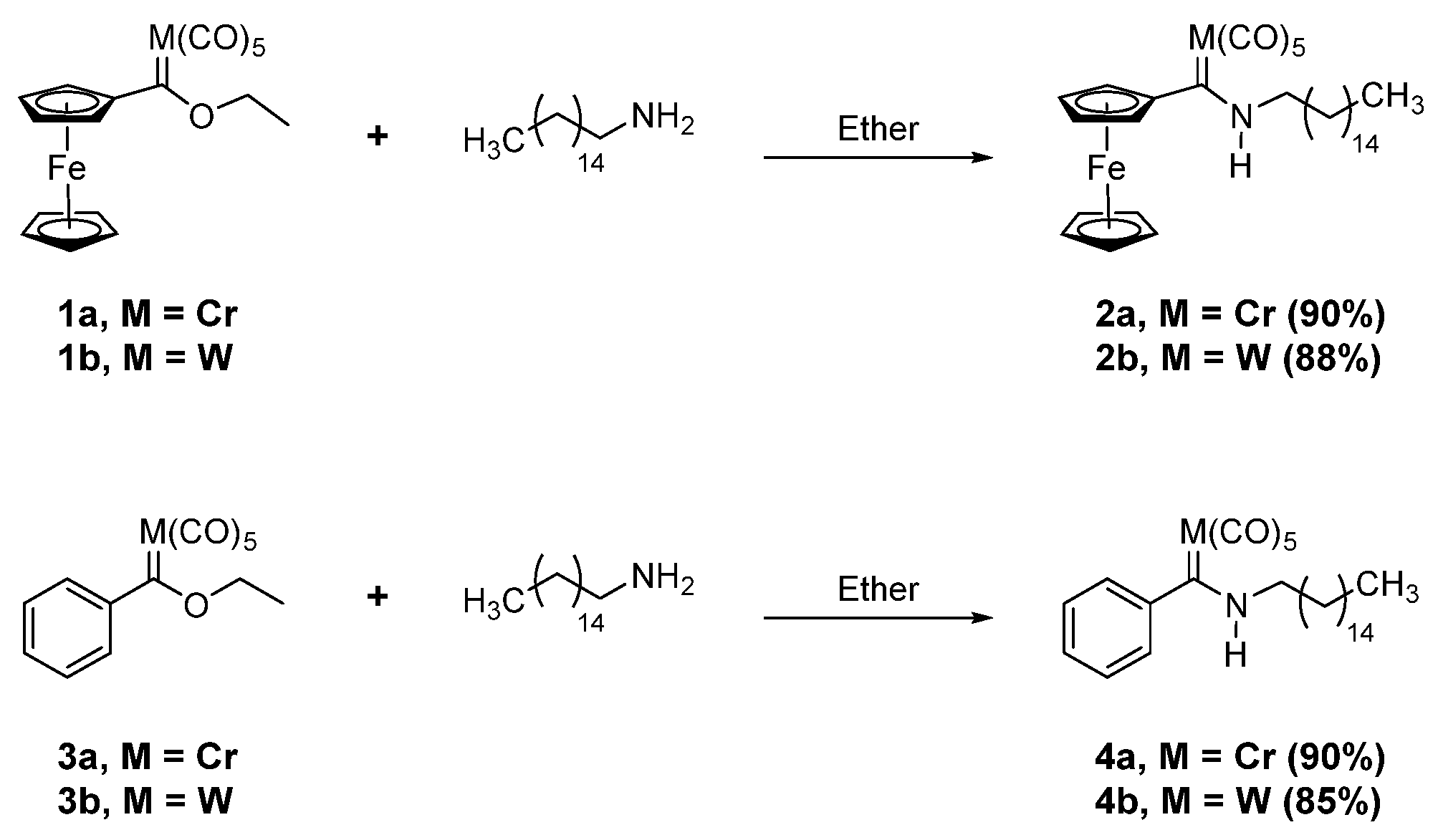
2.1. Synthesis of Organometallic Precursors
2.2. Langmuir Monolayer Surface
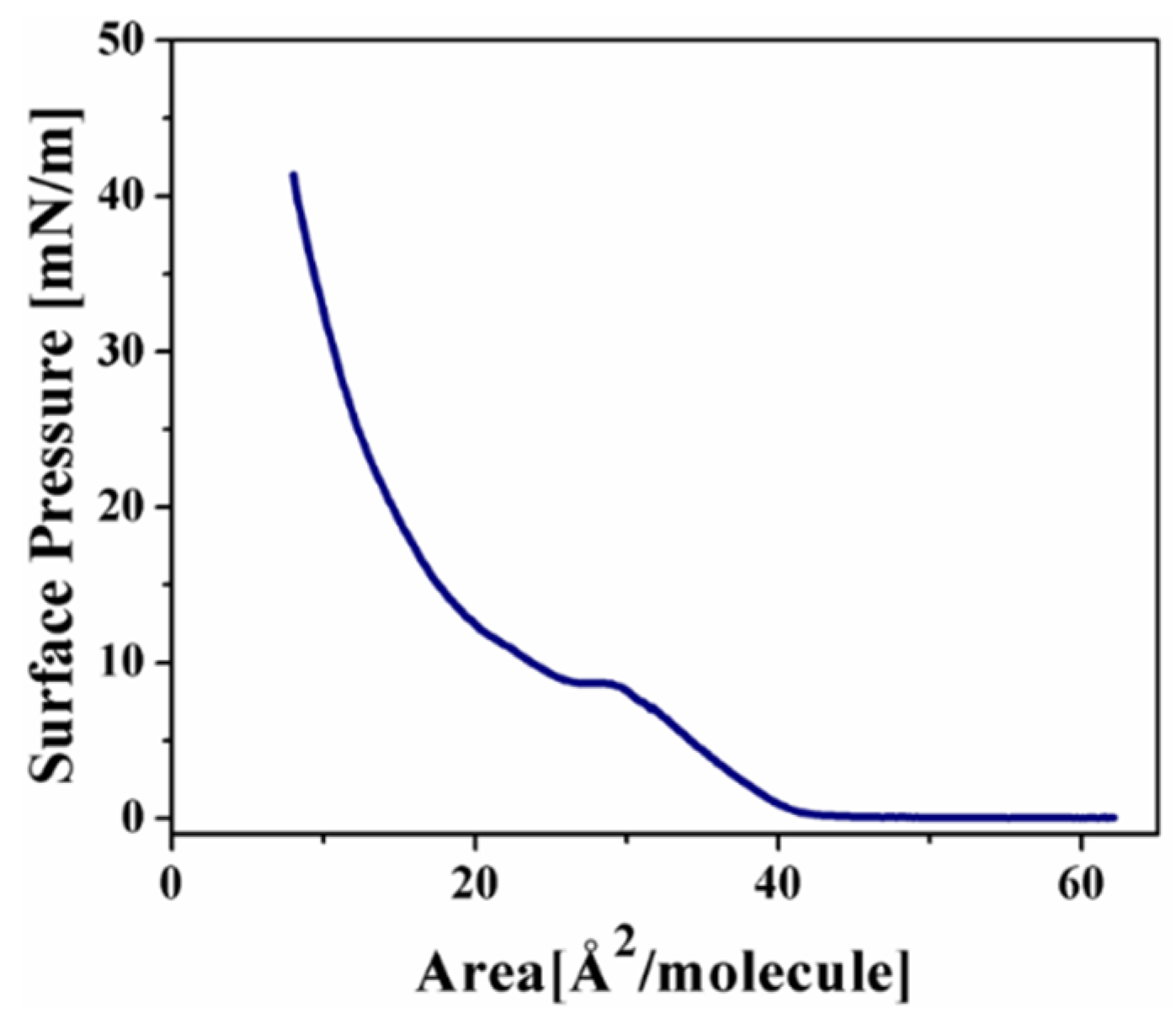
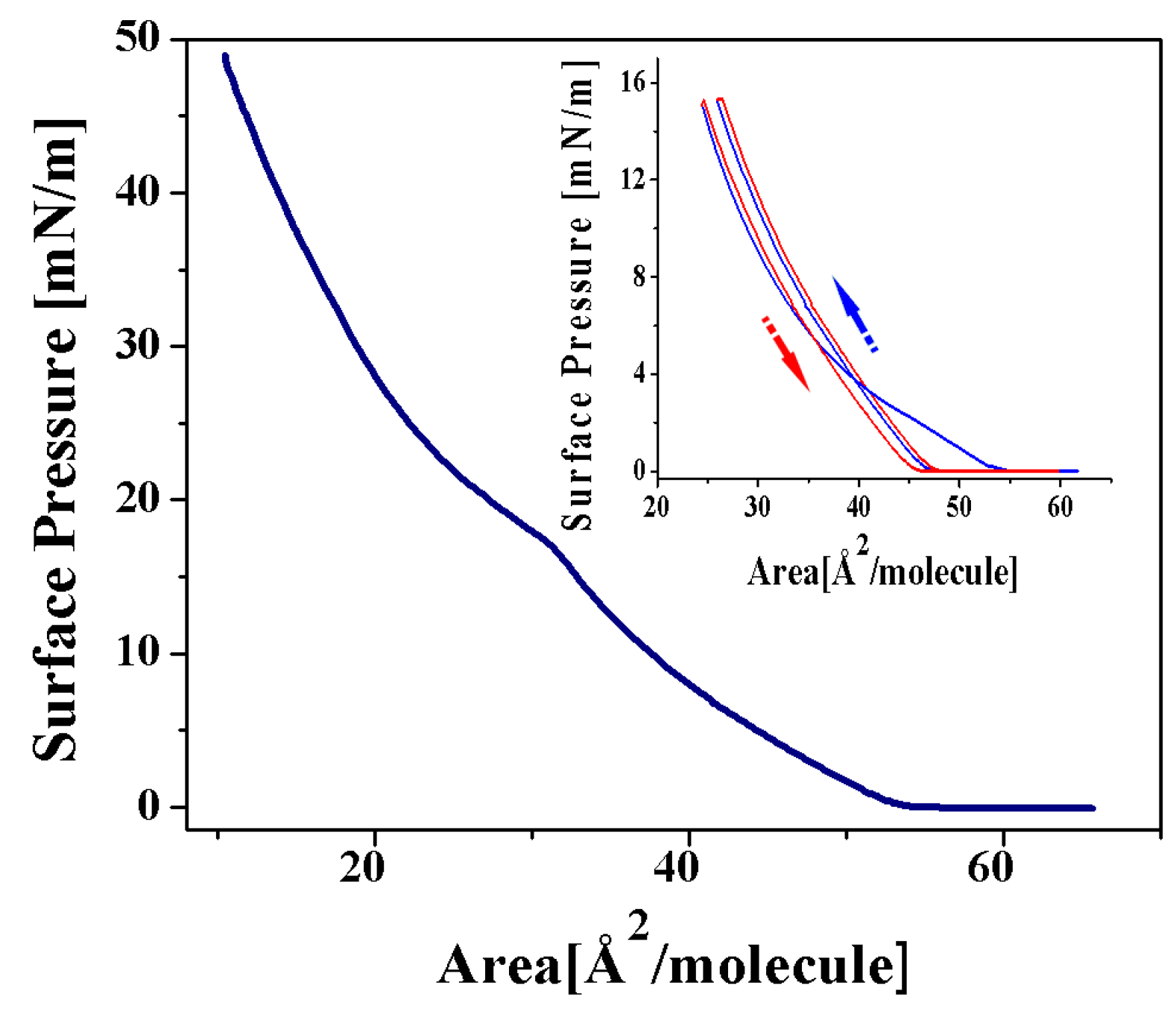
2.2.1. Langmuir Monolayers of 2a and 4a Complexes, with Chromium as Transition Metal
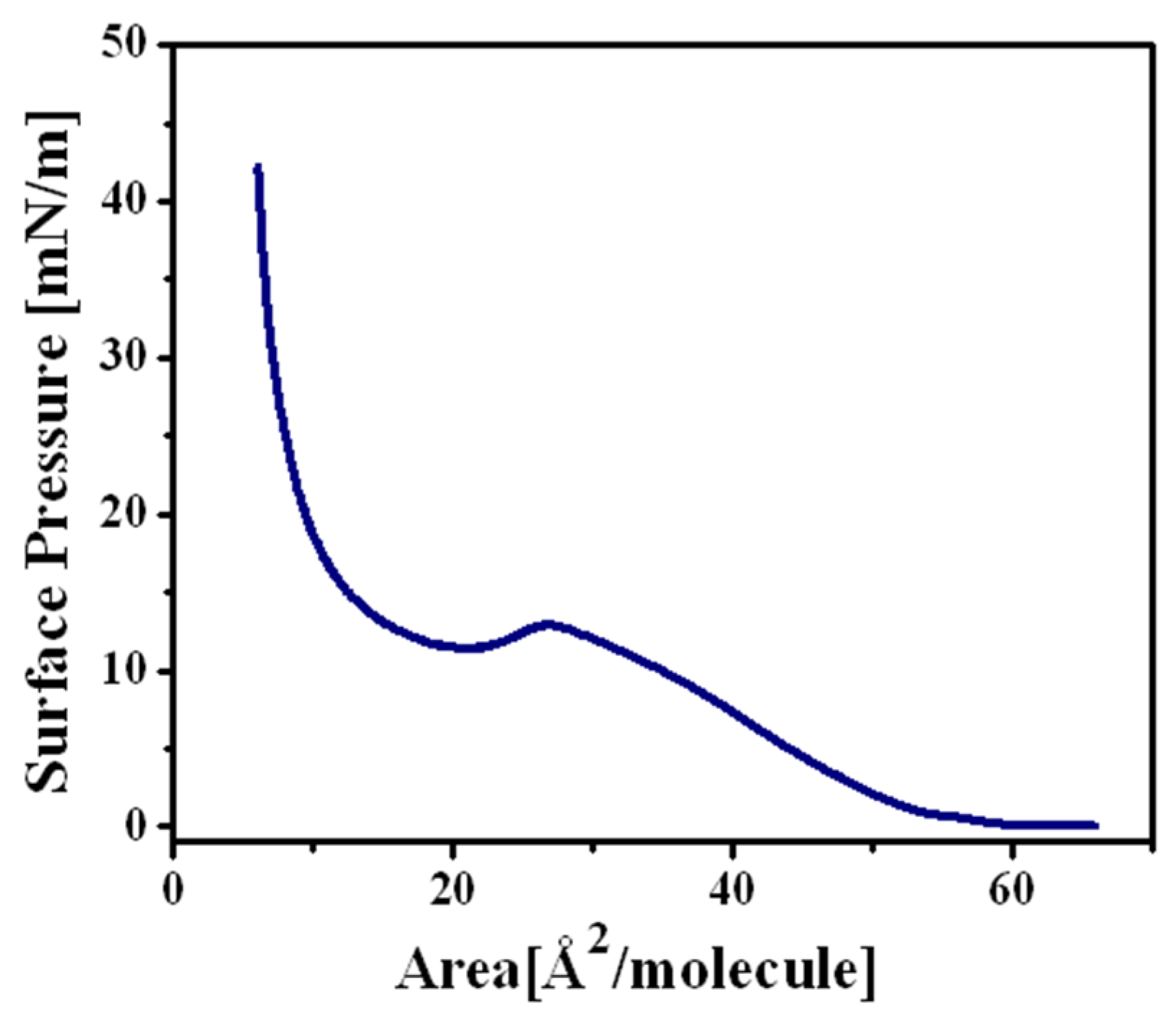
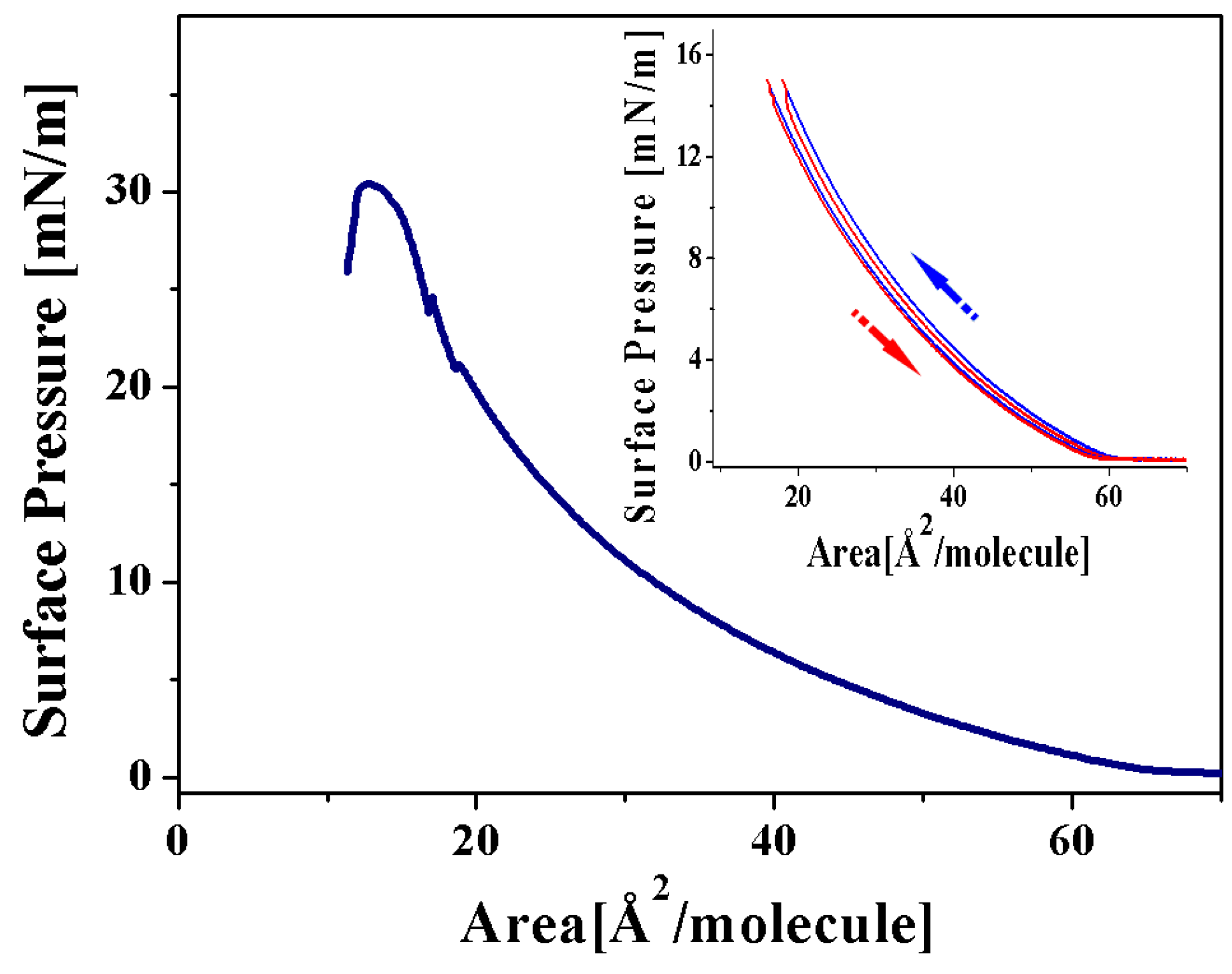
Langmuir Isotherm for the 2a Complex
Langmuir Isotherm for the 4a Complex
2.2.2. Langmuir Monolayers of Aminocarbene 2b and 4b Complexes, with Tungsten as Transition Metal
Langmuir Isotherm for the 2b Complex
Langmuir Isotherm for the 4b Complex
2.3. Langmuir–Blodgett films
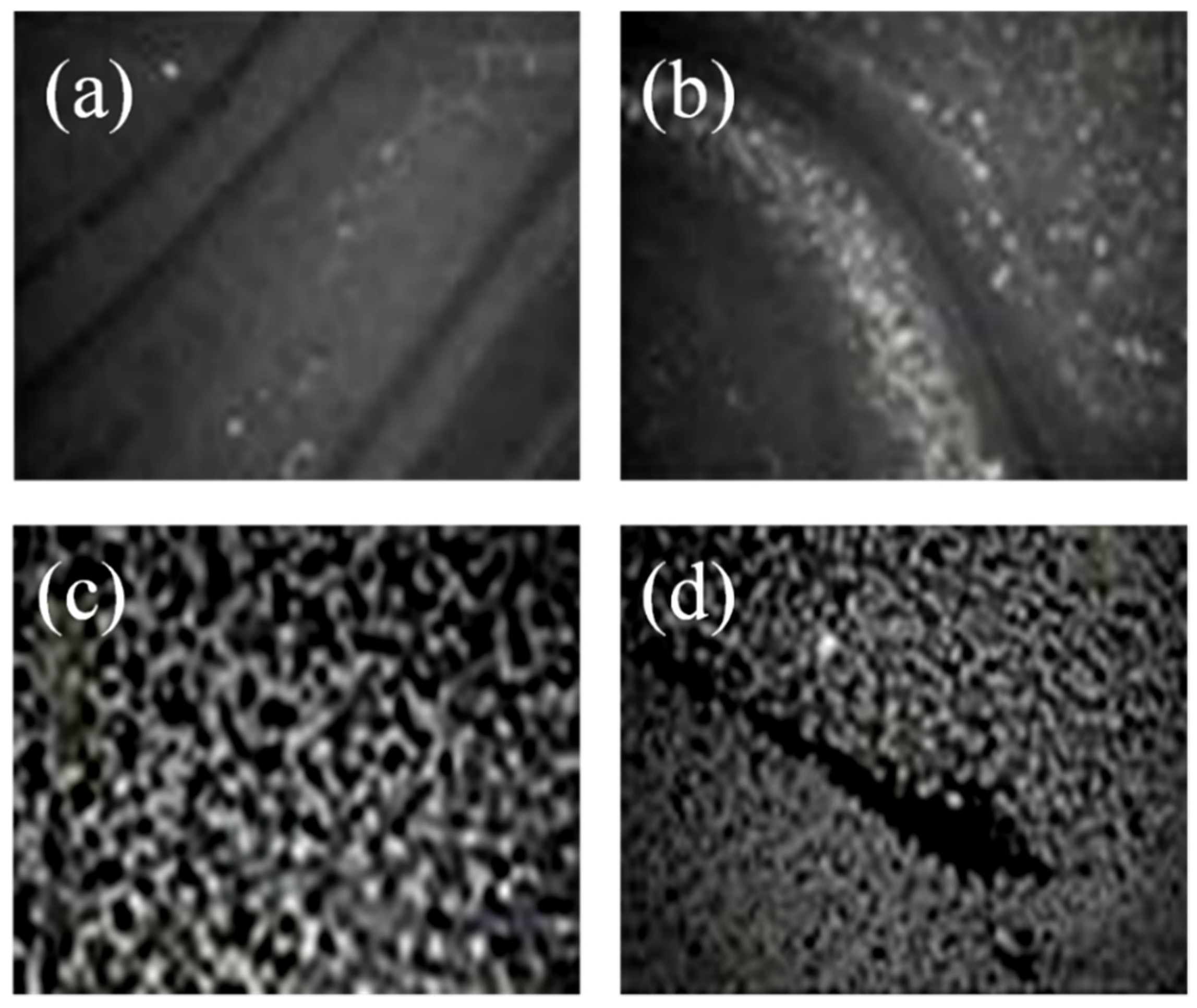
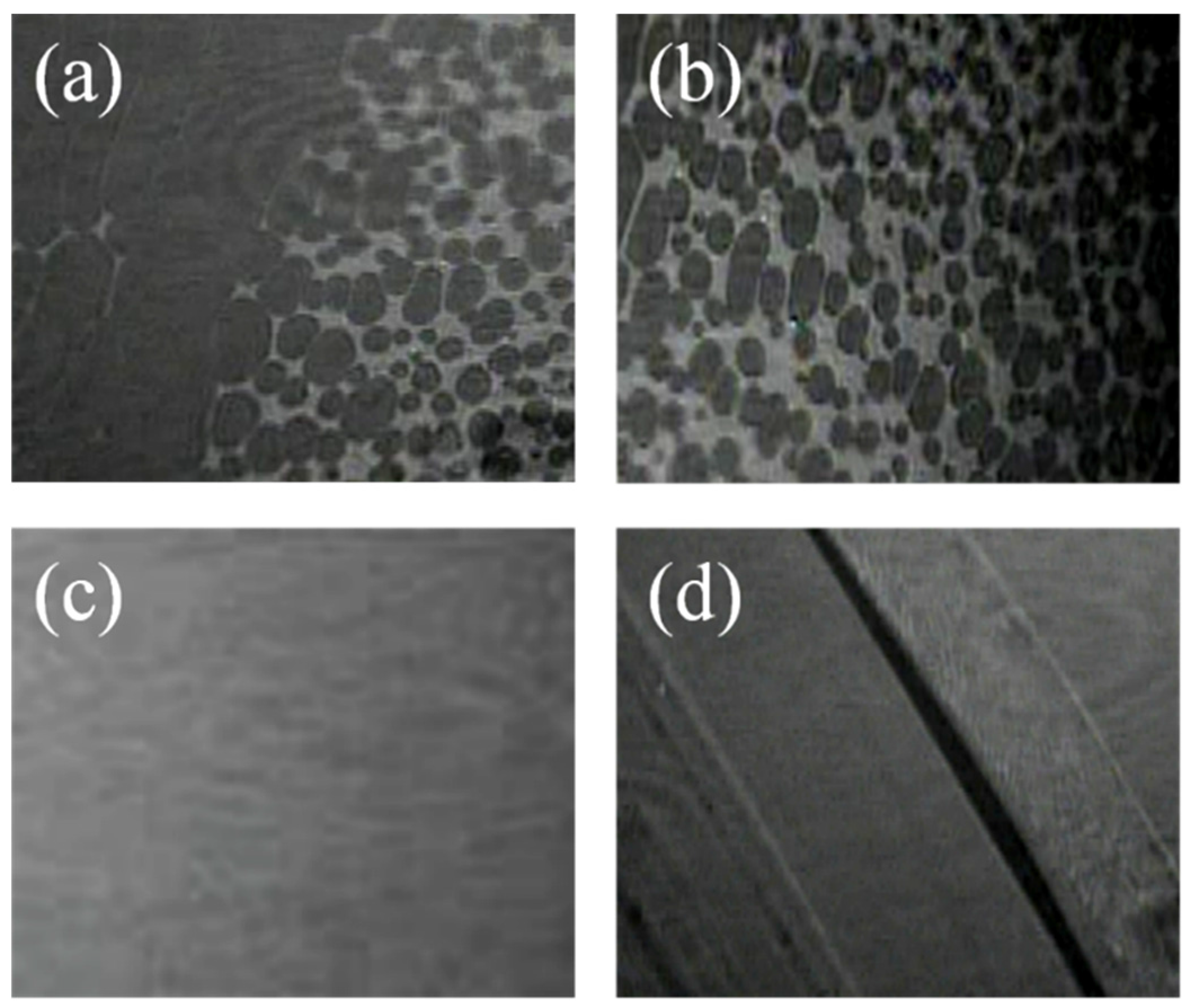
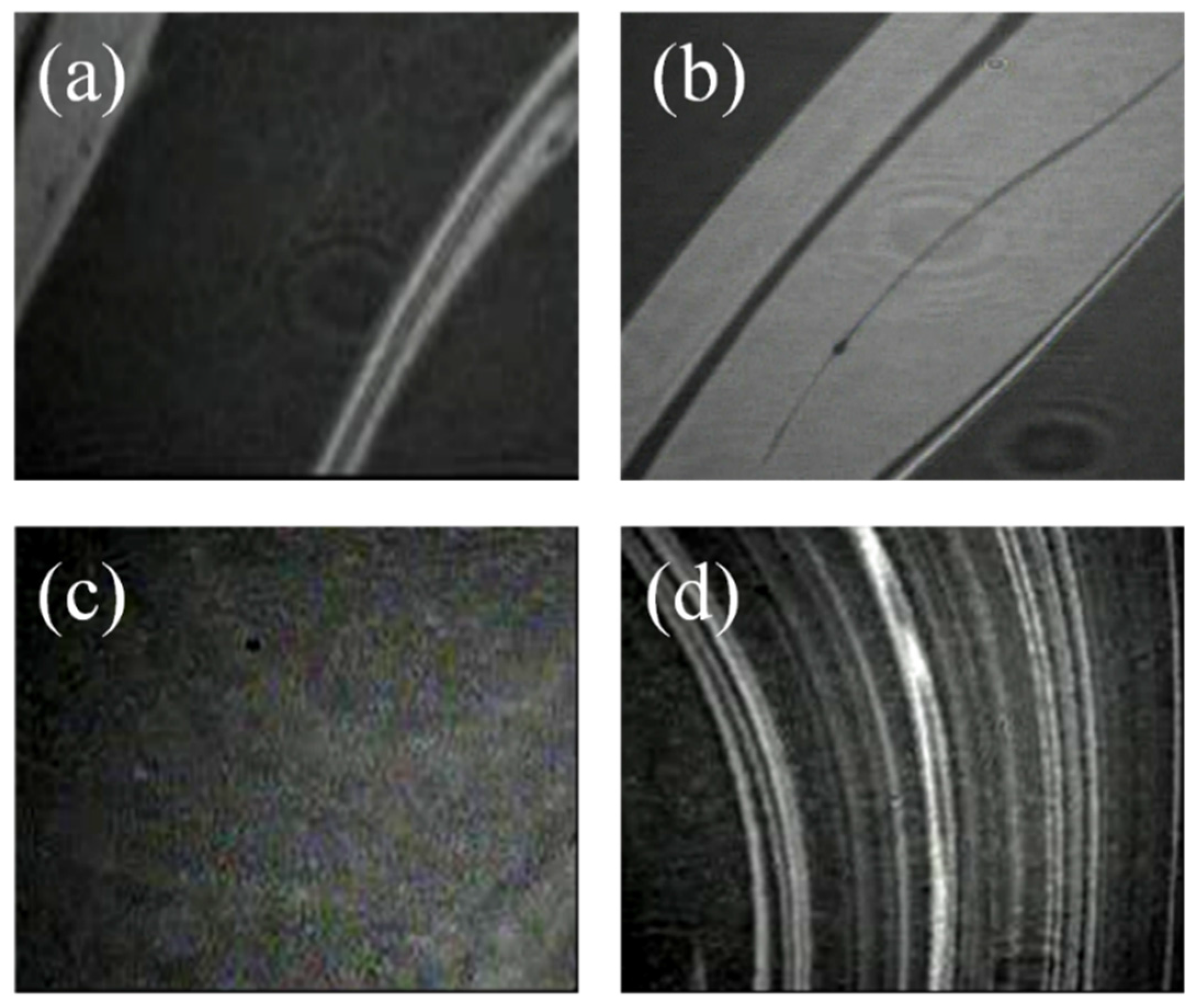
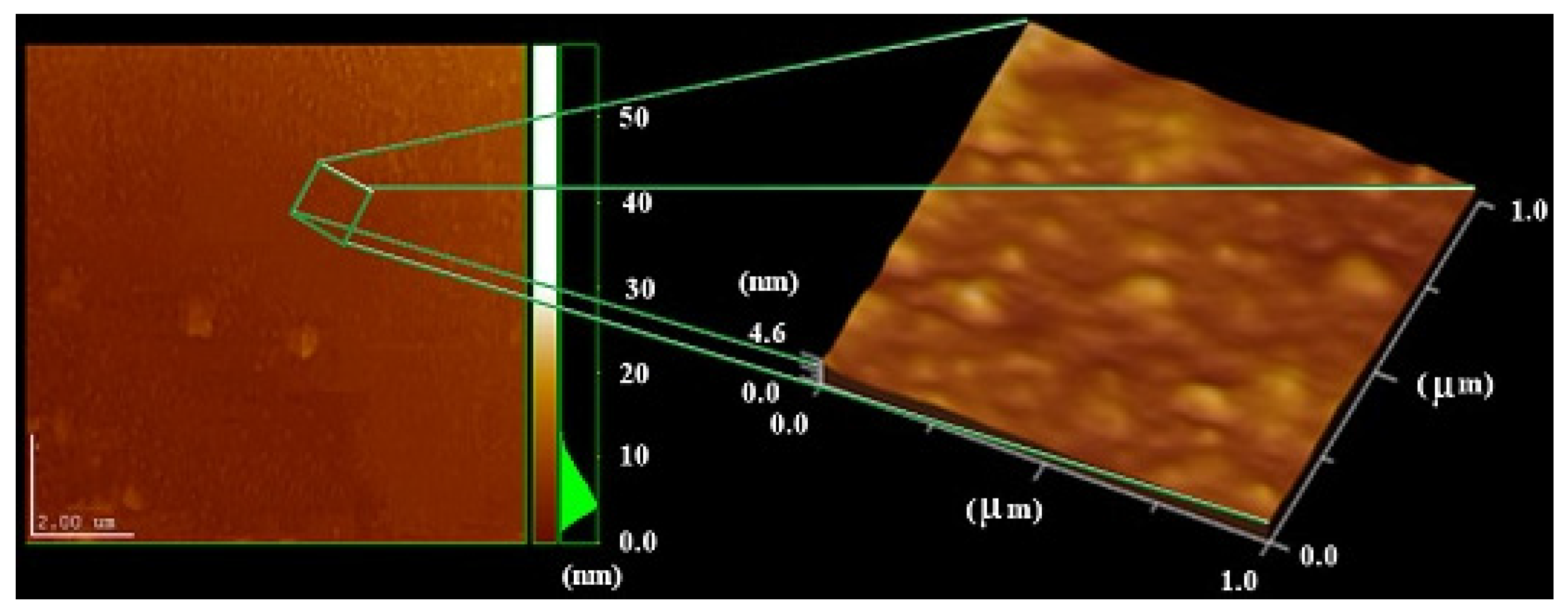
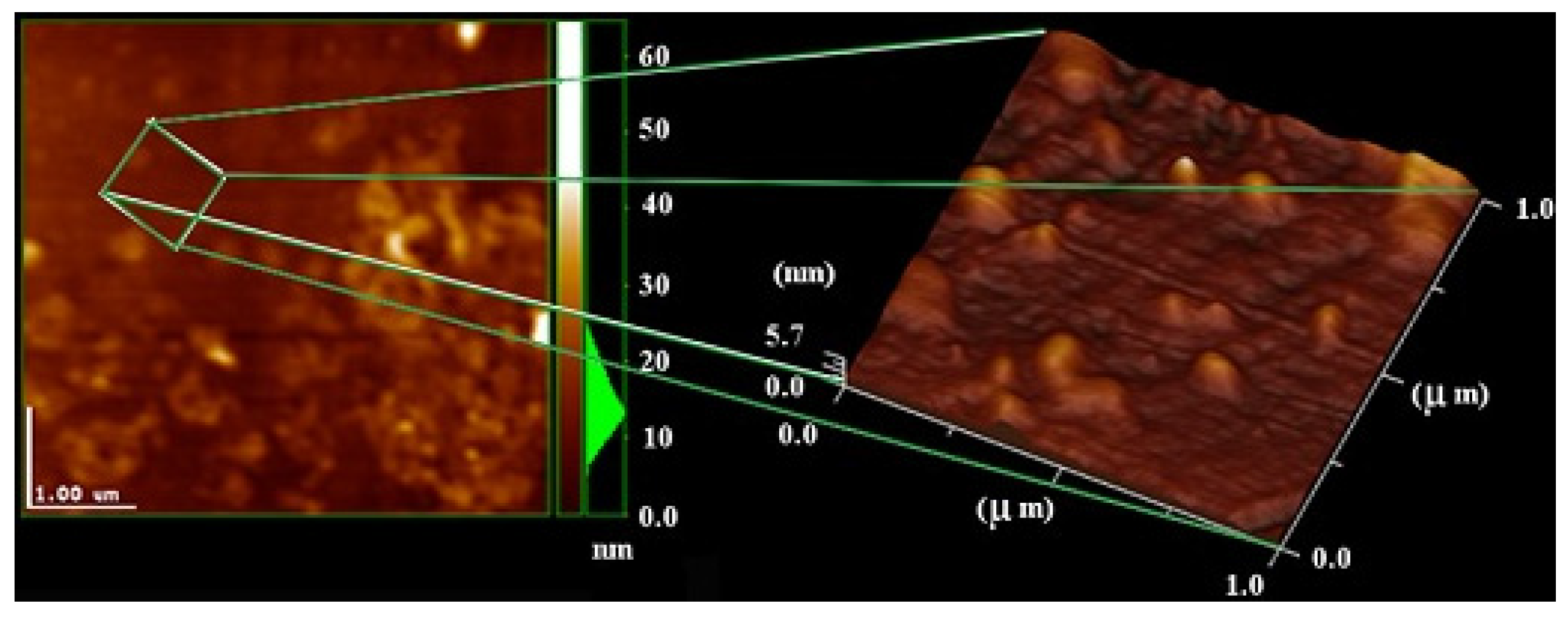
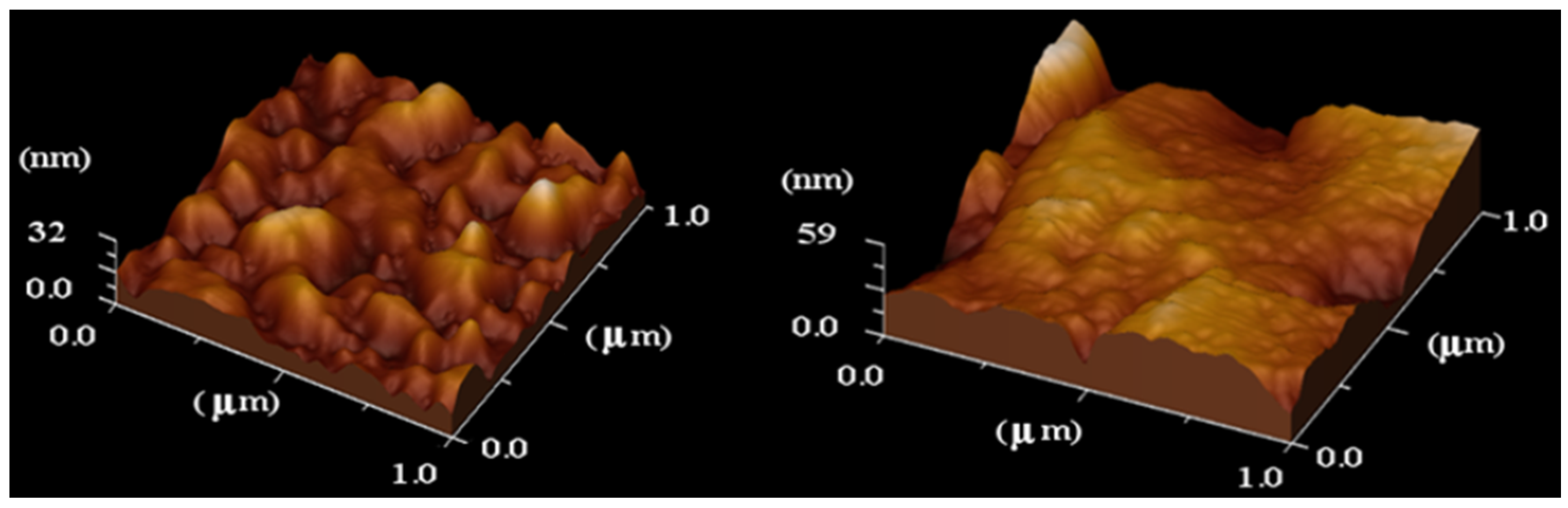
2.4 Atomic Force Microscopy (AFM)
2.5 Small-Angle X-ray Scattering (SAXS)
3. Experimental Section
3.1. Materials
3.2. Synthesis of Organometallic Precursors
3.2.1. Preparation of Fischer Ferrocenyl Hexadecylamino 2(a-b) Complexes
3.2.2. Preparation of Fischer Hexadecylamino Phenyl Carbene 4(a-b) Complexes
3.3. Preparation of Langmuir Films
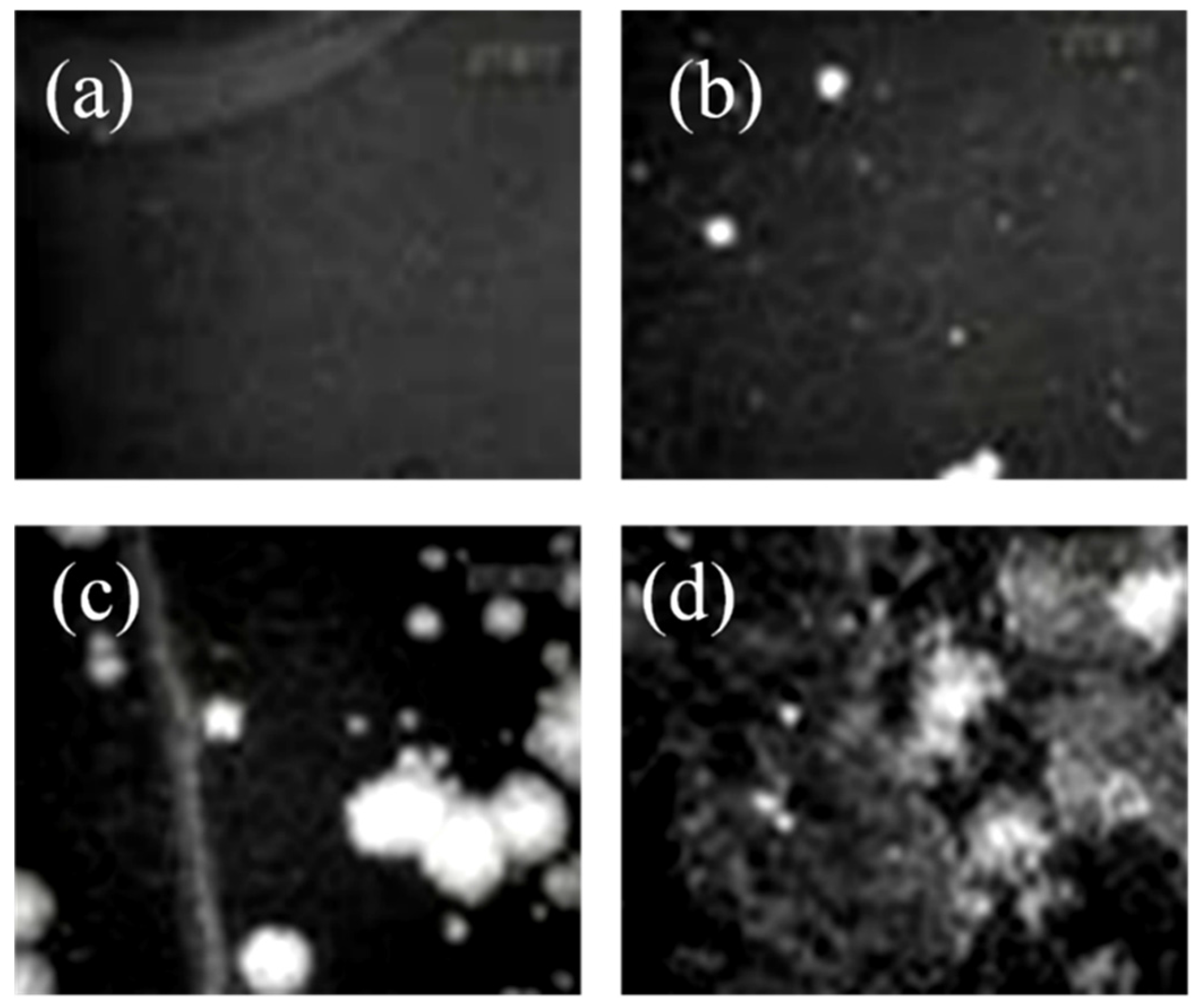
Characterization of Langmuir Films through BAM
3.4. Preparation of LB Films
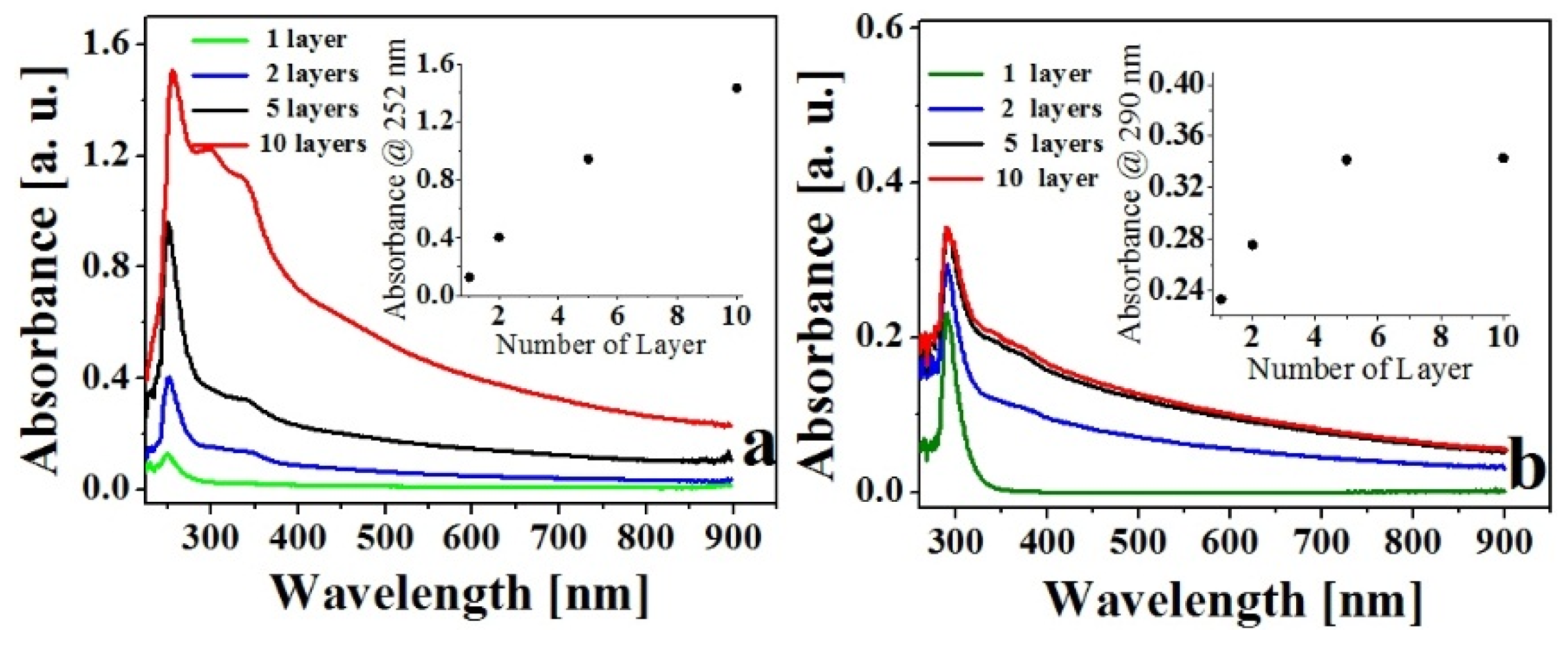
Characterization of LB Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hussain, S.A.; Bhattacharjee, D. Langmuir-Blodgett films and molecular electronics. Mod. Phys. Lett. B 2009, 23, 3437–3451. [Google Scholar] [CrossRef]
- Rigaut, S.; Massue, J.; Touchard, D.; Fillaut, J.-L.; Golhen, S.; Dixneuf, P.H. Unprecedented coupling of allenydene and diynyl metal complexes: A bimetallic ruthenium system with a C7 conjugated bridge. Angew. Chem. Int. Ed. 2002, 41, 4513–4517. [Google Scholar] [CrossRef]
- Lage, M.L.; Fernández, I.; Mancheño, M.J.; Sierra, M.A. Electronic structure of alkoxychromium (0) Carbene complexes: A joint TD-DFT/experimental Study. Inorg. Chem. 2008, 47, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Li, T.; Li, C.; Liu, P.; Shang, W.; Wu, Y. Langmuir-Blodgett films of cyclopalladated ferrocenylimine: Preparation, characterization, and application in Suzuki coupling reaction. Tetrahedron 2009, 65, 2599–2604. [Google Scholar] [CrossRef]
- Chai, X.D.; Yang, W.S.; Lu, R.; Cao, Y.W.; Lu, N.; Jiang, Y.S.; Bai, Y.B.; Li, T.J. A novel amphiphilic push-pull ferrocene derivative: Langmuir-Blodgett films and second-order optical nonlinearity. Supramol. Sci. 1998, 5, 679–682. [Google Scholar] [CrossRef]
- Amer, W.A.; Wang, L.; Amin, A.M.; Ma, L.; Yu, H. Recent progress in the synthesis and applications of some ferrocene derivatives and ferrocene-base polymers. J. Inorg. Organomet. Polym. 2010, 20, 605–615. [Google Scholar] [CrossRef]
- Astruc, D. From organotransition-metal chemistry toward molecular electronics: Electronic communication between ligand-bridged metals. Acc. Chem. Res. 1997, 30, 383–391. [Google Scholar] [CrossRef]
- Sakakibara, K.; Kamitakahara, H.; Takano, T.; Nakatsubo, F. Redox-active cellulose Langmuir-Blodgett Films containing β-carotene as a molecular wire. Biomacromolecules 2007, 8, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Fery-Forgues, S.; Delavaux-Nicot, B. Ferrocene and ferrocenyl derivatives in luminescent systems. J. Photochem. Photobiol. A Chem. 2000, 132, 137–159. [Google Scholar] [CrossRef]
- Oh, S.Y.; Jung, G.-Y.; Choi, J-W.; Lee, S.B.; Kim, H.S.; Lee, W.H. Synthesis of ferrocene derivative with long alkyl chains and properties of the thin films prepared by Langmuir-Blodgett technique. J. Ind. Eng. Chem. 1996, 2, 1–6. [Google Scholar]
- Takahashi, S.; Anzai, J.-I. Recent progress in ferrocene-modified thin films and nanoparticles for biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Pandey, R.; Gupta, R.K.; Shahid, M.; Maiti, B.; Misra, A.; Pandey, D.S. Synthesis and characterization of electroactive ferrocene derivatives: ferrocenylimidazoquinazoline as a multichannel chemosensor selective for Hg2+ and Pb 2+ ions in an aqueous environment. Inorg. Chem. 2012, 51, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nakahara, H.; Kiyoshige, F.; Akabori, S. Synthesis and some properties of ferrocene derivatives having a long alkyl chain. Formation of regularly oriented organometallic complex in monolayer assemblies. J. Chem. Chem. Commun. 1988, 1, 24–25. [Google Scholar] [CrossRef]
- Begley, M.J.; Mountford, P.; Stewart, P.J.; Swallow, D.; Wan, S. Synthesis, structure and properties of new iron-molybdenum and tungsten fulvalene-bridged heterobimetallic complexes. J. Chem. Soc. Dalton Trans. 1996, 7, 1323–1332. [Google Scholar] [CrossRef]
- Bezuidenhout, D.I.; Barnard, W.; van der Westhuizen, B.; van der Watt, E.; Liles, D.C. Multimetal Fischer carbene complexes of group VI transition metals: Synthesis, structure and substituent effect investigation. Dalton Trans. 2011, 40, 6711–6721. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, K.N.; Ray, P.C.; Matsuoka, I.; Bhadbhade, M.M.; Puranik, V.G.; Das, P.K.; Nishihara, H.; Sarkar, A. Ferrocene in conjugation with a Fischer carbene: Synthesis, NLO, and electrochemical behavior of a novel organometallic push-pull system. Organometallics 1999, 18, 3851–3858. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.; Gómez-Gallego, M.; Fernandez, I.; Mancheño, M.J.; Sierra, M.A. ESI mass spectrometry as a tool for the study of electron transfer in nonconventional media: The case of bi- and polymetallic carbene complexes. Organometallics 2004, 23, 4647–4654. [Google Scholar] [CrossRef]
- Aoki, A.; Miyashita, T. Electrochemical characterization of redox polymer Langmuir-Blodgett films containing ferrocene derivatives. Macromolecules 1996, 29, 4662–4667. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Ortiz, A.; Álvarez-Toledano, C.; López-Cortés, J.G.; Moreno, A.; Álvarez, J.R. Corrigendum to thin films of molecular materials synthesized from fischer’s carbene ferrocenyl: Film formation and electrical properties. Thin Solid Films 2008, 516, 6382–6387. [Google Scholar] [CrossRef]
- Dynarowicz-Latka, P.; Dhanabalan, A.; Oliveira, O.N., Jr. Modern physicochemical research on Langmuir monolayers. Adv. Colloid Interface 2001, 91, 221–302. [Google Scholar] [CrossRef]
- Schuatz, D.K. Langmuir-Blodgett film structure. Surf. Sci. Rep. 1997, 27, 241–334. [Google Scholar]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction, 1st ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1996; pp. 13–166. [Google Scholar]
- Nalwa, H.S.; Kakuta, A. Organometallic Langmuir-Blodgett films for electronics and photonics. Appl. Organomet. Chem. 1992, 6, 645–678. [Google Scholar] [CrossRef]
- Ando, Y.; Hiroike, T.; Miyashita, T.; Miyazki, T. Magnetic properties of ferrocenylmethylacrylate-N-dodecylacryl-amide copolymer Langmuir-Blodgett films. Thin solid Films 1999, 350, 232–237. [Google Scholar] [CrossRef]
- Ifuku, S.; Tsujii, Y.; Kamitakahara, H.; Takano, T.; Nakatsubo, F. Preparation and characterization of redox cellulose Langmuir-Blodgett films containing a ferrocene derivative. J. Polym. Sci. A Polym. Chem. 2005, 43, 5023–5031. [Google Scholar] [CrossRef]
- Fisher, E.O.; Masböl, A. On the existence of a tungsten carbonyl carbene complex. Angew. Chem. Int. Ed. 1964, 3, 580–581. [Google Scholar] [CrossRef]
- Andrada, D.M.; Jiménez-Halla, J.O.; Sola, M. Mechanism of the aminolysis of Fischer alkoxy and thiocarbene complexes: A DFT study. J. Org. Chem. 2010, 75, 5821–5836. [Google Scholar] [CrossRef] [PubMed]
- Veya, P.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. Reaction of metal carbonyls with naked enolates to make a metallocarbene enolate and alkylcarbonylmetalates. Organometallics 1994, 13, 214–223. [Google Scholar] [CrossRef]
- López-Cortés, J.G.; Contreras de la Cruz, L.F.; Ortega-Alfaro, M.C.; Toscano, R.A.; Alvarez-Toledano, C.; Rudler, H. Improved approaches and structures of new ferrocenyl carbene complexes of chromium, tungsten, and molybdenum. J. Organomet. Chem. 2005, 690, 2229–2237. [Google Scholar] [CrossRef]
- Schobert, R.; Kempe, R.; Schmalz, T.; Gmeiner, A. Syntheses, structures and electrochemistry of some 1-(η2-allylamino)-1-ferrocenylcarbene complexes of chromium(0),molybdenum(0) and tungsten(0) Part 13: The chemistry of metallacyclic alkenylcarbene complexes. J. Organomet. Chem. 2006, 691, 859–868. [Google Scholar] [CrossRef]
- Schobert, R.; Schmalz, T. Synthesis and selective silylation of x-hydroxy functionalized (η2-alkenyl)carbene complexes of chromium(0) and tungsten(0) Part 11. The chemistry of metallacyclic alkenylcarbene complexes. J. Organomet. Chem. 2004, 689, 1771–1779. [Google Scholar] [CrossRef]
- Fernández, I.; Cossío, F.P.; Sierra, M.A. Photochemistry of group 6 Fischer carbene complexes: Beyond the photocarbonylation reaction. Acc. Chem. Res. 2011, 44, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Arnett, E.M.; Bushick, R.D. Quantitative estimates of the strong electron donor properties of metallocenyl nuclei. J. Org. Chem. 1962, 27, 111–115. [Google Scholar] [CrossRef]
- Foley, H.C.; Strubinger, L.M.; Targos, T.S.; Geoffroy, G.L. Photochemistry of [W(CO)5{C(OMe)Ph}]. Formation of alkyne-carbene complexes and studies of their decomposition reactions. J. Am. Chem. Soc. 1983, 105, 3064–3073. [Google Scholar] [CrossRef]
- Hegedus, L.S. Chromium carbene complex photochemistry in organic synthesis. Tetrahedron 1997, 53, 4105–4128. [Google Scholar] [CrossRef]
- Ybert, C.; Lu, W.; Möller, G.; Knobler, C.M. Collapse of a monolayer by three mechanisms. J. Phys. Chem. B 2002, 106, 2004–2008. [Google Scholar] [CrossRef]
- Ries, H.E., Jr.; Swif, H. Twisted double-layer ribbons and the mechanism for monolayer collapse. Langmuir 1987, 3, 853–855. [Google Scholar] [CrossRef]
- Israelachvili, J. Self-assembly in two dimensions: Surface micelles and domain formation in monolayers. Langmuir 1994, 10, 3774–3781. [Google Scholar] [CrossRef]
- Siegel, S.; Hönig, D.; Vollhardt, D.; Möbius, D. Direct observation of three-dimensional transformation of insoluble monolayers. J. Phys. Chem. 1992, 96, 8157–8160. [Google Scholar] [CrossRef]
- Dechenaux, R.; Megert, S.; Zumbrunn, C.; Ketterer, J.; Steiger, R. Organized molecular films from new ferrocene-containing molecular units. Langmuir 1997, 13, 2363–2372. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Brezesinski, G. Brewster Angle Microscopy (BAM) for in situ characterization of ultrathin films at air/liquid interfaces. In Current Microscopy Contributions to Advances in Science and Technology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2012; Volume 2, pp. 1007–1012. [Google Scholar]
- Hönig, D.; Möbius, D. Direct visualization of monolayers at the air-water interface by Brewster Angle Microscopy. J. Phys. Chem. 1991, 195, 4590–4592. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazo-Jiménez, R.E.; Ortega-Alfaro, M.C.; López-Cortés, J.G.; Alvarez-Toledano, C.; Chávez-Carvayar, J.Á.; Ignés-Mullol, J.; González-Torres, M.; Carreón-Castro, P. Nanostructured Thin Films Obtained from Fischer Aminocarbene Complexes. Materials 2016, 9, 167. https://doi.org/10.3390/ma9030167
Lazo-Jiménez RE, Ortega-Alfaro MC, López-Cortés JG, Alvarez-Toledano C, Chávez-Carvayar JÁ, Ignés-Mullol J, González-Torres M, Carreón-Castro P. Nanostructured Thin Films Obtained from Fischer Aminocarbene Complexes. Materials. 2016; 9(3):167. https://doi.org/10.3390/ma9030167
Chicago/Turabian StyleLazo-Jiménez, Rosa E., M. Carmen Ortega-Alfaro, José G. López-Cortés, Cecilio Alvarez-Toledano, José Á. Chávez-Carvayar, Jordi Ignés-Mullol, Maykel González-Torres, and Pilar Carreón-Castro. 2016. "Nanostructured Thin Films Obtained from Fischer Aminocarbene Complexes" Materials 9, no. 3: 167. https://doi.org/10.3390/ma9030167





