Polystyrene-Encapsulated Carbonyl Iron Microcapsules: A Corrosion-Resistant Microwave Absorber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CIP@PS
2.3. Characterization
3. Results and Discussion
3.1. Structure and Morphology Analysis
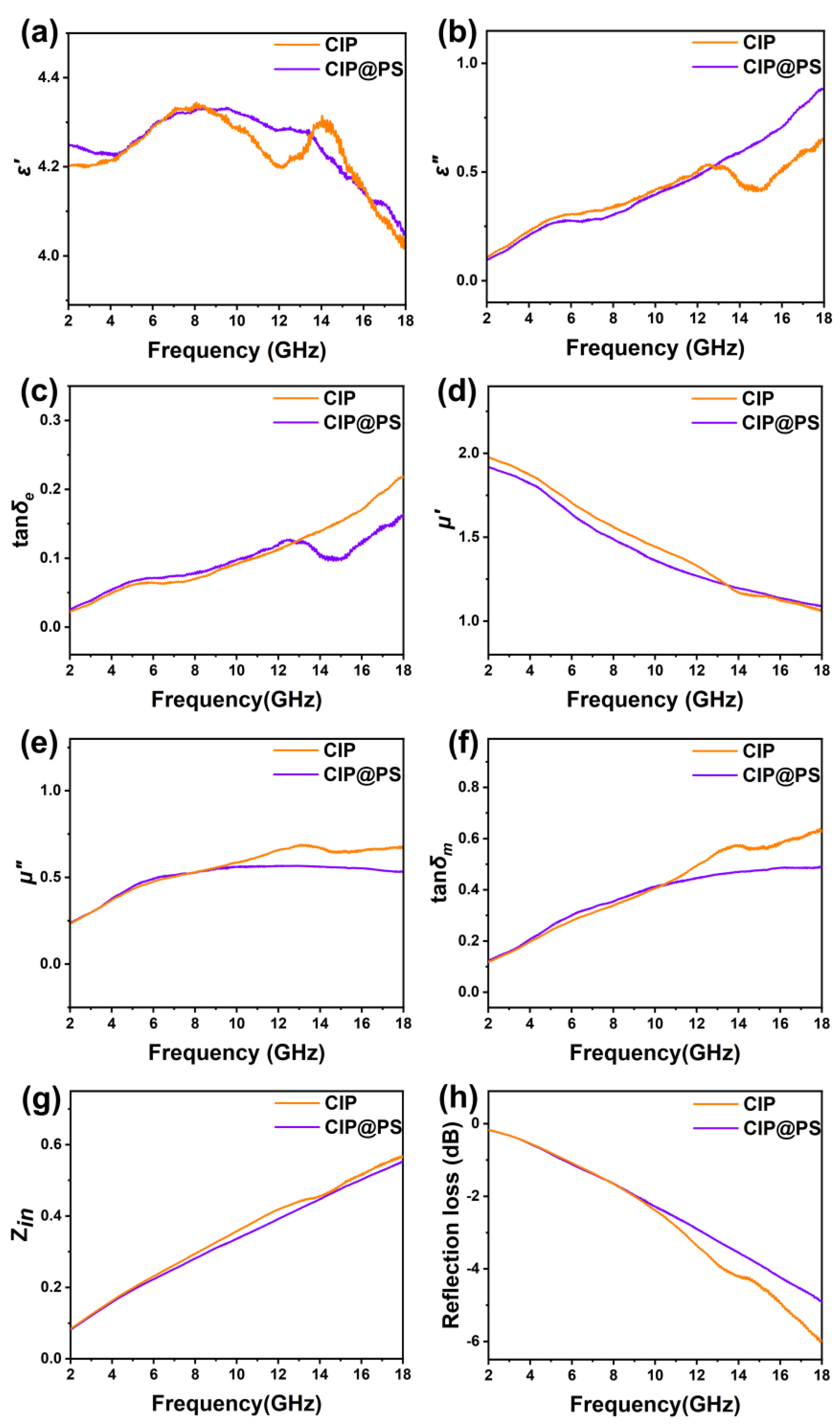
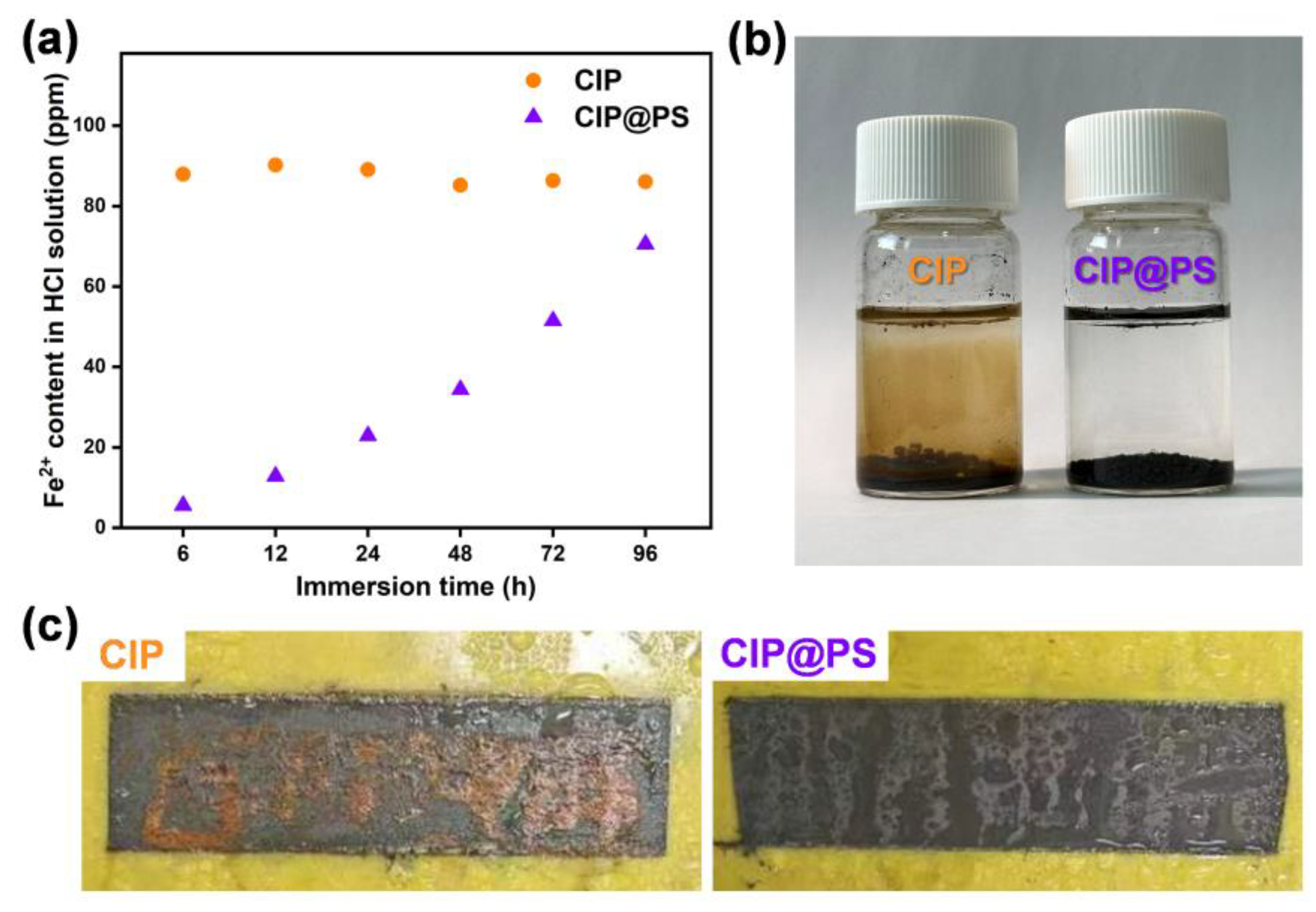
3.2. Wave-Absorption and Corrosion-Resistance Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Yang, M.; Yan, H.; Qi, S. The characterization and preparation of core-shell structure particles of carbon-sphere@NiFe2O4@PPy as microwave absorbing materials in X band. J. Mater. Sci. Mater. Electron. 2017, 28, 14988–14995. [Google Scholar] [CrossRef]
- Cui, X.J.; Jiang, Q.R.; Wang, C.S.; Wang, S.H.; Jiang, Z.Y.; Li, X.A.; Deng, D.H. Encapsulating FeCo alloys by single layer graphene to enhance microwave absorption performance. Mater. Today Nano 2021, 16, 100138. [Google Scholar] [CrossRef]
- Ji, C.; Liu, Y.; Xu, J.; Li, Y.; Shang, Y.; Su, X. Enhanced microwave absorption properties of biomass-derived carbon decorated with transition metal alloy at improved graphitization degree. J. Alloys Compd. 2022, 890, 161834. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Yousaf, M.; Lu, Y.; Baqir, M.A.; Azhar Khan, M.; Ahmad, M.; Sarosh, A.; Shahid Nazir, M. Highly efficient absorber with enhanced magnetoelectric properties based on Y, Gd, and Pr doped NMZ nanoferrites. J. Magn. Magn. Mater. 2021, 537, 168232. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Xue, R.; Qi, S. A polypyrrole/CoFe2O4/hollow glass microspheres three-layer sandwich structure microwave absorbing material with wide absorbing bandwidth and strong absorbing capacity. J. Mater. Sci. Mater. Electron. 2016, 28, 519–525. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Wang, Z.; Peng, Y.; Xia, L.; Ma, S.; Yin, Z.; Huang, Y. A review on metal-organic framework-derived porous carbon-based novel microwave absorption materials. Nano-Micro Lett. 2021, 13, 56. [Google Scholar] [CrossRef]
- Falegnami, A.; Romano, E.; Tomassi, A. The emergence of the greenscent competence framework. In The European Green Deal in Education, 1st ed.; Routledge: Abingdon, UK, 2024; pp. 204–216. [Google Scholar]
- Sun, J.; Wang, L.; Yang, Q.; Shen, Y.; Zhang, X. Preparation of copper-cobalt-nickel ferrite/graphene oxide/polyaniline composite and its applications in microwave absorption coating. Prog. Org. Coat. 2020, 141, 105552. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Luo, F.; Su, X.; Xu, J.; Wang, J.; He, X.; Qu, Y. Electromagnetic and microwave absorption properties of SiO2-coated Ti3SiC2 powders with higher oxidation resistance. J. Alloys Compd. 2017, 715, 21–28. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2001, 6, 73–84. [Google Scholar] [CrossRef]
- Pang, R.; Hu, X.; Zhou, S.; Sun, C.; Yan, J.; Sun, X.; Xiao, S.; Chen, P. Preparation of multi-shelled conductive polymer hollow microspheres by using Fe3O4 hollow spheres as sacrificial templates. Chem. Commun. 2014, 50, 12493–12496. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Y.; Lin, X.; Liu, Y.; Gong, H.; Zhang, Y. The dielectric and microwave absorption properties of polymer-derived SiCN ceramics. J. Eur. Ceram. Soc. 2018, 38, 1327–1333. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.; Li, X.; Zhu, Y.; Zhu, B. Enhanced electromagnetic wave absorption for Y2O3-doped SiBCN ceramics. ACS Appl. Mater. Interfaces 2021, 13, 55440–55453. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Zhou, X.; Tian, D.; Zhang, G.; Gan, Y.; Shi, J.; He, J. Facile preparation and microwave absorption properties of RGO/MWCNTs/ZnFe2O4 hybrid nanocomposites. J. Alloys Compd. 2018, 743, 163–174. [Google Scholar] [CrossRef]
- Kaur, P.; Narang, S.B.; Bahel, S. Enhanced microwave absorption properties of Ni-Zr doped La-Sr hexagonal ferrites in 18-40 GHz frequency range. Mater. Sci. Eng. B 2021, 268, 115141. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, J.; Che, R.; Xu, J.; Liu, M.; Liu, Z. Synthesis and microwave absorption properties of yolk-shell microspheres with magnetic iron oxide cores and hierarchical copper silicate shells. ACS Appl. Mater. Interfaces 2013, 5, 2503–2509. [Google Scholar] [CrossRef]
- Ali, N.N.; Atassi, Y.; Salloum, A.; Charba, A.; Malki, A.; Jafarian, M. Comparative study of microwave absorption characteristics of (Polyaniline/NiZn ferrite) nanocomposites with different ferrite percentages. Mater. Chem. Phys. 2018, 211, 79–87. [Google Scholar] [CrossRef]
- Chen, Q.; Li, L.; Wang, Z.; Ge, Y.; Zhou, C.; Yi, J. Synthesis and enhanced microwave absorption performance of CIP@SiO2@Mn0.6Zn0.4Fe2O4 ferrite composites. J. Alloys Compd. 2019, 779, 720–727. [Google Scholar] [CrossRef]
- Małecki, P.; Kolman, K.; Pigłowski, J.; Kaleta, J.; Krzak, J. Sol-gel method as a way of carbonyl iron powder surface modification for interaction improvement. J. Solid State Chem. 2015, 226, 224–230. [Google Scholar] [CrossRef]
- Qing, Y.; Zhou, W.; Jia, S.; Luo, F.; Zhu, D. Microwave electromagnetic property of SiO2-coated carbonyl iron particles with higher oxidation resistance. Phys. B 2011, 406, 777–780. [Google Scholar] [CrossRef]
- Guo, X.; Yao, Z.; Lin, H.; Zhou, J.; Zuo, Y.; Xu, X.; Wei, B.; Chen, W.; Qian, K. Epoxy resin addition on the microstructure, thermal stability and microwave absorption properties of core-shell carbonyl iron@epoxy composites. J. Magn. Magn. Mater. 2019, 485, 244–250. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xiong, J.; Yang, Y.; Lu, H. Microwave-absorbing properties of de-aggregated flake-shaped carbonyl-iron particle composites at 2-18 GHz. IEEE Trans. Magn. 2006, 42, 1778–1781. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Abshinova, M.A.; Kazantseva, N.E.; Sáha, P.; Sapurina, I.; Kovářová, J.; Stejskal, J. The enhancement of the oxidation resistance of carbonyl iron by polyaniline coating and consequent changes in electromagnetic properties. Polym. Degrad. Stab. 2008, 93, 1826–1831. [Google Scholar] [CrossRef]
- Zhu, H.R.; Tang, W.; Gao, C.Z.; Han, Y.; Li, T.; Cao, X.; Wang, Z.L. Self-powered metal surface anti-corrosion protection using energy harvested from rain drops and wind. Nano Energy 2015, 14, 193–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Bu, A.; Xiang, Y.; Yang, Y.; Chen, W.; Cheng, H.; Wang, L. Improving corrosion resistance of carbonyl iron powders by plasma electrolytic deposition. Mater. Des. 2020, 188, 108480. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, J. Preparation and characterization of graphene oxide/polyaniline/carbonyl iron nanocomposites. Mater. 2022, 15, 484. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Jin, K.; Wang, C.; Guo, W.; Tian, K.; Wang, H. Enduring effect mechanism of Co(Ni) layers on excellent microwave absorption performance of carbonyl iron in seawater. J. Magn. Magn. Mater. 2022, 564, 170202. [Google Scholar] [CrossRef]
- Duan, W.; Li, X.; Wang, Y.; Qiang, R.; Tian, C.; Wang, N.; Han, X.; Du, Y. Surface functionalization of carbonyl iron with aluminum phosphate coating toward enhanced anti-oxidative ability and microwave absorption properties. Appl. Surf. Sci. 2018, 427, 594–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, S.; Li, C.; Dong, T.; Yu, Q. Enhanced anti-corrosion and microwave absorption performance of coating with TiO2-wrapped carbonyl iron-modified composites. Ceram. Inter. 2024, 50, 25216–25227. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, W.; Jiang, X.; Wang, B.; Yin, L.; Agathopoulos, S.; Xie, J.; Zhang, L.; Lu, H.; Deng, L. Enhancement of oxidation and corrosion resistance of flaky carbonyl-iron powder via SiO2/KH560/PDMS coating applied with sol-gel. Surf. Coat. Technol. 2022, 437, 128346. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Lee, I.Y.; Park, S.; Willis, J.; Haldolaarachchige, N.; Young, D.P.; Luo, Z.; Guo, Z. Silica stabilized iron particles toward anti-corrosion magnetic polyurethane nanocomposites. RSC Adv. 2012, 2, 1136–1143. [Google Scholar] [CrossRef]
- Tian, W.; Li, J.; Liu, Y.; Deng, L.; Guo, Y.; Jian, X. Large-scale synthesis of fluorine-free carbonyl iron-organic silicon hydrophobic absorbers with long term corrosion protection property. Nano Res. 2022, 15, 9479–9491. [Google Scholar] [CrossRef]
- Jiang, X.; Wan, W.; Wang, B.; Zhang, L.; Yin, L.; Van Bui, H.; Xie, J.; Zhang, L.; Lu, H.; Deng, L. Enhanced anti-corrosion and microwave absorption performance with carbonyl iron modified by organic fluorinated chemicals. Appl. Surf. Sci. 2022, 572, 151320. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Yin, L.; Yang, G.; Xie, J.; Zhang, L.; Lu, H.; Liang, D.; Deng, L. Corrosion behavior of fluorinated carbonyl iron-hydrophobic composites in neutral salt spray environment. Corros. Sci. 2023, 210, 110823. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, J.; Yin, L.; Peng, J.; Li, J.; Zhang, P.; Zhang, M.; Qin, H.; Jing, Z.; Yuan, L.; et al. In-depth insight into the inhibition mechanism in iron-based composites with bifunction of anti-corrosion and electromagnetic wave absorption based on experiments and theoretical calculations. Appl. Surf. Sci. 2024, 665, 160362. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Q.; Yang, W.; Zeng, W.; Jiang, Y.; Chai, L.; Deng, L. Corrosion resistance enhancement and superhydrophobic surface realization of flake magnetic particles with organic polyolefin copolymer modification. Mater. Today Commun. 2022, 30, 103111. [Google Scholar] [CrossRef]
- Li, K.; Ge, J.; Zhang, D.; Zhao, R.; Wu, C.; Cheng, Z.; Xie, Z.; Liu, Y. “Dual-armor” strategy to prepare multifunctional carbonyl iron particle with corrosion resistance, self-healing and high microwave absorbing performances. Compos. Sci. Technol. 2024, 255, 110699. [Google Scholar] [CrossRef]
- Tang, J.; Ma, L.; Tian, N.; Gan, M.; Xu, F.; Zeng, J.; Tu, Y. Synthesis and electromagnetic properties of PANI/PVP/CIP core-shell composites. Mater. Sci. Eng. B 2014, 186, 26–32. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Choudhary, H.K.; Sahoo, B. Graphene-oxide coating for corrosion protection of iron particles in saline water. Carbon 2018, 140, 477–487. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Pandey, U.P.; Sahoo, B. Role of oxygen functionalities of GO in corrosion protection of metallic Fe. Carbon 2021, 173, 350–363. [Google Scholar] [CrossRef]
- Chen, J.; Lei, W.; Huang, C.; Wang, J.; Zhang, Y.; Liu, Z.; Xu, Z.; Wang, K.; Guo, S. Magneto-electric adjustable Co/C porous layer coated flaky carbonyl iron composites with bifunctions of anti-corrosion and microwave absorption. J. Alloys Compd. 2022, 927, 167104. [Google Scholar] [CrossRef]
- Li, Q.; Tian, X.; Yang, W.; Hou, L.; Li, Y.; Jiang, B.; Wang, X.; Li, Y. Fabrication of porous graphene-like carbon nanosheets with rich doped-nitrogen for high-performance electromagnetic microwave absorption. Appl. Surf. Sci. 2020, 530, 147298. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, X.; Xue, H.; Guo, H.; Fan, X.; Pan, X.; He, J. Graphene-carbonyl iron cross-linked composites with excellent electromagnetic wave absorption properties. J. Mater. Chem. C 2014, 2, 6582–6591. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, K.; Shi, J.; Li, W.; Liu, W.; He, W.; Wang, Q.; Zhao, T. Polystyrene-Encapsulated Carbonyl Iron Microcapsules: A Corrosion-Resistant Microwave Absorber. Materials 2025, 18, 1779. https://doi.org/10.3390/ma18081779
Gai K, Shi J, Li W, Liu W, He W, Wang Q, Zhao T. Polystyrene-Encapsulated Carbonyl Iron Microcapsules: A Corrosion-Resistant Microwave Absorber. Materials. 2025; 18(8):1779. https://doi.org/10.3390/ma18081779
Chicago/Turabian StyleGai, Ke, Junhe Shi, Wanxun Li, Weisen Liu, Weiping He, Qian Wang, and Tong Zhao. 2025. "Polystyrene-Encapsulated Carbonyl Iron Microcapsules: A Corrosion-Resistant Microwave Absorber" Materials 18, no. 8: 1779. https://doi.org/10.3390/ma18081779
APA StyleGai, K., Shi, J., Li, W., Liu, W., He, W., Wang, Q., & Zhao, T. (2025). Polystyrene-Encapsulated Carbonyl Iron Microcapsules: A Corrosion-Resistant Microwave Absorber. Materials, 18(8), 1779. https://doi.org/10.3390/ma18081779





