Concrete Durability Performance in Aggressive Salt and Deicing Environments—Case Study of Select Pavement and Bridge Concrete Mixtures
Abstract
:1. Introduction
Mechanisms of Concrete Deterioration
2. Materials, Specimen Preparation, and Curing
3. Test Methods
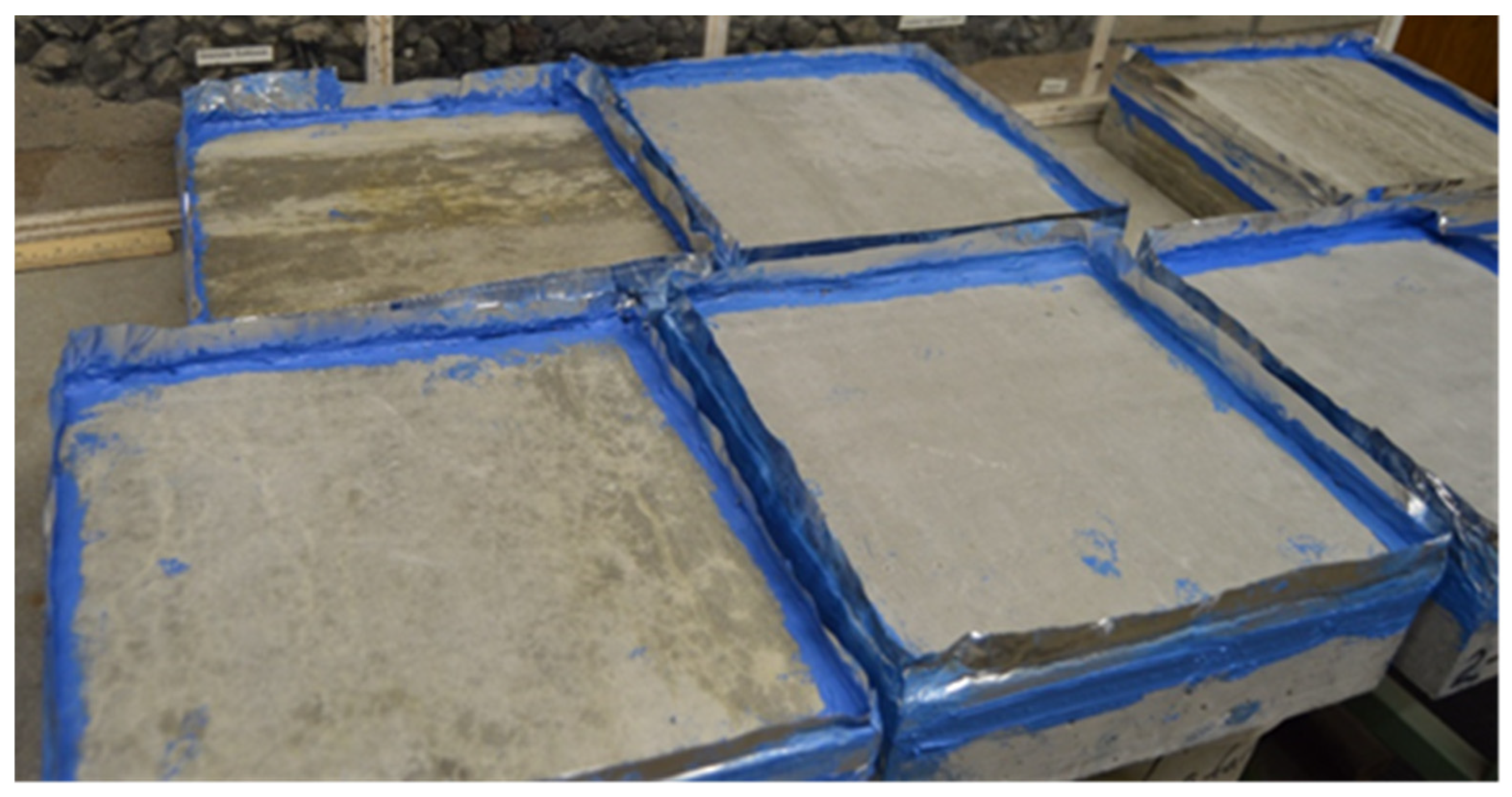
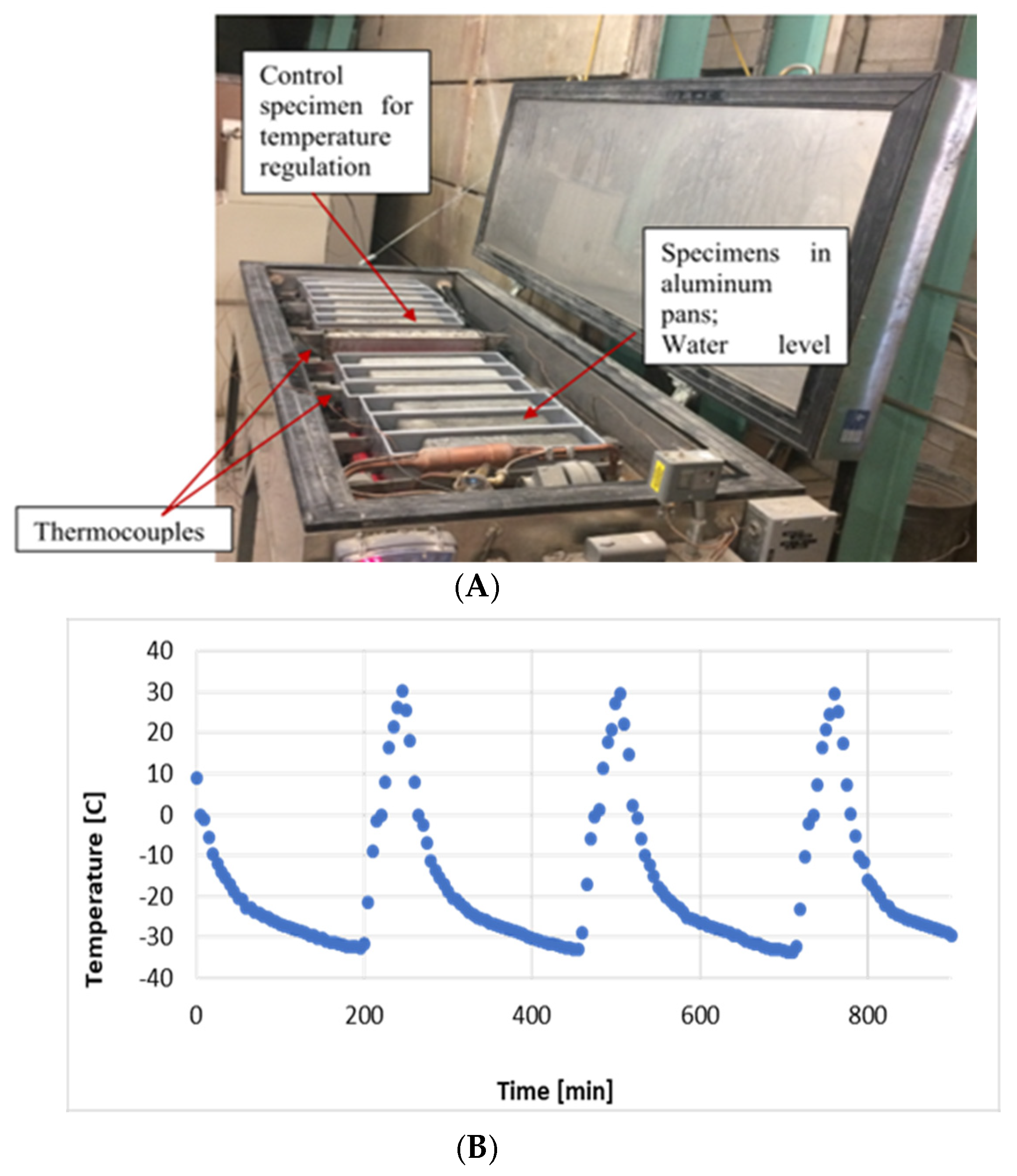
3.1. Environmental Exposure (Conditioning) for Deicing Scaling Evaluation
3.2. Acid Soluble Chloride Test
3.3. Calcium Hydroxide and Oxychloride Contents
3.4. Rapid Freeze–Thaw Cycling Test
3.5. Surface Resistivity Test
3.6. Scanning Electron Microscope (SEM)
4. Results and Discussion for Original Mixtures
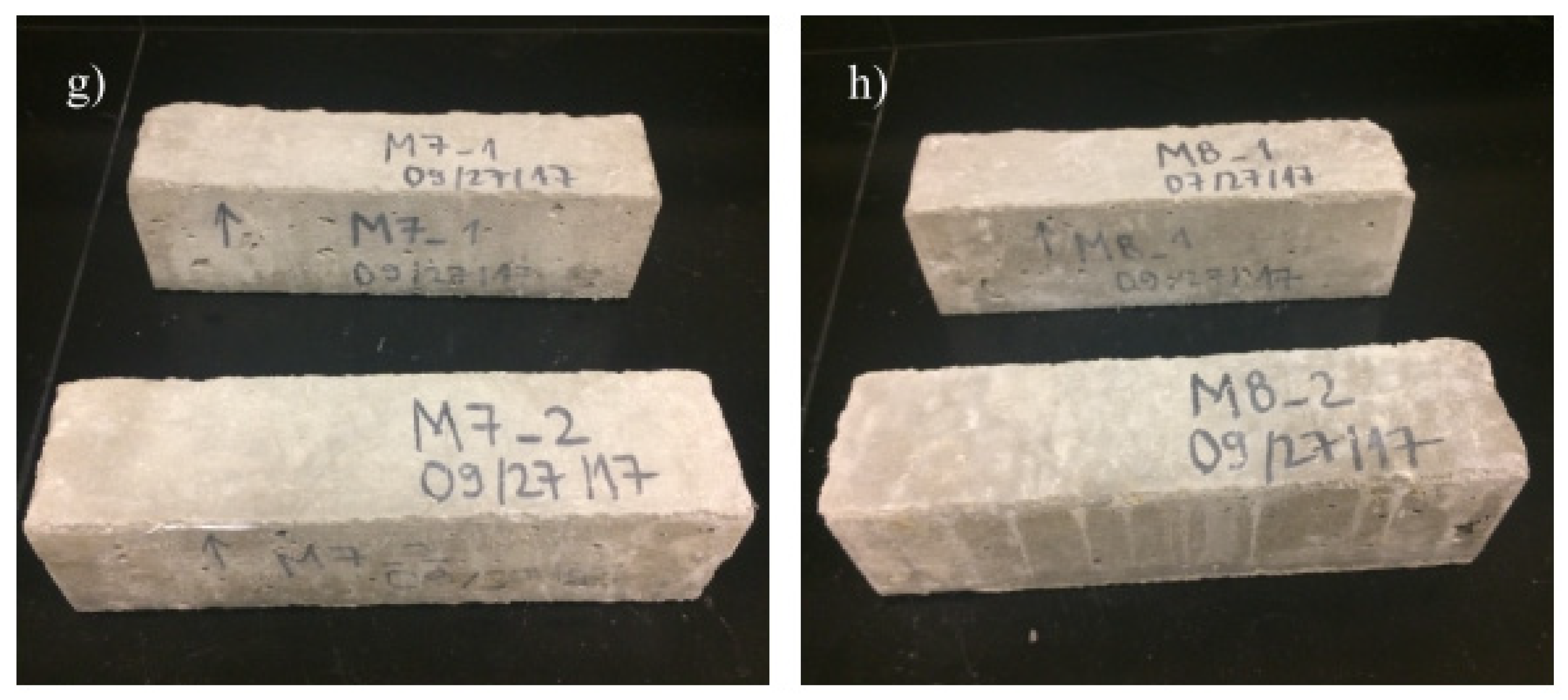
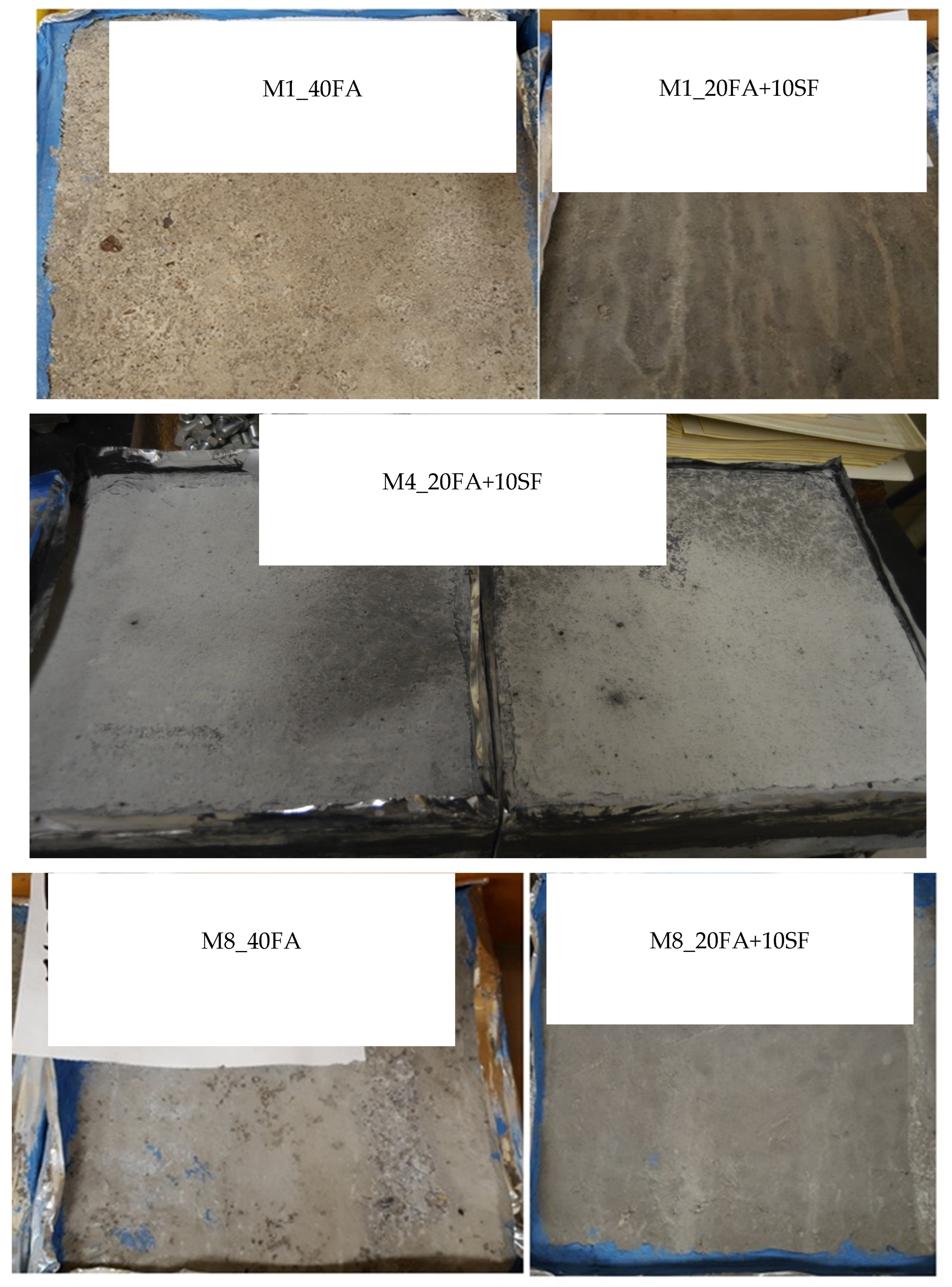
4.1. Deicing Scaling Resistance
4.2. Acid Soluble Chloride Test Results
4.3. TGA and LT-DSC Test Results
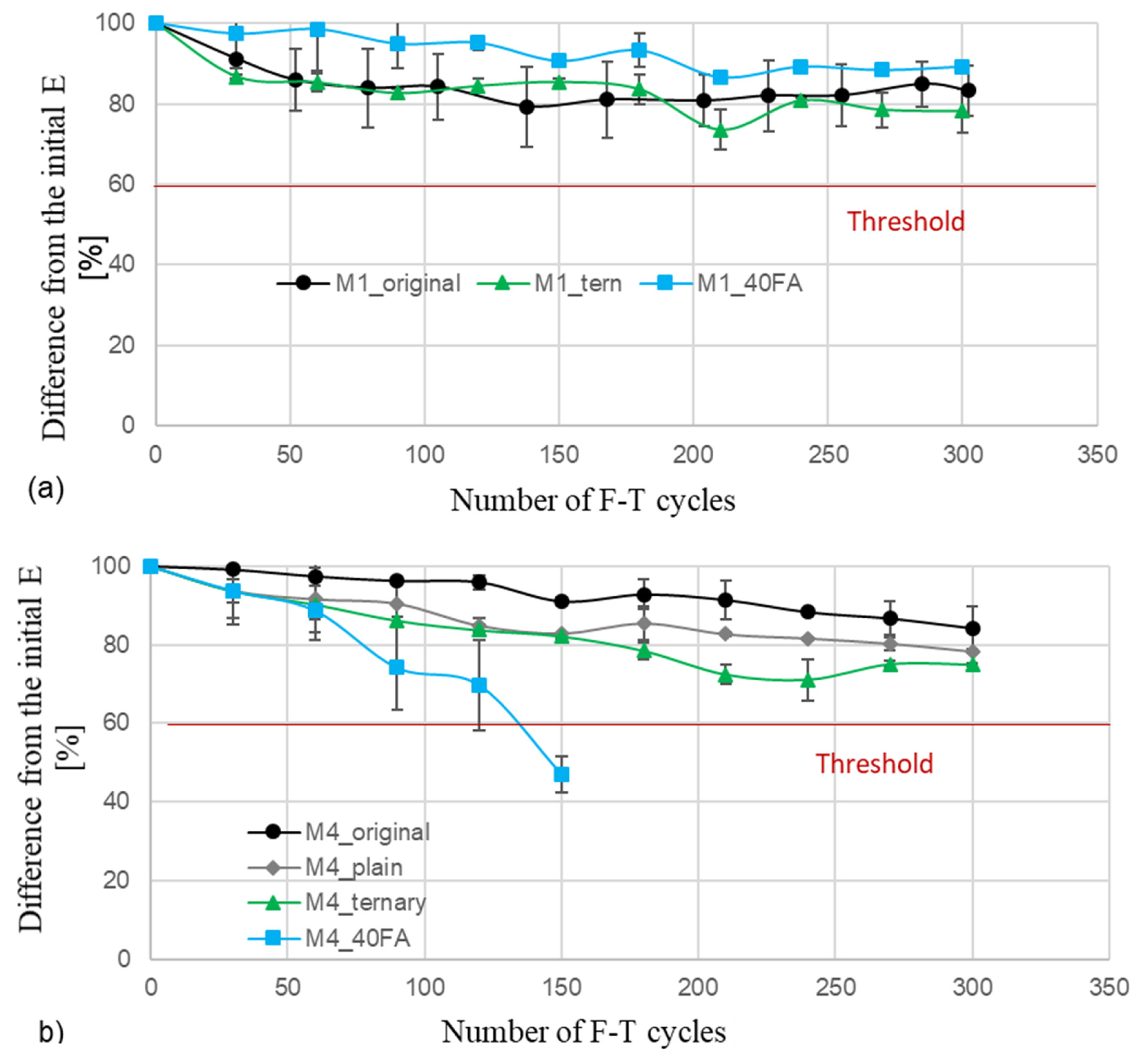
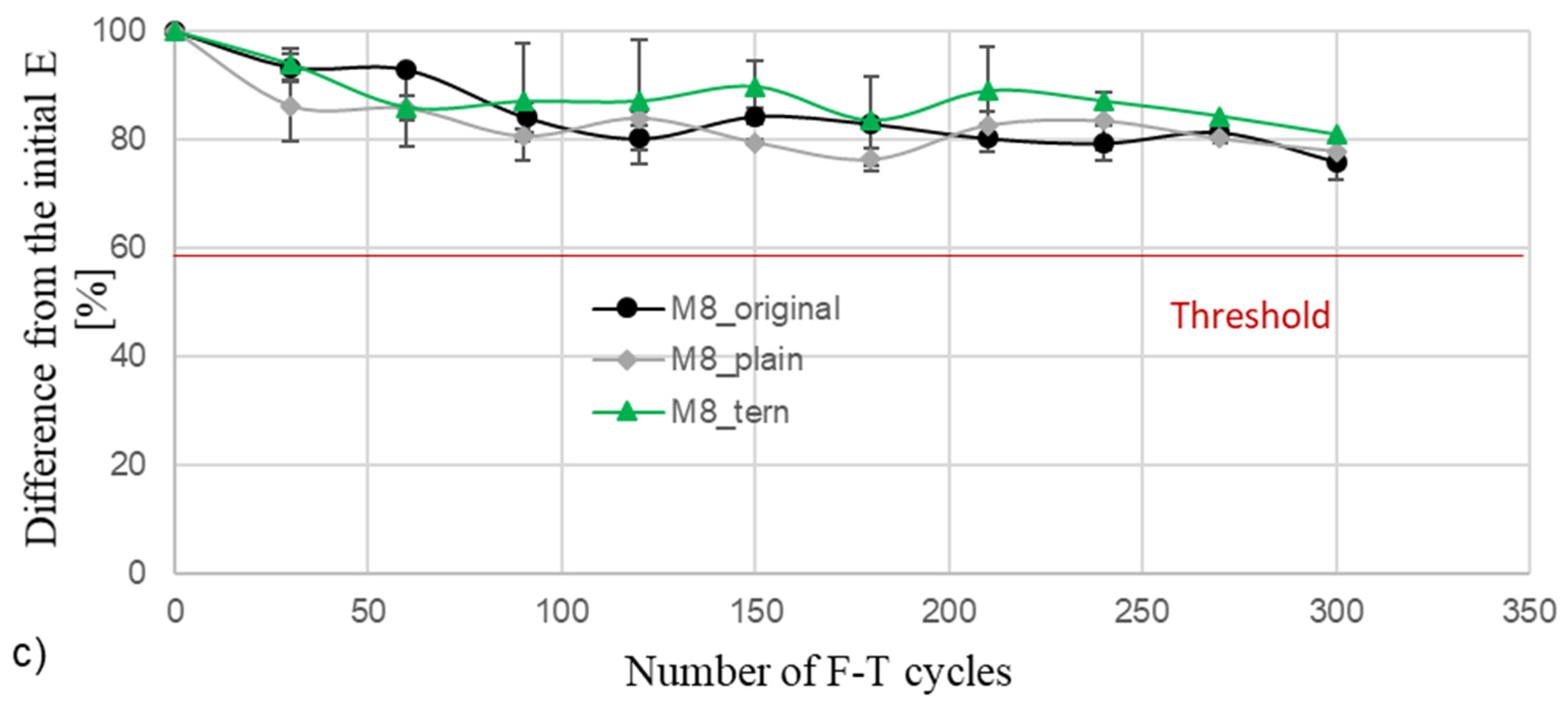
4.4. Rapid F-T Cycle Test Results
4.5. Surface Resistivity Results
4.6. Continuous Soaking
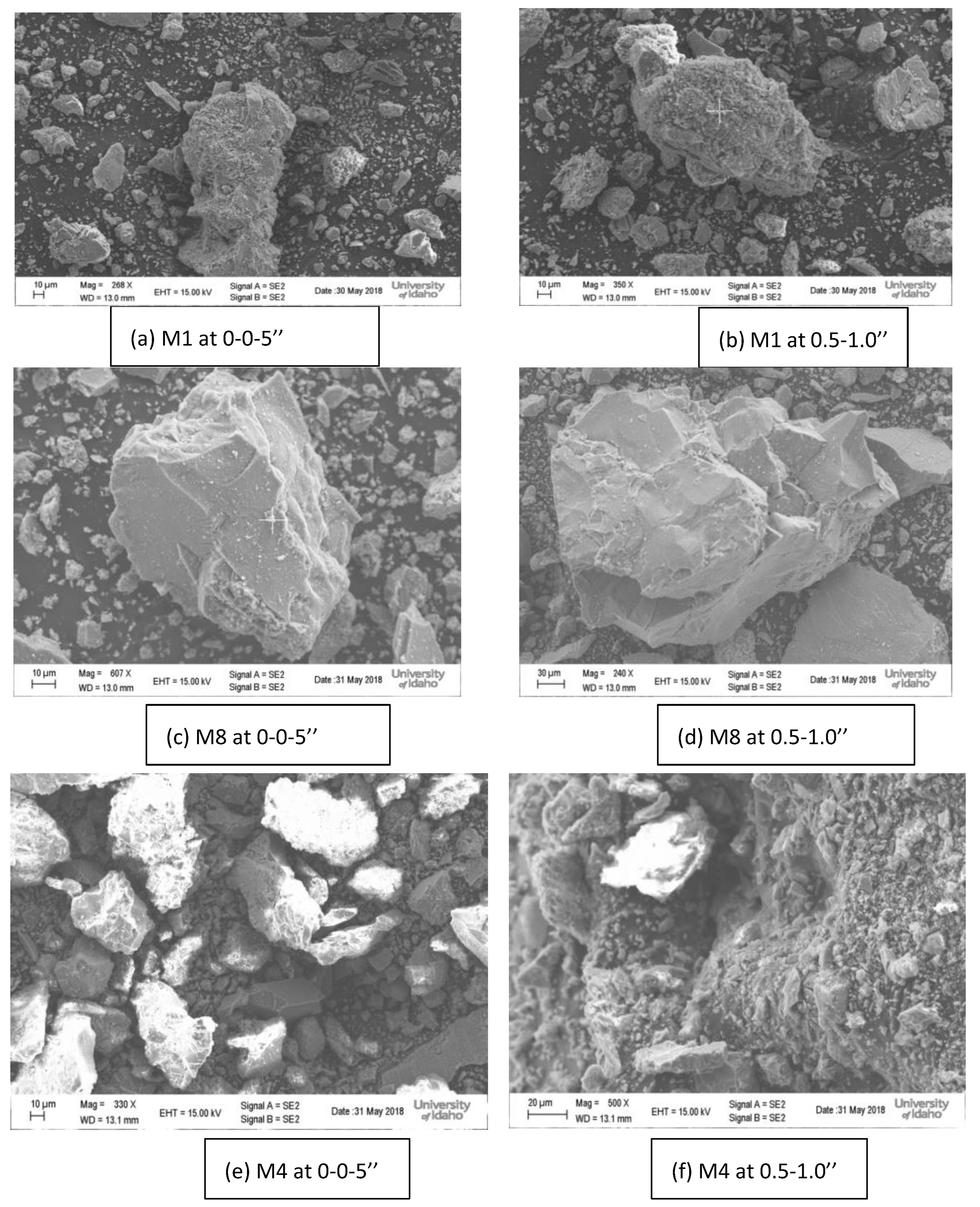
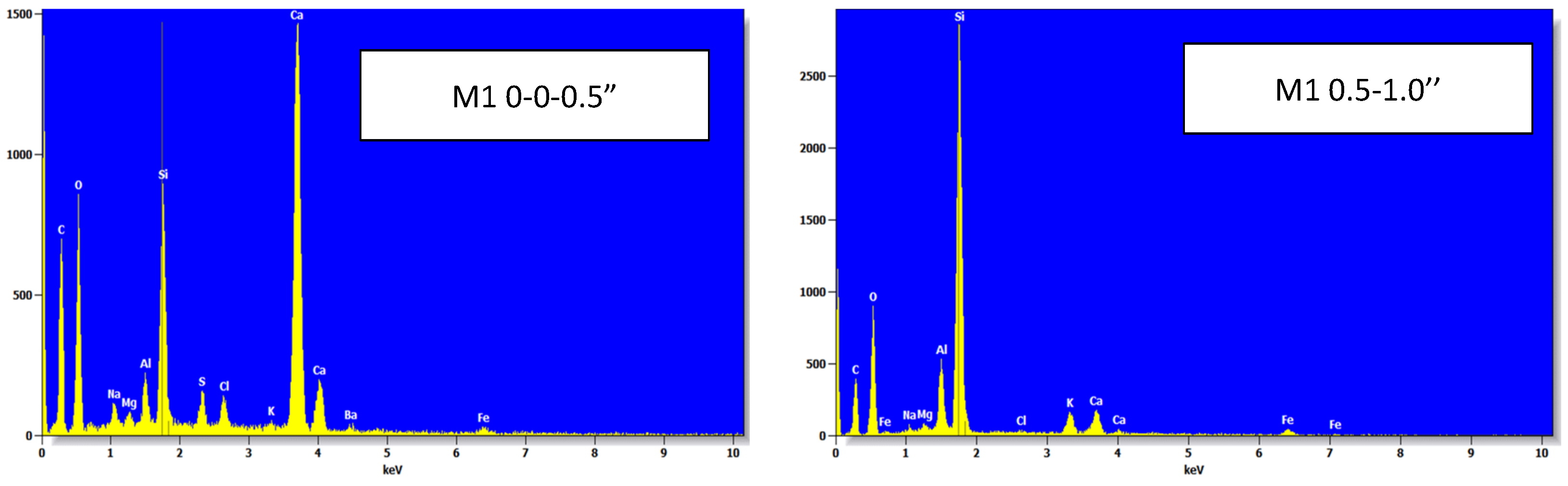
4.7. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Analysis (EDX)
4.8. Alternative Concrete Mixtures
5. Results and Discussions for Alternative Mixtures
5.1. SAM Number
5.2. Scaling Resistance
5.3. Rapid F-T Cycle Test
5.4. Surface Resistivity and Acid Soluble Chloride Test
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cooperative Highway Research Program. Guidelines for the Selection of Snow and Ice 430 Control Materials to Mitigate Environmental Impacts. In NCHRP-Report 577; Transportation 431 Research Board: Washington, DC, USA, 2007. [Google Scholar]
- Zhao, G.; Li, J.; Shao, W. Effect of mixed chlorides on the degradation and sulfate diffusion of cast-in-situ concrete due to sulfate attack. Constr. Build. Mater. 2018, 181, 49–58. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2012, 34, 903–910. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S.; Pavlou, A.; Chaniotakis, E.; Swamy, R.N. The effect of chlorides on the thaumasite form of sulfate attack of limestone cement concrete containing mineral admixtures at low temperature. Constr. Build. Mater. 2013, 43, 156–164. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete. CBI Research Report 4.82; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Farnam, Y.; Dick, S.; Davis, J.; Bentz, D.; Weiss, J. The influence of calcium chloride deicing salt on phase changes and damage development in cementitious materials. Cem. Concr. Compos. 2015, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; Julio-Betancourt, G.; Sutter, L.; Hooton, R.D.; Johnston, D. Observations of chloride ingress and calcium oxychloride formation in laboratory concrete and mortar at 5 °C. Cem. Concr. Res. 2013, 45, 79–90. [Google Scholar] [CrossRef]
- Ghazy, A.; Bassuoni, M.T.; Islam, A.K.M. Response of concrete with blended binders and Nano silica to freezing-thawing cycles and different concentrations of deicing Salts. ASCE J. Mater. Civ. Eng. 2017, 30, 04018214. [Google Scholar] [CrossRef]
- Farnam, Y.; Bentz, D.; Sakulich, A.; Flynn, D.; Weiss, J. Measuring freeze and thaw damage in mortars containing deicing salt using a low-temperature longitudinal guarded comparative calorimeter and acoustic emission. Adv. Civ. Eng. Mater. 2014, 3, 316–337. [Google Scholar] [CrossRef]
- Farnam, Y.; Bentz, D.; Hampton, A.; Weiss, J. Acoustic emission and low temperature calorimetry study of freeze and thaw behavior in cementitious materials exposed to sodium chloride salt. Transp. Res. Rec. J. Transp. Res. Board 2014, 2441, 81–90. [Google Scholar] [CrossRef]
- Shi, C. Formation and stability of 3CaO·CaCl2·12H2O. Cem. Concr. Res. 2001, 31, 1373–1375. [Google Scholar] [CrossRef]
- Brown, P.; Bothe, J. The system CaO-Al2O3-CaCl2-H2O at 23 ± 2 °C and the mechanisms of chloride binding in concrete. Cem. Concr. Res. 2004, 34, 1549–1553. [Google Scholar] [CrossRef]
- Suraneni, P.; Azad, V.J.; Isgor, B.; Weiss, J. Role of Supplementary Cementitious Material Type in the Mitigation of Calcium Oxychloride Formation in Cementitious Paste. J. Mater. Civ. Eng. 2018, 30, 04018248. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Nehdi, M.L. Exploring effects of supplementary cementitious materials in concrete exposed to physical salt attack. Mag. Concr. Res. 2017, 69, 576–585. [Google Scholar] [CrossRef]
- ACI CODE-318-19(22); Building Code Requirements for Structural Concrete and Commentary (Reapproved 2022). American Concrete Institute: Indianapolis, IN, USA, 2011.
- Ibrahim, A.; Bayomy, F.; Arowojolu, O.; Nassiri, S.; Rangelov, M. Concrete Performance in Aggressive Salt and Deicing Environments. Idaho Transportation Department. RP 262. 2018. Available online: https://apps.itd.idaho.gov/apps/research/Completed/RP262.pdf (accessed on 1 January 2018).
- ASTM C192/C192M-19; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C39/C39M-20; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D559/D559M-15; Standard Test Methods for Wetting and Drying Compacted Soil-Cement Mixtures. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C1876-19; Standard Test Method for Bulk Electrical Resistivity or Bulk Conductivity of Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Shi, X.; Fay, L.; Peterson, M.M.; Berry, M.; Mooney, M. A FESEM/EDX investigation into how continuous deicer exposure affects the chemistry of Portland cement concrete. Constr. Build. Mater. 2010, 25, 957–966. [Google Scholar] [CrossRef]
- ASTM C666/C666M-15; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C1543-10a; Standard Test Method for Determining the Penetration of Chloride Ion into Concrete by Ponding (Withdrawn 2019). ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C143/C143M-12; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM C231-09a; Standard Test Method for Air Content of Freshly Mixed Concrete by the Pressure Method. ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM C138/C138M-17a; Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C511-21; Standard Specification for Mixing Rooms, Moist Cabinets, Moist Rooms, and Water Storage Tanks Used in the Testing of Hydraulic Cements and Concretes. ASTM International: West Conshohocken, PA, USA, 2021.
- ACI 201.2R-16; Guide to Durable Concrete. American Concrete Institute: Indianapolis, IN, USA, 2001.
- ASTM C672/C672M-12; Standard Test Method for Scaling Resistance of Concrete Surfaces Exposed to Deicing Chemicals. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM C1152/C1152M-04(2012) e1; Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- Monica, J.; Unai, E.; Barrett, T.; Farnam, Y.; Weiss, J. Reducing joint damage in concrete pavements- quantifying calcium oxychloride formation. Transp. Res. Rec. 2016, 2577, 17–24. [Google Scholar] [CrossRef]
- ACI PRC-209.2-08; Guide for Modeling and Calculating Shrinkage and Creep in Hardened Concrete. American Concrete Institute: Indianapolis, IN, USA, 2008.
- Katayama, T.; Tagami, M.; Sarai, Y.; Izumi, S.; Hira, T. Alkali-Aggregate Reaction under the Influence of Deicing Salts in the Hokuriku District, Japan. Mater. Charact. 2004, 53, 105–122. [Google Scholar] [CrossRef]
- Rangaraju, P.R.; Sompura, K.R.; Olek, J. Investigation into Potential of Alkali-Acetate-Based Deicers to Cause Alkali-Silica Reaction in Concrete. Transp. Res. Rec. 2006, 1979, 69–78. [Google Scholar] [CrossRef]
- Gillerman, V.S.; Weppner, K.N. Lithologic Characterization of Active ITD Aggregate Sources and Implications for Aggregate Quality; Idaho Transportation Department: Boise, ID, USA, 2014.
- Tingting Zhang, C.R.; Cheeseman, L.J. Vandeperre, Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- ACI 222R-01; Protection of Metals in Concrete Against Corrosion. Technical Report for ACI Committee 222: Farmington Hills, MI, USA, 2001.
- Shi, X.; Fay, L.; Peterson, M.M.; Yang, Z. Freeze–thaw damage and chemical change of a Portland cement concrete in the presence of diluted deicers. Mater Struct 2010, 43, 933–946. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Xiao, H. Self-deicing road system with a CNFP high-efficiency thermal source and MWCNT/cement-based high-thermal conductive composites. J. Cold Reg. Sci. Technol. 2013, 86, 22–35. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, A.; Palomo, A.; Fernández-Jiménez, D.; Macphee, E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]




| (a) | |||||||||
| Mixture No | Mixture Application | Binder Type | Cement Content: kg/m3 | Entrained Air | SAM * Number | Spacing Factor | |||
| SCMs Content kg/m3 | w/cm Ratio | Nominal Maximum Aggregate Size (mm) | (%) | (mm) | |||||
| M1 | Structural | OPC | 362 | 0.0 | 0.42 | 19 | 6.50 | 0.20 | 0.24 |
| M2 | Paving | 80OPC + 20FA | 326 | 82 | 0.38 | 38 | 5.00 | 0.10 | 0.16 |
| M3 | Paving | 80OPC + 20FA | 290 | 72 | 0.43 | 38 | 6.50 | 0.36 | 0.34 |
| M4 | Structural | 80OPC + 20FA | 297 | 74 | 0.40 | 19 | 6.50 | 0.39 | 0.36 |
| M5 | Structural | 80OPC + 20FA | 312 | 78 | 0.39 | 19 | 6.50 | 0.10 | 0.15 |
| M6 | Structural | 70OPC + 20FA + 10SF | 253 | 109 | 0.42 | 19 | 7.50 | 0.35 | 0.15 |
| M7 | Structural | 70OPC + 20FA + 10SF | 208 | 118 | 0.40 | 19 | 7.50 | 0.25 | 0.18 |
| M8 | Structural | 70OPC + 20FA + 10SF | 218 | 94 | 0.39 | 19 | 7.50 | 0.37 | 0.16 |
| (b) | |||||||||
| Mixtures | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | |
| Coarse Aggregates (kg/m3) | 1100 | 1070 | 1020 | 985 | 1045 | 1100 | 1070 | 1045 | |
| Fine Aggregates (kg/m3) | 640 | 685 | 740 | 800 | 596 | 640 | 685 | 596 | |
| Chemical Composition | Cement (% by Mass) | Silica Fume (% by Mass) | Fly Ash-Class F (% by Mass) |
|---|---|---|---|
| CaO | 61.18 | 0.49 | 1.41 |
| SiO2 | 20.01 | 95.30 | 60.56 |
| Al2O3 | 4.98 | 0.20 | 32.67 |
| Fe2O3 | 4.88 | 0.10 | 4.44 |
| MgO | 1.78 | 0.27 | 0.23 |
| SO3 | 2.36 | 0.24 | 0.02 |
| Loss on ignition | 2.18 | 1.99 | 0.21 |
| Total Chloride Content | 0.03 | - | 0.01 |
| Na2O | 0.20 | - | 0.02 |
| K2O | 0.60 | - | 0.03 |
| Insoluble residue | 1.23 | - | 0.46 |
| Bogue compound composition of cement | |||
| Compound | % (by mass) | ||
| C3S | 49.82 | ||
| C2S | 19.78 | ||
| C3A | 4.94 | ||
| C4AF | 14.84 | ||
| Mixture | Deicing Chemical |
|---|---|
| M1 | Salt brine |
| M2 | Salt brine |
| M3 | Freeze Guard + bud MgCl2 |
| M4 | Freeze Guard + bud MgCl2 |
| M5 | Salt brine |
| M6 | Salt brine |
| M7 | Freeze Guard + bud MgCl2 |
| M8 | Salt brine |
| Rating Grade | Rating Description |
| 0 | No scaling |
| 1 | Very slight scaling (3 mm depth, max, no coarse aggregate visible) |
| 2 | Slight to moderate scaling |
| 3 | Moderate scaling (some coarse aggregate visible) |
| 4 | Moderate to severe scaling |
| 5 | Severe scaling (coarse aggregate visible over the entire surface |
| Mixture | Depth (mm) | Acid Soluble Chloride (% by Mass of Cement) | ACI 222R (% by Mass of Cement)- Table 3.1 Threshold |
|---|---|---|---|
| M1 | 0.0–12.5 | 0.1240 | 0.1000 |
| 12.5–25.0 | 0.1050 | ||
| 25.0–37.5 | 0.0850 | ||
| 37.5–62.5 | 0.0628 | ||
| 62.5–75.0 | 0.0587 | ||
| M4 | 0.0–12.5 | 0.0754 | 0.1000 |
| 12.5–25.0 | 0.0563 | ||
| 25.0–37.5 | 0.0432 | ||
| 37.5–62.5 | 0.0382 | ||
| 62.5–75.0 | 0.0105 | ||
| M5 | 0.0–12.5 | 0.1109 | 0.1000 |
| 12.5–25.0 | 0.1010 | ||
| 25.0–37.5 | 0.0795 | ||
| 37.5–62.5 | 0.0653 | ||
| 62.5–75.0 | 0.0553 | ||
| M6 | 0.0–12.5 | 0.0533 | 0.1000 |
| 12.5–25.0 | 0.0533 | ||
| 25.0–37.5 | 0.0531 | ||
| 37.5–62.5 | 0.0529 | ||
| 62.5–75.0 | 0.0515 | ||
| M7 | 0.0–12.5 | 0.0534 | 0.1000 |
| 12.5–25.0 | 0.0533 | ||
| 25.0–37.5 | 0.0532 | ||
| 37.5–62.5 | 0.0531 | ||
| 62.5–75.0 | 0.0526 | ||
| M8 | 0.0–12.5 | 0.0530 | 0.1000 |
| 12.5–25.0 | 0.0528 | ||
| 25.0–37.5 | 0.0526 | ||
| 37.5–62.5 | 0.0525 | ||
| 62.5–75.0 | 0.0519 |
| Mixture | Deicing Chemical | Average CH (g/100 g Paste) * | Reaction Product | Amount of Reaction Product (g/100 g Paste) * |
|---|---|---|---|---|
| M1 | Salt brine | 19.54 (1.12) | N-A-S-H | 38.65 (4.32) |
| M2 | Salt brine | 15.65 (1.06 | N-A-S-H | 29.65 (4.28) |
| M3 | Freeze Guard + bud MgCl2 | 14.63 (1.23) | M-O-C | 28.65 (3.68) |
| M4 | Freeze Guard + bud MgCl2 | 13.65 (1.11) | M-O-C | 25.06 (4.63) |
| M5 | Salt brine | 13.64 (1.01) | M-O-C | 24.96 (3.87) |
| M6 | Salt brine | 13.12 (0.86) | M-O-C | 14.01 (0.69) |
| M7 | Freeze Guard + bud MgCl2 | 12.98 (0.85) | M-O-C | 13.66 (1.01) |
| M8 | Salt brine | 11.45 (0.78) | N-A-S-H | 12.46 (1.24) |
| Mixture ID | Percentage Mass Loss | Percentage Elastic Modulus Retained |
|---|---|---|
| M1 | 0.38 | 82 |
| M2 | 0.24 | 78 |
| M3 | 0.58 | 80 |
| M4 | 0.45 | 76 |
| M5 | 0.20 | 80 |
| M6 | 0.20 | 85 |
| M7 | 0.15 | 80 |
| M8 | 0.16 | 80 |
| Mixture | Average Resistivity (Kilo-Ohms Cm) | Standard Error | Risk Level |
|---|---|---|---|
| M1 | 17.9 | 0.4 | Moderate risk |
| M2 | 73.2 | 0.5 | Low risk |
| M3 | 64.6 | 1.2 | Low risk |
| M4 | 93.9 | 5.4 | Low risk |
| M5 | 109.8 | 6.9 | Negligible risk |
| M6 | 145.0 | 2.5 | Negligible risk |
| M7 | 163.5 | 4.8 | Negligible risk |
| M8 | 178.0 | 6.5 | Negligible risk |
| Mixture ID | f′c-28 Days (MPa) (Standard Deviation [MPa]) | f′c-548 Days (Control) (MPa) (Standard Deviation [MPa]) | f′c-548 Days (Soaked) (MPa) (Standard Deviation [MPa]) | Percentage Loss (%) |
|---|---|---|---|---|
| M1 | 33.58 (1.10) | 38.50 (0.14) | 34.84 (0.25) | 9.50 |
| M2 | 37.99 (1.65) | 39.54 (0.25) | 34.20 (0.21) | 13.50 |
| M3 | 35.58 (1.79) | 37.68 (0.85) | 37.08 (0.15) | 1.60 |
| M4 | 47.57 (0.90) | 48.65 (1.25) | 37.48 (1.50) | 22.95 |
| M5 | 29.72 (1.03) | 32.35 (0.98) | 25.78 (0.19) | 20.30 |
| M6 | 52.12 (0.97) | 54.32 (1.32) | 49.38 (2.65) | 9.10 |
| M7 | 56.64 (0.97) | 58.23 (0.85) | 50.89 (3.50) | 12.60 |
| M8 | 55.65 (1.62) | 58.36 (0.95) | 53.40 (3.40) | 8.05 |
| Basic Mixture ID | Description | Slump (in) | Air Content (%) | Unit Weight (kg/m3) | SAM Number | Type of Deicer | Visual Rating |
|---|---|---|---|---|---|---|---|
| M1 | Original mix (M1_original) | 1 1/2 | N/A | N/A | N/A | Salt brine | 5.0 (Severe) |
| Ternary mix (M1_20FA + 10SF) | 2 | 3.25 | 2290.64 | 0.43 | Salt brine | 0 (No scaling) | |
| 40% fly ash (M1_40FA) | N/A | 3.5 | 2287.44 | 0.54 | Salt brine | 5.0 (Severe) | |
| M4 | Original mix (M4_original) | 6 ¾ | 5.3 | 2377.14 | 0.47 | Mag Bud Converse | 2.0 and ASR |
| Plain mix (M4_15FA) | 6.5 | 4.3 | 2426.79 | 0.47 | Mag Bud Converse | 4.0 (severe scaling) and ASR | |
| Ternary mix (M4_20FA + 10SF) | 6 ¾ | 5.6 | 2410.77 | 0.25 | Mag Bud Converse | 0 (No scaling) | |
| 40% fly ash (M4_40FA) | N/A | 2 | 2324.28 | 0.4 | Mag Bud Converse | 1.0 (very slight scaling) and ASR | |
| M8 | Plain mix (M8_15FA) | 2 | 5.2 | 2298.64 | 0.27 | Salt brine | 4.0 (Moderate to severe scaling) |
| Ternary mix (M8_20FA + 10SF) | 1 ¾ | 5.2 | 2240.98 | 0.41 | Salt brine | 0 (No Scaling) | |
| 40% fly ash (M8_40FA) | 6 ¾ | 4.9 | 2281.03 | 0.26 | Salt brine | 5.0 (Severe) |
| District | Basic Mixture ID | Description | Resistivity (Kilo-Ohms-Cm) | Standard Error | Remarks (Chloride Penetrability) |
|---|---|---|---|---|---|
| 1 | (M1) | Original mix (M1_original) | 17.9 | 0.4 | Moderate risk |
| Ternary mix (M1_ternary) | 145.0 | 2.5 | Negligible | ||
| 40% fly ash (M1_40FA) | 63.0 | 1.5 | Low risk | ||
| 2 | (M4) | Original mix (M4_original) | 93.9 | 5.4 | Low risk |
| Plain mix (M4_plain) | 45.8 | 4.3 | Moderate risk | ||
| Ternary mix (M4_ternary) | 163.5 | 4.8 | Negligible | ||
| 40% fly ash (M4_40FA) | 65.4 | 2.6 | Low risk | ||
| 6 | (M8) | Original mix (M8_original) | 109.8 | 6.9 | Negligible |
| Plain mix (M8_plain) | 58.7 | 1.5 | Low risk | ||
| Ternary mix (M8_ternary) | 178 | 6.5 | Negligible | ||
| 40% fly ash (M8_40FA) | 79 | 2.7 | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arowojolu, O.S.; Rangelov, M.; Nassiri, S.; Bayomy, F.; Ibrahim, A. Concrete Durability Performance in Aggressive Salt and Deicing Environments—Case Study of Select Pavement and Bridge Concrete Mixtures. Materials 2025, 18, 1266. https://doi.org/10.3390/ma18061266
Arowojolu OS, Rangelov M, Nassiri S, Bayomy F, Ibrahim A. Concrete Durability Performance in Aggressive Salt and Deicing Environments—Case Study of Select Pavement and Bridge Concrete Mixtures. Materials. 2025; 18(6):1266. https://doi.org/10.3390/ma18061266
Chicago/Turabian StyleArowojolu, Olaniyi S., Milena Rangelov, Somayeh Nassiri, Fouad Bayomy, and Ahmed Ibrahim. 2025. "Concrete Durability Performance in Aggressive Salt and Deicing Environments—Case Study of Select Pavement and Bridge Concrete Mixtures" Materials 18, no. 6: 1266. https://doi.org/10.3390/ma18061266
APA StyleArowojolu, O. S., Rangelov, M., Nassiri, S., Bayomy, F., & Ibrahim, A. (2025). Concrete Durability Performance in Aggressive Salt and Deicing Environments—Case Study of Select Pavement and Bridge Concrete Mixtures. Materials, 18(6), 1266. https://doi.org/10.3390/ma18061266








