Phase-Controlled Synthesis of Ru Supported on Carbon Nitride and the Application in Photocatalytic H2 Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of C3N4 Nanosheets
2.2.2. Synthesis of C3N4-Ru Samples
2.3. Photocatalytic Hydrogen Activity Measurement
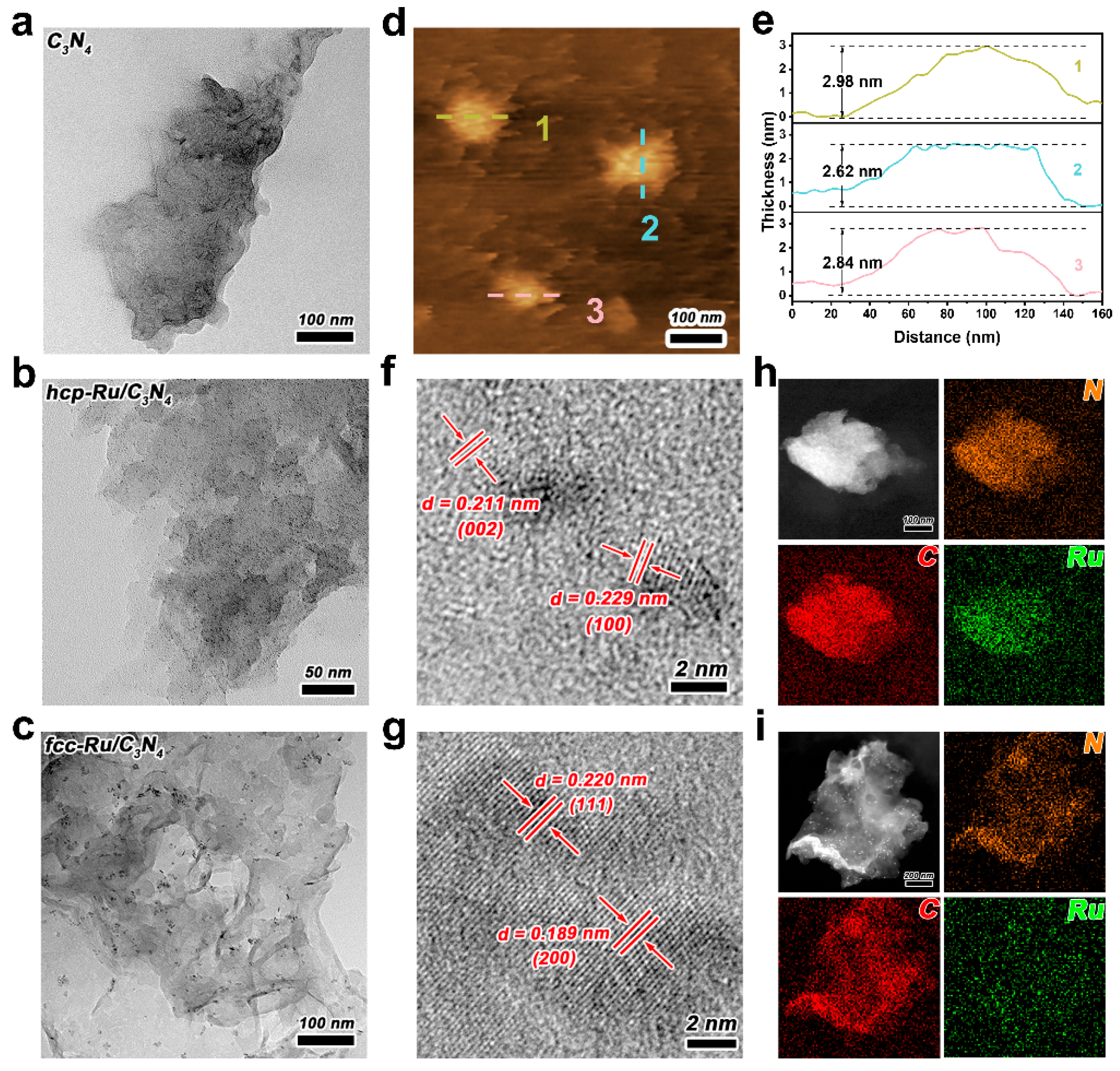
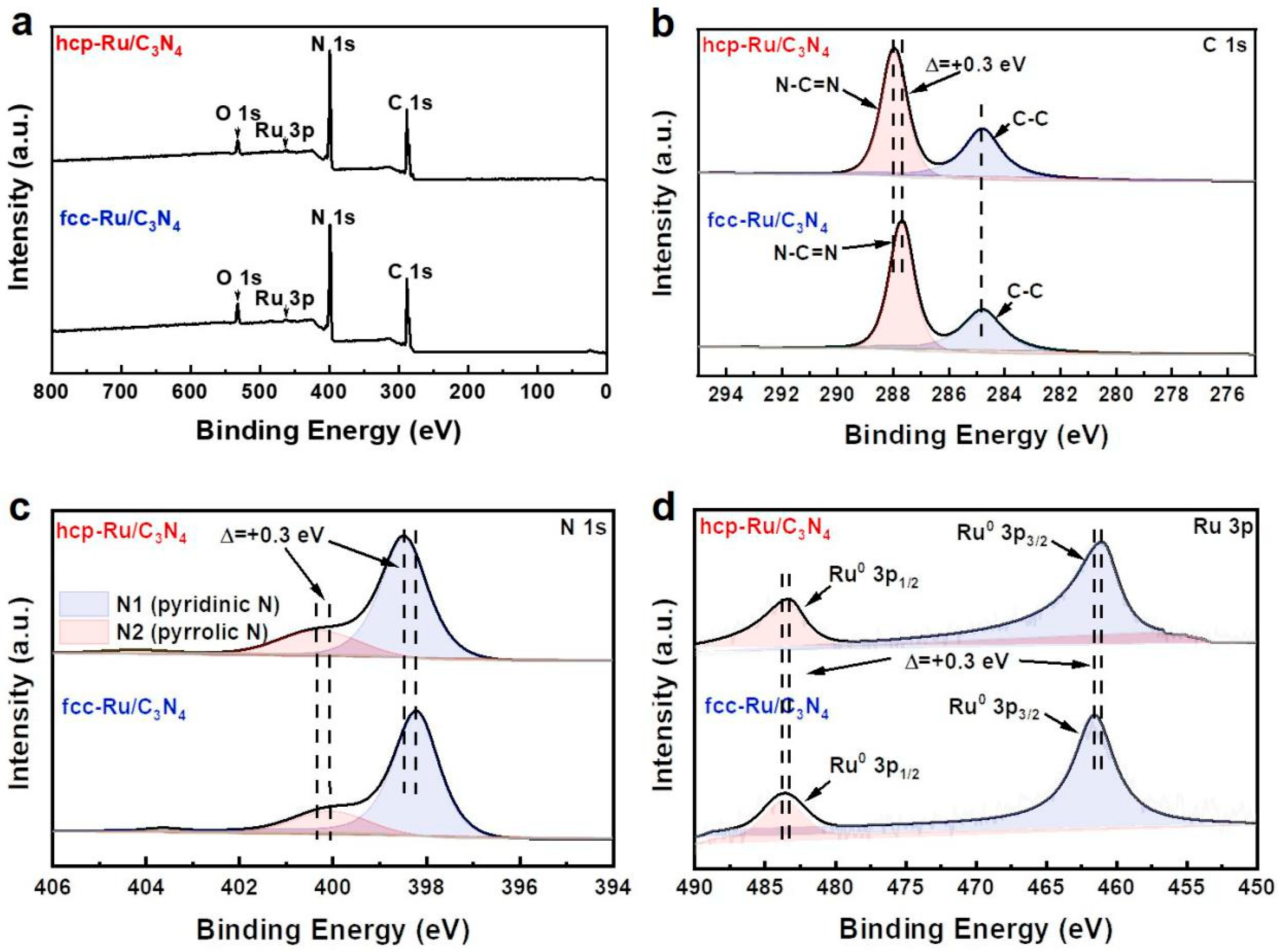
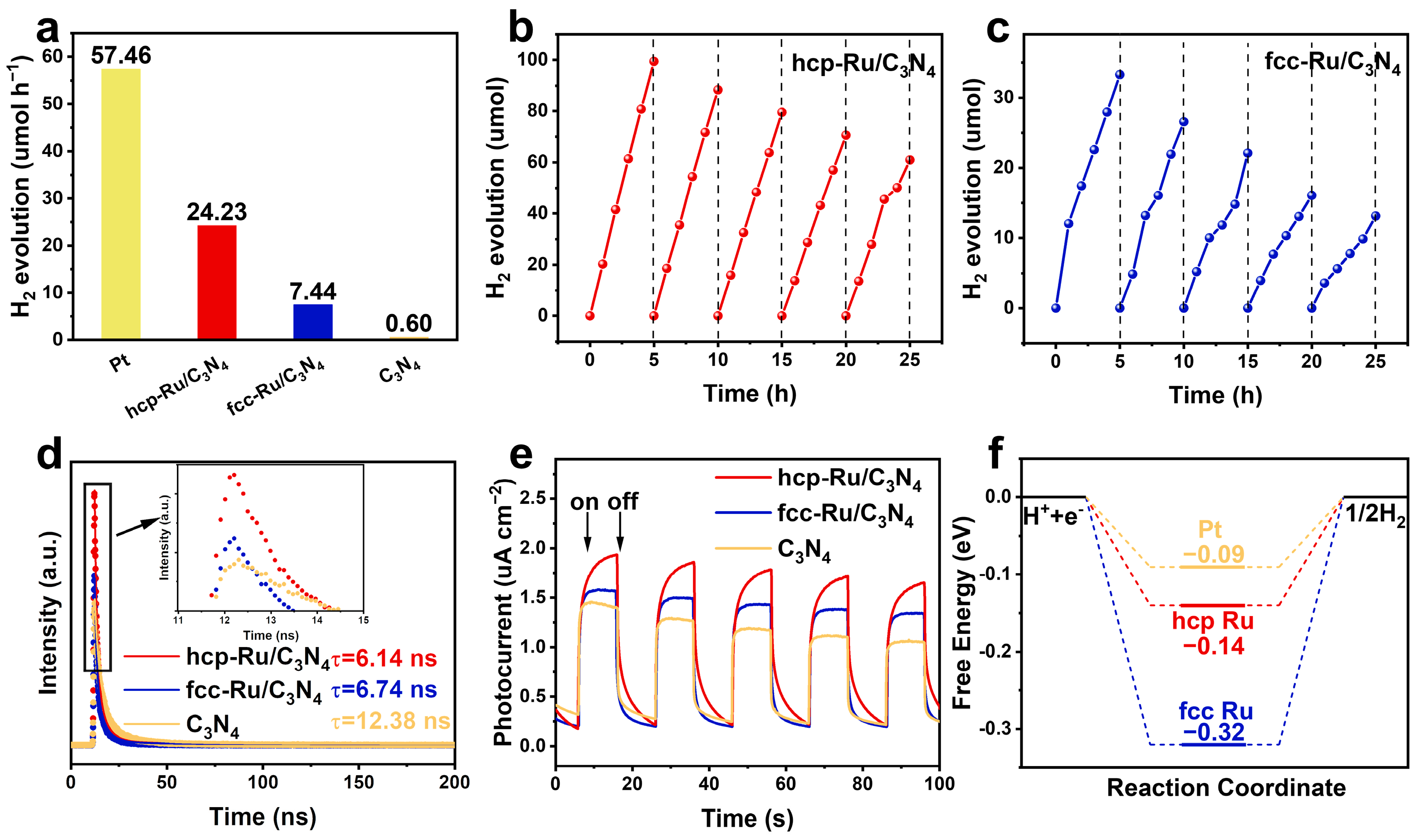
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben-Shahar, Y.; Scotognella, F.; Kriegel, I.; Moretti, L.; Cerullo, G.; Rabani, E.; Banin, U. Optimal metal domain size for photocatalysis with hybrid semiconductor-metal nanorods. Nat. Commun. 2016, 7, 10413. [Google Scholar] [CrossRef] [PubMed]
- Ham, R.; Nielsen, C.J.; Pullen, S.; Reek, J.N.H. Supramolecular Coordination Cages for Artificial Photosynthesis and Synthetic Photocatalysis. Chem. Rev. 2023, 123, 5225–5261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, C.; Fang, J. Noble-Metal Based Random Alloy and Intermetallic Nanocrystals: Syntheses and Applications. Chem. Rev. 2021, 121, 736–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Wang, Y.; Xin, C.; Zhang, P.; Liu, D.; Mamba, B.B.; Kefeni, K.K.; Kuvarega, A.T.; Gui, J. Hollow β-Bi2O3@CeO2 heterostructure microsphere with controllable crystal phase for efficient photocatalysis. Chem. Eng. J. 2020, 387, 124100. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Wang, C.; Chen, F.; Ma, T.; Huang, H. Defects in photoreduction reactions: Fundamentals, classification, and catalytic energy conversion. eScience 2024, 4, 100228. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, Y. Crystal-phase and surface-structure engineering of ruthenium nanocrystals. Nat. Rev. Mater. 2020, 5, 440–459. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, S.; Li, Z.; Wu, Y. Atomic level engineering of noble metal nanocrystals for energy conversion catalysis. J. Energy Chem. 2021, 63, 604–624. [Google Scholar] [CrossRef]
- Zhai, W.; Li, Z.; Wang, Y.; Zhai, L.; Yao, Y.; Li, S.; Wang, L.; Yang, H.; Chi, B.; Liang, J.; et al. Phase Engineering of Nanomaterials: Transition Metal Dichalcogenides. Chem. Rev. 2024, 124, 4479–4539. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, X.; Zhou, M.; Zhang, S.; Li, L.; Song, Y.; Chen, H.; Chen, Z.; Li, H.; Xu, H. Crystal phase dependent solar driven hydrogen evolution catalysis over cobalt diselenide. Chem. Eng. J. 2020, 396, 125244. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Zhai, W.; Lu, S.; Liang, J.; He, Z.; Long, H.; Xiong, T.; Sun, H.; He, Q.; et al. Phase Engineering of Nanomaterials for Clean Energy and Catalytic Applications. Adv. Energy Mater. 2020, 10, 2002019. [Google Scholar] [CrossRef]
- Yun, Q.; Ge, Y.; Huang, B.; Wa, Q.; Zhang, H. Ligand-Assisted Phase Engineering of Nanomaterials. ACC Chem. Res. 2023, 56, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal Phase and Architecture Engineering of Lotus-Thalamus-Shaped Pt-Ni Anisotropic Superstructures for Highly Efficient Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, e1801741. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, W.; Huang, Y.; Huang, Q.; Li, J.; Zhao, S.; Tian, S. Unconventional Phase Synergies with Doping Engineering over Ni Electrocatalyst Featuring Regulated Electronic State for Accelerated Urea Oxidation. ChemSusChem 2023, 16, e202201921. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, G.; Cao, X.; Zhu, X.; Li, L.; Wang, X.; Zhu, X.; Song, Y.; Xu, H.; Wang, X. Structurally disordered MoSe2 with rich 1T phase as a universal platform for enhanced photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 668, 492–501. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Saruyama, M.; Takahata, R.; Sato, R.; Kitahama, Y.; Matsuzaki, H.; Yamada, T.; Hisatomi, T.; Domen, K.; Teranishi, T. Bimetallic Synergy in Ultrafine Cocatalyst Alloy Nanoparticles for Efficient Photocatalytic Water Splitting. Adv. Funct. Mater. 2022, 32, 2202987. [Google Scholar] [CrossRef]
- She, P.; Qin, J.S.; Sheng, J.; Qi, Y.; Rui, H.; Zhang, W.; Ge, X.; Lu, G.; Song, X.; Rao, H. Dual-Functional Photocatalysis for Cooperative Hydrogen Evolution and Benzylamine Oxidation Coupling over Sandwiched-Like Pd@TiO2@ZnIn2S4 Nanobox. Small 2022, 18, 2105114. [Google Scholar] [CrossRef]
- Li, R.; Wu, D.; Rao, P.; Deng, P.; Li, J.; Luo, J.; Huang, W.; Chen, Q.; Kang, Z.; Shen, Y.; et al. General approach for atomically dispersed precious metal catalysts toward hydrogen reaction. Carbon Energy 2023, 5, e294. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Ye, B.; Ma, H.; Wang, C.; Zhuang, T.; Lv, Z. Droplet microreactor continuous synthesis of hierarchical Rh on CdZnS snowflakes for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2024, 499, 156123. [Google Scholar] [CrossRef]
- Su, K.; Wang, Y.; Zhang, C.; Gao, Z.; Han, J.; Wang, F. Tuning the Pt species on Nb2O5 by support-induced modification in the photocatalytic transfer hydrogenation of phenylacetylene. Appl. Catal. B Environ. Energy 2021, 298, 120554. [Google Scholar] [CrossRef]
- Ding, Z.; Li, X.; Kang, C.; Yan, S.; Zhao, D.; Cai, H.; Zhang, S.-Y.; Zeng, Y.-J. Single Ru atoms confined into MOF/C3N4 for dual improved photocatalytic carbon dioxide reduction and nitrogen fixation. Chem. Eng. J. 2023, 473, 145256. [Google Scholar] [CrossRef]
- Saito, D.; Yamazaki, Y.; Tamaki, Y.; Ishitani, O. Photocatalysis of a Dinuclear Ru(II)-Re(I) Complex for CO2 Reduction on a Solid Surface. J. Am. Chem. Soc. 2020, 142, 19249–19258. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Evans, J.D.; Andersson, G.G.; Metha, G.F.; Shearer, C.J. Ultrathin Ru-CdIn2S4 nanosheets for simultaneous photocatalytic green hydrogen production and selective oxidation of furfuryl alcohol to furfural. Chem. Eng. J. 2024, 493, 152603. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.; Hou, L.; Kim, M.G.; Jang, H.; Liu, S.; Liu, X. Revealing The Role of Electronic Asymmetricity on Supported Ru Nanoclusters for Alkaline Hydrogen Evolution Reaction. Adv. Funct. Mater. 2023, 34, 2314899. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, W.; Hu, Z.; Ng, Y.H.; Wei, Z.; Liu, Y.; Deng, J.; Dai, H.; Jing, L. Advancing CO2 to CH4 conversion: The pivotal role of RuCu alloy in crystalline red phosphorus photocatalysis. Appl. Catal. B Environ. Energy 2024, 357, 124347. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Kim, E.M.; Ding, Y.; Janssen, A.; Wang, C.; Li, K.K.; Kim, J.; Fichthorn, K.A.; Xia, Y. Elucidating the Role of Reduction Kinetics in the Phase-Controlled Growth on Preformed Nanocrystal Seeds: A Case Study of Ru. J. Am. Chem. Soc. 2024, 146, 12040–12052. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, Y.-H.; Tsao, C.-Y.; Wu, C.-Y.; Hsieh, C.-J.; Chen, M.-Z.; Chang, C.-W.; Hsiao, Y.-C.; Chen, H.-L.; Yang, T.-H. Toward a Quantitative Understanding of Crystal-Phase Engineering of Ru Nanocrystals. Chem. Mater. 2023, 35, 4276–4285. [Google Scholar] [CrossRef]
- Fan, Z.; Guo, X.; Yang, M.; Jin, Z. Mechanochemical preparation and application of graphdiyne coupled with CdSe nanoparticles for efficient photocatalytic hydrogen production. Chin. J. Catal. 2022, 43, 2708–2719. [Google Scholar] [CrossRef]
- Yu, B.; Li, H.; White, J.; Donne, S.; Yi, J.B.; Xi, S.B.; Fu, Y.; Henkelman, G.; Yu, H.; Chen, Z.L.; et al. Tuning the Catalytic Preference of Ruthenium Catalysts for Nitrogen Reduction by Atomic Dispersion. Adv. Funct. Mater. 2020, 30, 1905665. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, L.; Chen, D.; Hao, Z.; Deng, B.; Sun, Y.; Liu, X.; Jia, B.; Chen, L.; Liu, H. Twin S-Scheme g-C3N4/CuFe2O4/ZnIn2S4 Heterojunction with a Self-Supporting Three-Phase System for Photocatalytic CO2 Reduction: Mechanism Insight and DFT Calculations. ACS Catal. 2024, 14, 5326–5343. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, L.; Xia, Y.; Yin, W.; Zhu, X.; Hou, J.; Wang, S.; Ning, X.; Wang, X. Remarkably enhanced hydrogen evolution of g-C3N4 nanosheet under simulated sunlight via AgPt alloy co-catalyst with low amount of Pt. J. Clean. Prod. 2024, 434, 139950. [Google Scholar] [CrossRef]
- Chen, Z.; Bu, Y.; Wang, L.; Wang, X.; Ao, J.-P. Single-sites Rh-phosphide modified carbon nitride photocatalyst for boosting hydrogen evolution under visible light. Appl. Catal. B Environ. Energy 2020, 274, 119117. [Google Scholar] [CrossRef]
- Kusada, K.; Kobayashi, H.; Yamamoto, T.; Matsumura, S.; Sumi, N.; Sato, K.; Nagaoka, K.; Kubota, Y.; Kitagawa, H. Discovery of Face-Centered-Cubic Ruthenium Nanoparticles: Facile Size-Controlled Synthesis Using the Chemical Reduction Method. J. Am. Chem. Soc. 2013, 135, 5493–5496. [Google Scholar] [CrossRef]
- Saruyama, M.; Pelicano, C.M.; Teranishi, T. Bridging electrocatalyst and cocatalyst studies for solar hydrogen production via water splitting. Chem. Sci. 2022, 13, 2824–2840. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Tong, H. Recent advances in cocatalyst engineering for solar-driven overall water splitting. Appl. Res. 2023, 3, e202300080. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, J.; Liu, M.; Shi, L.; Chen, M.; Wu, L. High-Density Atomically Dispersed Metals Activate Adjacent Nitrogen/Carbon Sites for Efficient Ammonia Electrosynthesis from Nitrate. ACS Nano 2024, 18, 26722–26732. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Chen, Q.; Ming, M.; Wang, Y.; Sun, T.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Nitrogen-Doped Carbon-Stabilized Ru Nanoclusters as Excellent Catalysts for Hydrogen Production. ACS Sustain. Chem. Eng. 2019, 7, 1178–1184. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Wang, G.C.; Liu, M.; Zhang, C.; Liu, S. Ultrafine Nanoclusters Unlocked 3d-4f Electronic Ladders for Efficient Electrocatalytic Water Oxidation. ACS Nano 2024, 18, 20518–20529. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Emrick, T.; Russell, T.P. Polymer design to promote low work function surfaces in organic electronics. Prog. Polym. Sci. 2020, 103, 101222. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Guan, Q.; Ran, W.; Zhang, D.; Li, W.; Li, N.; Huang, B.; Yan, T. Non-Metal Sulfur Doping of Indium Hydroxide Nanocube for Selectively Photocatalytic Reduction of CO2 to CH4: A “One Stone Three Birds” Strategy. Adv. Sci. 2024, 11, 2401990. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Fei, T.; Li, L.; Yu, Q.; Zhang, S.; Song, Y.; Lian, J.; Zhu, X.; Deng, J.; Xu, H.; et al. Large-scale production of ultrathin carbon nitride-based photocatalysts for high-yield hydrogen evolution. Appl. Catal. B Environ. Energy 2021, 281, 119475. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Hu, L.; Zhou, G.; Xia, Y.; Hu, Q.; Yin, W.; Zhu, X.; Yi, J.; Wang, X. Phase Control of Cobalt Selenide: Unraveling the Relationship Between Phase Property and Hydrogen Evolution Catalysis. Adv. Mater. Interfaces 2022, 9, 2201473. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, W.; Zhou, G.; Zhang, S.; Zhu, X.; Li, L.; Zhu, X.; Wang, X.; Han, X.; Yi, J. MOF-derived phase-selective synthesis of ln2O3 with appropriate surface atomic arrangement for CO2 photoreduction. Chem. Eng. J. 2024, 501, 157513. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Li, A.; Si, Z.; Wu, X.; Ran, R.; Weng, D. A strategy to construct (reduced graphene oxide, γ-Fe2O3)/C3N4 step-scheme photocatalyst for visible-light water splitting. Catal. Commun. 2021, 157, 106327. [Google Scholar] [CrossRef]
- Shen, J.; Luo, C.; Qiao, S.; Chen, Y.; Tang, Y.; Xu, J.; Fu, K.; Yuan, D.; Tang, H.; Zhang, H.; et al. Single-Atom Cu Channel and N-Vacancy Engineering Enables Efficient Charge Separation and Transfer between C3N4 Interlayers for Boosting Photocatalytic Hydrogen Production. ACS Catal. 2023, 13, 6280–6288. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.; Ci, Q.; Du, L.; Ren, X.; Phillips, D.L. Near-Field Drives Long-Lived Shallow Trapping of Polymeric C3N4 for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 2103978. [Google Scholar] [CrossRef]
- Xing, F.; Wang, C.; Liu, S.; Jin, S.; Jin, H.; Li, J. Interfacial Chemical Bond Engineering in a Direct Z-Scheme g-C3N4/MoS2 Heterojunction. ACS Appl. Mater. Interfaces 2023, 15, 11731–117340. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Zhao, X.; Zhang, J.; Ao, Z.; Liu, W.; Wu, H.; Shi, L.; Yin, Y.; Xu, X.; et al. Efficient photocatalytic overall water splitting on metal-free 1D SWCNT/2D ultrathin C3N4 heterojunctions via novel non-resonant plasmonic effect. Appl. Catal. B Environ. Energy 2020, 278, 119312. [Google Scholar] [CrossRef]
- Shao, M.; Chen, W.; Ding, S.; Lo, K.H.; Zhong, X.; Yao, L.; Ip, W.F.; Xu, B.; Wang, X.; Pan, H. WXy/g-C3N4 (WXy = W2C, WS2, or W2N) Composites for Highly Efficient Photocatalytic Water Splitting. ChemSusChem 2019, 12, 3355–3362. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Alraeesi, A.; Kumar, N.; Al-Marzouqi, M. Synergistic effect of bimetallic RuCo loaded N-defective g-C3N4 nanosheets with cleavage of metal-hydrogen bonds for H2 production in a continuous flow photoreactor. Int. J. Hydrogen Energy 2024, 95, 402–416. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; Peng, L.; Luo, J.; Ning, X.; Fan, X.; Zhou, X.; Zhou, X. Pd(II) coordination molecule modified g-C3N4 for boosting photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 671, 134–144. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Cao, X.; Zhou, G.; Lv, T.; Xu, J.; Zhou, Y.; Wang, Z.; Yi, J. Phase-Controlled Synthesis of Ru Supported on Carbon Nitride and the Application in Photocatalytic H2 Evolution. Materials 2025, 18, 1259. https://doi.org/10.3390/ma18061259
Sun X, Cao X, Zhou G, Lv T, Xu J, Zhou Y, Wang Z, Yi J. Phase-Controlled Synthesis of Ru Supported on Carbon Nitride and the Application in Photocatalytic H2 Evolution. Materials. 2025; 18(6):1259. https://doi.org/10.3390/ma18061259
Chicago/Turabian StyleSun, Xiaohu, Xiangyang Cao, Ganghua Zhou, Tiaolong Lv, Jian Xu, Yubo Zhou, Zhigang Wang, and Jianjian Yi. 2025. "Phase-Controlled Synthesis of Ru Supported on Carbon Nitride and the Application in Photocatalytic H2 Evolution" Materials 18, no. 6: 1259. https://doi.org/10.3390/ma18061259
APA StyleSun, X., Cao, X., Zhou, G., Lv, T., Xu, J., Zhou, Y., Wang, Z., & Yi, J. (2025). Phase-Controlled Synthesis of Ru Supported on Carbon Nitride and the Application in Photocatalytic H2 Evolution. Materials, 18(6), 1259. https://doi.org/10.3390/ma18061259






