Mucorales Species and Macrophages
Abstract
1. Introduction.
2. Mucoralean Species Involved in Mucormycosis
3. Host Defense against Mucorales
3.1. Epithelial Cells
3.2. Phagocytes
3.3. Natural Killer Cells
3.4. Platelets
3.5. Dendritic and Lymphocyte T Cells
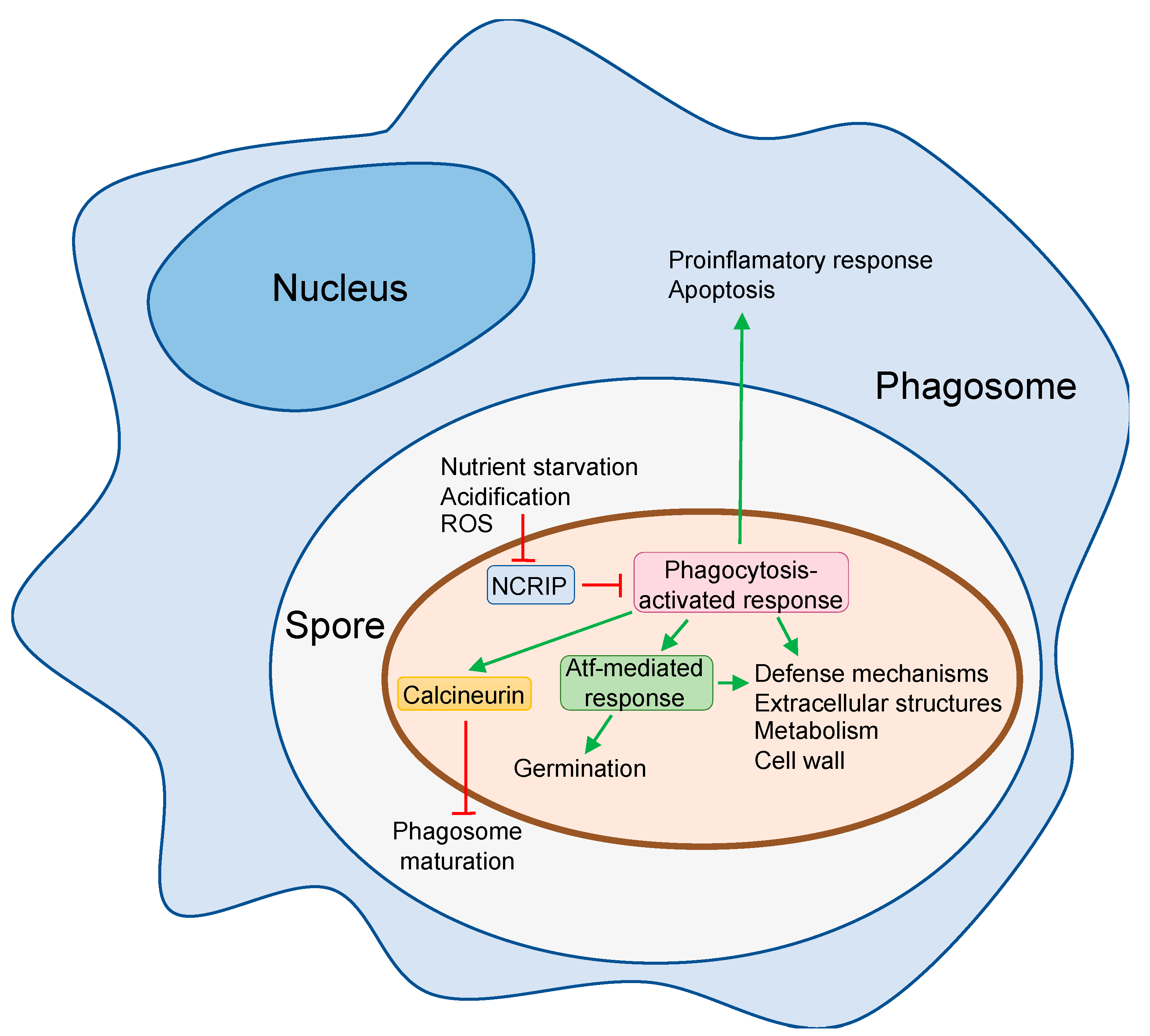
4. The Responses of Mucorales Spores to Phagocytosis
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef]
- Casadevall, A. Fungi and the rise of mammals. PLoS Pathog. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Greene, J.; Pak, J.; Tucci, V.; Vincent, A.; Sandin, R. Mucormycosis in immunochallenged patients. J. Emergencies Trauma Shock 2008, 1, 106. [Google Scholar] [CrossRef]
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef] [PubMed]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-Tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Calo, S.; Nicolás, F.E.; Lee, S.C.; Vila, A.; Cervantes, M.; Torres-Martinez, S.; Ruiz-Vazquez, R.M.; Cardenas, M.E.; Heitman, J. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Moxon, S.; de Haro, J.P.; Calo, S.; Grigoriev, I.V.; Torres-Martínez, S.; Moulton, V.; Ruiz-Vázquez, R.M.; Dalmay, T. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010, 38, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for genera: Basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers. 2018, 92, 43–129. [Google Scholar] [CrossRef]
- Wagner, L.; de Hoog, S.; Alastruey-Izquierdo, A.; Voigt, K.; Kurzai, O.; Walther, G. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob. Agents Chemother. 2019, 63, 1–8. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Outbreaks of mucorales and the species involved. Mycopathologia 2019. [Google Scholar] [CrossRef] [PubMed]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Al-Ajam, M.R.; Bizri, A.R.; Mokhbat, J.; Weedon, J.; Lutwick, L. Mucormycosis in the Eastern Mediterranean: A seasonal disease. Epidemiol. Infect. 2006, 134, 341–346. [Google Scholar] [CrossRef]
- Hoffmann, K.; Discher, S.; Voigt, K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 2007, 111, 1169–1183. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef]
- Schipper, M.A.A. On certain species of Mucor with a key to all accepted species. Stud. Mycol. 1978, 17, 1–52. [Google Scholar]
- Vellanki, S.; Navarro-Mendoza, M.I.; Garcia, A.B.; Murcia, L.; Perez-Arques, C.; Garre, V.; Nicolas, F.E.; Lee, S.C. Mucor circinelloides: Growth, maintenance and genetic manipulation. Curr. Protoc. Microbiol. 2018, 49, e53. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Ghuman, H.; Voelz, K. Innate and adaptive immunity to Mucorales. J. Fungi 2017, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Voelz, K. The mucormycete–host interface. Curr. Opin. Microbiol. 2017, 40, 40–45. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Oumeziane, N.A.; Lissitzky, J.C.; Larcher, G.; Tronchin, G.; Chabasse, D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur. J. Cell Biol. 1996, 70, 76–83. [Google Scholar]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 12218. [Google Scholar] [CrossRef]
- Watkins, T.N.; Gebremariam, T.; Swidergall, M.; Shetty, A.C.; Graf, K.T.; Alqarihi, A.; Alkhazraji, S.; Alsaadi, A.I.; Edwards, V.L.; Filler, S.G.; et al. Inhibition of EGFR signaling protects from mucormycosis. MBio 2018, 9, e01384-18. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- López-Muñoz, A.; Nicolás, F.E.; García-Moreno, D.; Pérez-Oliva, A.B.; Navarro-Mendoza, M.I.; Hernández-Oñate, M.A.; Herrera-Estrella, A.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V.; et al. An adult zebrafish model reveals that mucormycosis induces apoptosis of infected macrophages. Sci. Rep. 2018, 8, 12802. [Google Scholar] [CrossRef] [PubMed]
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018, 9, 3333. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Kraibooj, K.; Park, H.R.; Dahse, H.M.; Skerka, C.; Voigt, K.; Figge, M.T. Virulent strain of Lichtheimia corymbifera shows increased phagocytosis by macrophages as revealed by automated microscopy image analysis. Mycoses 2014, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D.; Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 1986, 154, 483–489. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Invest. 1984, 74, 150–160. [Google Scholar] [CrossRef]
- Jorens, P.G.; Boelaert, J.R.; Halloy, V.; Zamora, R.; Schneider, Y.J.; Herman, A.G. Human and rat macrophages mediate fungistatic activity against Rhizopus species differently: In vitro and ex vivo studies. Infect. Immun. 1995, 63, 4489–4494. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef]
- Trieu, T.A.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Sanchis, M.; Capilla, J.; Navarro-Rodriguez, P.; Lopez-Fernandez, L.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M.; et al. RNAi-based functional genomics identifies new virulence determinants in mucormycosis. PLoS Pathog. 2017, 13, e1006150. [Google Scholar] [CrossRef]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. Br. J. Haematol. 1997, 99, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Diamond, R.D. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect.Immun. 1985, 48, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. North. Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis - from the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef]
- Inglesfield, S.; Jasiulewicz, A.; Hopwood, M.; Tyrrell, J.; Youlden, G.; Mazon-Moya, M.; Millington, O.R.; Mostowy, S.; Jabbari, S.; Voelz, K. Robust phagocyte recruitment controls the opportunistic fungal pathogen Mucor circinelloides in innate granulomas in vivo. MBio 2018, 9, e02010-17. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Tramsen, L.; Perkhofer, S.; Lass-Flörl, C.; Hanisch, M.; Röger, F.; Klingebiel, T.; Koehl, U.; Lehrnbecher, T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 2013, 218, 939–944. [Google Scholar] [CrossRef]
- Speth, C.; Rambach, G.; Lass-Flörl, C. Platelet immunology in fungal infections. Thromb. Haemost. 2014, 112, 632–639. [Google Scholar] [CrossRef]
- Schmidt, S.; Schneider, A.; Demir, A.; Lass-Flörl, C.; Lehrnbecher, T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses 2016, 59, 34–38. [Google Scholar] [CrossRef]
- Perkhofer, S.; Kainzner, B.; Kehrel, B.E.; Dierich, M.P.; Nussbaumer, W.; Lass-Flörl, C. Potential antifungal effects of human platelets against zygomycetes in vitro. J. Infect. Dis. 2009, 200, 1176–1179. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Forghieri, F.; Zanetti, E.; Quadrelli, C.; Candoni, A.; Maertens, J.; Rossi, G.; et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood 2011, 118, 5416–5419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Georgiadou, S.P.; Sampsonas, F.; Chamilos, G.; Kontoyiannis, D.P. Risk factors for early mortality in haematological malignancy patients with pulmonary mucormycosis. Mycoses 2014, 57, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: A propensity score analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Hejazi, A.S.; Wei, S.H.; Cahalan, M.D.; Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA 2004, 101, 998–1003. [Google Scholar] [CrossRef]
- Chamilos, G.; Ganguly, D.; Lande, R.; Gregorio, J.; Meller, S.; Goldman, W.E.; Gilliet, M.; Kontoyiannis, D.P. Generation of IL-23 producing Dendritic Cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of TH-17 responses. PLoS ONE 2010, 5, e12955. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Martínez-García, P.; Heitman, J.; Nicolás, F.E.; Garre, V. Mucor circinelloides thrives inside the phagosome through an Atf-mediated germination pathway. MBio 2019, 10, e02765-18. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. A non-canonical RNAi pathway controls virulence and genome stability in Mucorales. PLoS Genet. 2020. [Google Scholar] [CrossRef]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kyrmizi, I.; Gresnigt, M.S.; Akoumianaki, T.; Samonis, G.; Sidiropoulos, P.; Boumpas, D.; Netea, M.G.; van de Veerdonk, F.L.; Kontoyiannis, D.P.; Chamilos, G. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting Dectin-1/Syk kinase signaling. J. Immunol. 2013, 191, 1287–1299. [Google Scholar] [CrossRef]
- Vellanki, S.; Billmyre, R.B.; Lorenzen, A.; Campbell, M.; Turner, B.; Huh, E.Y.; Heitman, J.; Lee, S.C. A novel resistance pathway for calcineurin inhibitors in the human-pathogenic mucorales Mucor circinelloides. MBio 2020, 11, e02949-19. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Murcia, L.; Martínez-García, P.; Lax, C.; Sanchis, M.; Capilla, J.; Nicolás, F.E.; Garre, V. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci. Rep. 2018, 8, 7660. [Google Scholar] [CrossRef]
- Shiozaki, K.; Russell, P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996, 10, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. MBio 2019, 10, e02579-19. [Google Scholar] [CrossRef]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M. A non-canonical RNA silencing pathway promotes mRNA degradation in basal fungi. PLoS Genet. 2015, 11, 1005168. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Díaz-Pérez, A.L.; Martínez-Cadena, G.; Campos-García, J.; Ramírez-Díaz, M.I.; Reyes-De la Cruz, H.; Riveros-Rosas, H.; Díaz-Pérez, C.; Meza-Carmen, V. Phylogenetic analysis of fungal heterotrimeric G protein-encoding genes and their expression during dimorphism in Mucor circinelloides. Fungal Biol. 2015, 119, 1179–1193. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Reyes-Mares, N.Y.; Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Pérez-Arques, C.; Nuñez-Anita, R.E.; Campos-García, J.; Anaya-Martínez, V.; Ortiz-Alvarado, R.; Ramírez-Díaz, M.I.; et al. Heterotrimeric G-alpha subunits Gpa11 and Gpa12 define a transduction pathway that control spore size and virulence in Mucor circinelloides. PLoS ONE 2019, 14, e0226682. [Google Scholar] [CrossRef]
- Patiño-Medina, J.A.; Vargas-Tejeda, D.; Valle-Maldonado, M.I.; Alejandre-Castañeda, V.; Jácome-Galarza, I.E.; Villegas-Moreno, J.; Nuñez-Anita, R.E.; Ramírez-Díaz, M.I.; Ortiz-Alvarado, R.; Meza-Carmen, V. Sporulation on blood serum increases the virulence of Mucor circinelloides. Microb. Pathog. 2019, 137, 103737. [Google Scholar] [CrossRef]
| Host Cell | Mucoralean Species | Interactions | References |
|---|---|---|---|
| Alveolar macrophages and BMDMs | R. oryzae R. delemar | Macrophages fail to kill resting spores, but they inhibit spore germination via iron starvation. Spores block phagosome maturation via cell wall melanin. | [32,35,36,37] |
| Murine cell line and BMDMs | M. circinelloides | Macrophages fail to kill resting spore and do not inhibit spore germination. Spores block phagosome maturation via calcineurin signal pathway. Spores kill macrophages. | [38,39,40,60] |
| Murine alveolar cell line | L. corymbifera | A virulent strain shows increased phagocytosis | [34] |
| Zebrafish macrophages | M. circinelloides | Formation of early granulomas in vivo. Induction of macrophages apoptosis in vivo. | [31,33,46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolás, F.E.; Murcia, L.; Navarro, E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Garre, V. Mucorales Species and Macrophages. J. Fungi 2020, 6, 94. https://doi.org/10.3390/jof6020094
Nicolás FE, Murcia L, Navarro E, Navarro-Mendoza MI, Pérez-Arques C, Garre V. Mucorales Species and Macrophages. Journal of Fungi. 2020; 6(2):94. https://doi.org/10.3390/jof6020094
Chicago/Turabian StyleNicolás, Francisco E., Laura Murcia, Eusebio Navarro, María Isabel Navarro-Mendoza, Carlos Pérez-Arques, and Victoriano Garre. 2020. "Mucorales Species and Macrophages" Journal of Fungi 6, no. 2: 94. https://doi.org/10.3390/jof6020094
APA StyleNicolás, F. E., Murcia, L., Navarro, E., Navarro-Mendoza, M. I., Pérez-Arques, C., & Garre, V. (2020). Mucorales Species and Macrophages. Journal of Fungi, 6(2), 94. https://doi.org/10.3390/jof6020094







