Regulation of Actin Dynamics in the C. elegans Somatic Gonad
Abstract
1. Introduction
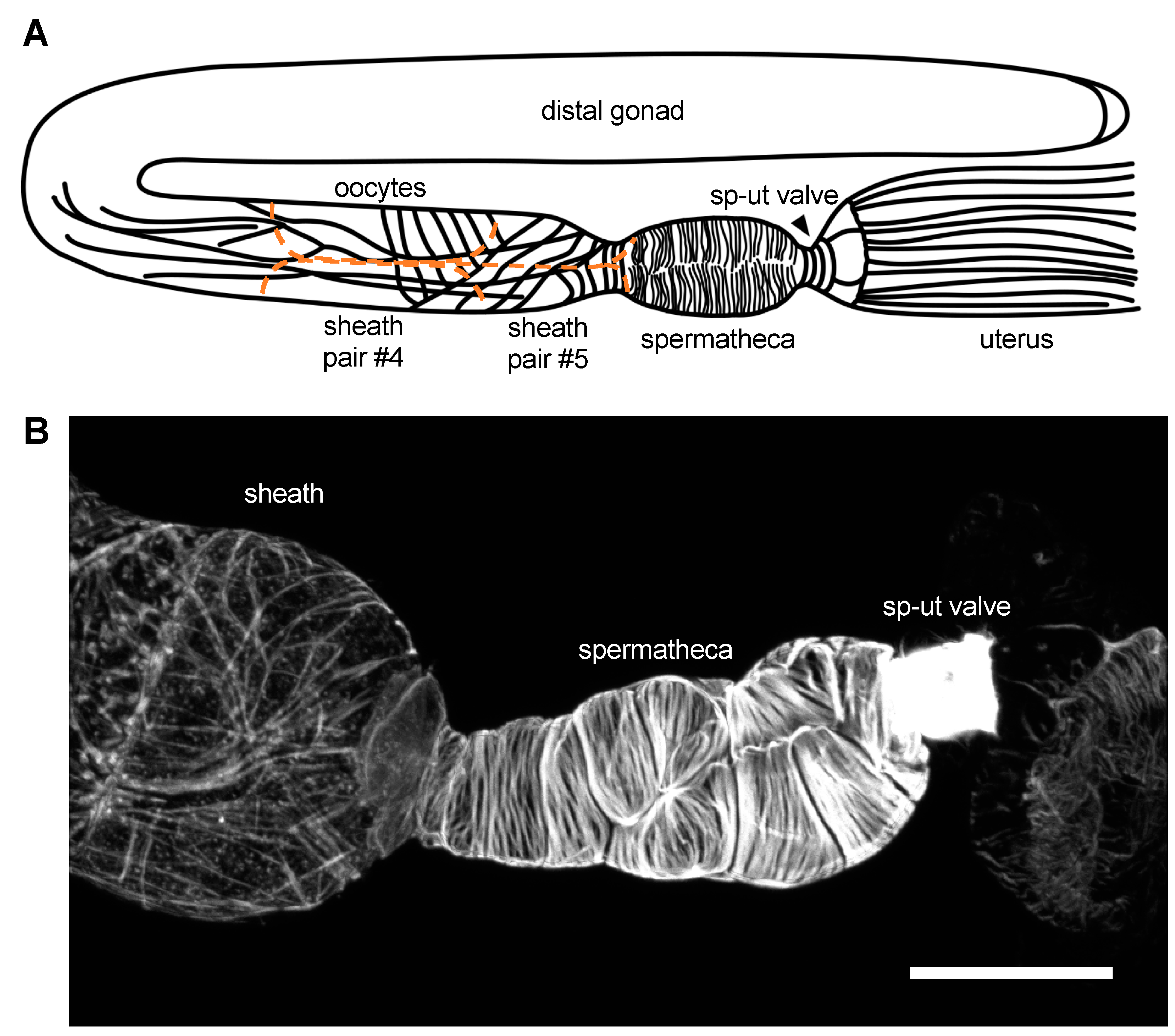
2. The Gonadal Sheath Cells
3. The Spermatheca
4. The SP–UT Valve
5. Shared Features, Unanswered Questions, and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Plastino, J.; Blanchoin, L. Dynamic stability of the actin ecosystem. J. Cell Sci. 2018, 132, jcs219832. [Google Scholar] [CrossRef] [PubMed]
- Velarde, N.; Gunsalus, K.C.; Piano, F. Diverse roles of actin in C. elegans early embryogenesis. BMC Dev. Biol. 2007, 7, 142. [Google Scholar] [CrossRef]
- Reymann, A.-C.; Staniscia, F.; Erzberger, A.; Salbreux, G.; Grill, S.W. Cortical flow aligns actin filaments to form a furrow. Elife 2016, 5, e17807. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. The contractile ring. Curr. Biol. 2011, 21, R976–R978. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Szep, G.; Nemethova, M.; de Vries, I.; Lieber, A.D.; Winkler, C.; Kruse, K.; Small, J.V.; Schmeiser, C.; Keren, K.; et al. Load Adaptation of Lamellipodial Actin Networks. Cell 2017, 171, 188–200.e16. [Google Scholar] [CrossRef]
- Williams-Masson, E.M.; Malik, A.N.; Hardin, J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 1997, 124, 2889–2901. [Google Scholar]
- Burridge, K.; Wittchen, E.S. The tension mounts: Stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013, 200, 9–19. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef]
- Nelson, C.M.; Gleghorn, J.P. Sculpting Organs: Mechanical Regulation of Tissue Development. Annu. Rev. Biomed. Eng. 2012, 14, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.N.; Coravos, J.S.; Perkins, L.A.; Perrimon, N.; Correspondence, A.C.M.; Chanet, S.; Vasquez, C.G.; Tworoger, M.; Kingston, E.R.; Martin, A.C. Stable Force Balance between Epithelial Cells Arises from F-Actin Turnover Article. Dev. Cell 2015, 35, 685–697. [Google Scholar] [CrossRef]
- Brugues, A.; Anon, E.; Conte, V.; Veldhuis, J.H.; Gupta, M.; Colombelli, J.; Munoz, J.J.; Brodland, G.W.; Ladoux, B.; Trepat, X. Forces driving epithelial wound healing. Nat. Phys. 2014, 10, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Wu, X.; Nurkiewicz, T.R.; Kawasaki, J.; Davis, G.E.; Hill, M.A.; Meininger, G.A. Integrins and mechanotransduction of the vascular myogenic response. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1427–H1433. [Google Scholar] [CrossRef]
- Boudou, T.; Legant, W.R.; Mu, A.; Borochin, M.A.; Thavandiran, N.; Radisic, M.; Zandstra, P.W.; Epstein, J.A.; Margulies, K.B.; Chen, C.S. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 2012, 18, 910–919. [Google Scholar] [CrossRef]
- Skwarek-Maruszewska, A.; Hotulainen, P.; Mattila, P.K.; Lappalainen, P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J. Cell Sci. 2009, 122, 2119–2126. [Google Scholar] [CrossRef]
- Greiner, A.M.; Chen, H.; Spatz, J.P.; Kemkemer, R. Cyclic Tensile Strain Controls Cell Shape and Directs Actin Stress Fiber Formation and Focal Adhesion Alignment in Spreading Cells. PLoS ONE 2013, 8, e77328. [Google Scholar] [CrossRef]
- Hannezo, E.; Dong, B.; Recho, P.; Joanny, J.-F.; Hayashi, S. Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes. Proc. Natl. Acad. Sci. USA 2015, 112, 8620–8625. [Google Scholar] [CrossRef]
- Mason, F.M.; Tworoger, M.; Martin, A.C. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 2013, 15, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Hosono, C.; Matsuda, R.; Adryan, B.; Samakovlis, C. Transient junction anisotropies orient annular cell polarization in the Drosophila airway tubes. Nat. Cell Biol. 2015, 17, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, S.; Mellor, H. Actin stress fibres. J. Cell Sci. 2007, 120, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Naumanen, P.; Lappalainen, P.; Hotulainen, P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008, 231, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Cetera, M.; Ramirez-San Juan, G.R.; Oakes, P.W.; Lewellyn, L.; Fairchild, M.J.; Tanentzapf, G.; Gardel, M.L.; Horne-Badovinac, S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 2014, 5, 5511. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.E.; Farrell, D.L.; Zallen, J.A. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. USA 2014, 111, 11732–11737. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Arnold, T.R.; Stephenson, R.E.; Dinshaw, K.M.; Miller, A.L. Maintenance of the Epithelial Barrier and Remodeling of Cell-Cell Junctions during Cytokinesis. Curr. Biol. 2016, 26, 1829–1842. [Google Scholar] [CrossRef]
- Stephenson, R.E.; Higashi, T.; Erofeev, I.S.; Arnold, T.R.; Leda, M.; Goryachev, A.B.; Miller, A.L. Rho Flares Repair Local Tight Junction Leaks. Dev. Cell 2019, 48, 445–459.e5. [Google Scholar] [CrossRef] [PubMed]
- Mandato, C.A.; Bement, W.M. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 2001, 154, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Merriam, R.W.; Christensen, K. A contractile ring-like mechanism in wound healing and soluble factors affecting structural stability in the cortex of Xenopus eggs and oocytes. J. Embryol. Exp. Morphol. 1983, 75, 11–20. [Google Scholar] [PubMed]
- Sonnemann, K.J.; Bement, W.M. Wound Repair: Toward Understanding and Integration of Single-Cell and Multicellular Wound Responses. Annu. Rev. Cell Dev. Biol. 2011, 27, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, D.R.; Plastino, J. Invading, leading and navigating cells in caenorhabditis elegans: Insights into cell movement in vivo. Genetics 2018, 208, 53–78. [Google Scholar] [CrossRef]
- Tang, N.H.; Jin, Y. Shaping neurodevelopment: Distinct contributions of cytoskeletal proteins. Curr. Opin. Neurobiol. 2018, 51, 111–118. [Google Scholar] [CrossRef]
- Ono, S. Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans. Anat. Rec. 2014, 297, 1548–1559. [Google Scholar] [CrossRef]
- Hubbard, E.J.A.; Greenstein, D. The Caenorhabditis elegans Gonad: A Test Tube for Cell and Developmental Biology. Dev. Dyn. 2000, 218, 2–22. [Google Scholar] [CrossRef]
- Whitten, S.J.; Miller, M.A. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev. Biol. 2006, 301, 432–446. [Google Scholar] [CrossRef]
- Altun, Z.F.; Chen, B.; Wang, Z.-W.; Hall, D.H. High resolution map of Caenorhabditis elegans gap junction proteins. Dev. Dyn. 2009, 238, 1936–1950. [Google Scholar] [CrossRef]
- Kimble, J.; Hirsh, D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 1979, 70, 396–417. [Google Scholar] [CrossRef]
- Lints, R.; Hall, D.H. Reproductive system, somatic gonad. WormAtlas 2009. [Google Scholar] [CrossRef]
- McCarter, J.; Bartlett, B.; Dang, T.; Schedl, T. Soma–Germ Cell Interactions in Caenorhabditis elegans: Multiple Events of Hermaphrodite Germline Development Require the Somatic Sheath and Spermathecal Lineages. Dev. Biol. 1997, 181, 121–143. [Google Scholar] [CrossRef]
- McCarter, J.; Bartlett, B.; Dang, T.; Schedl, T. On the Control of Oocyte Meiotic Maturation and Ovulation in Caenorhabditis elegans. Dev. Biol. 1999, 205, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.; Hirsh, D. Receptor-mediated Endocytosis in the Caenorhabditis elegans Oocyte. Mol. Biol. Cell 1999, 10, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.H.; Winfrey, V.P.; Blaeuer, G.; Hoffman, L.H.; Furuta, T.; Rose, K.L.; Hobert, O.; Greenstein, D. Ultrastructural Features of the Adult Hermaphrodite Gonad of Caenorhabditis elegans: Relations between the Germ Line and Soma. Dev. Biol. 1999, 212, 101–123. [Google Scholar] [CrossRef]
- Strome, S. Fluorescence Visualization of the Distribution of Microfilaments in Gonads and Early Embryos of the Nematode Caenorhabditis elegans. J. Cell Biol. 1986, 103, 2241–2252. [Google Scholar] [CrossRef]
- Yin, X.Y.; Gower, N.J.D.; Baylis, H.A.; Strange, K. Inositol 1,4,5-Trisphosphate Signaling Regulates Rhythmic Contractile Activity of Myoepithelial Sheath Cells in Caenorhabditis elegans. Mol. Biol. Cell 2004, 15, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, T.R.; DeModena, J.A.; Sternberg, P.W. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell 1998, 92, 523–533. [Google Scholar] [CrossRef]
- Norman, K.R.; Fazzio, R.T.; Mellem, J.E.; Espelt, M.V.; Strange, K.; Beckerle, M.C.; Maricq, A.V. The Rho/Rac-Family Guanine Nucleotide Exchange Factor VAV-1 Regulates Rhythmic Behaviors in C. elegans. Cell 2005, 123, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Ardizzi, J.P.; Epstein, H.F. Immunochemical Localization of Myosin Heavy Chain Isoforms and Paramyosin in Developmentally and Structurally Diverse Muscle Cell Types of the Nematode Caenorhabditis elegans. J. Cell Biol. 1987, 105, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yu, R.; Ono, S. Structural components of the nonstriated contractile apparatuses in the Caenorhabditis elegans gonadal myoepithelial sheath and their essential roles for ovulation. Dev. Dyn. 2007, 236, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ono, S. Two distinct myosin II populations coordinate ovulatory contraction of the myoepithelial sheath in the Caenorhabditis elegans somatic gonad. Mol. Biol. Cell 2016, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Obinata, T.; Ono, K.; Ono, S. Troponin I controls ovulatory contraction of non-striated actomyosin networks in the C. elegans somatic gonad. J. Cell Sci. 2010, 123, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ono, S. Tropomyosin and Troponin Are Required for Ovarian Contraction in the Caenorhabditis elegans Reproductive System. Mol. Biol. Cell 2004, 15, 2782–2793. [Google Scholar] [CrossRef]
- Myers, C.D.; Goh, P.Y.; Allen, T.S.; Bucher, E.A.; Bogaert, T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 1996, 132, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Actin Dynamics Associated with Focal Adhesions. Int. J. Cell Biol. 2012, 2012, 941292. [Google Scholar] [CrossRef]
- Xu, X.; Rongali, S.C.; Miles, J.P.; Lee, K.D.; Lee, M. pat-4/ILK and unc-112/Mig-2 are required for gonad function in Caenorhabditis elegans. Exp. Cell Res. 2006, 312, 1475–1483. [Google Scholar] [CrossRef]
- Cram, E.J.; Clark, S.G.; Schwarzbauer, J.E. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J. Cell Sci. 2003, 116, 3871–3878. [Google Scholar] [CrossRef]
- Wioland, H.; Guichard, B.; Senju, Y.; Myram, S.; Lappalainen, P.; Jégou, A.; Romet-Lemonne, G. ADF/Cofilin Accerlerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Curr. Biol. 2017, 27, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Baillie, D.L.; Benian, G.M. UNC-60B, an ADF/Cofilin Family Protein, Is Required for Proper Assembly of Actin into Myofibrils in Caenorhabditis elegans Body Wall Muscle. J. Cell Biol. 1999, 145, 491–502. [Google Scholar] [CrossRef]
- Ono, K.; Yamashiro, S.; Ono, S. Essential role of ADF/cofilin for assembly of contractile actin networks in the C. elegans somatic gonad. J. Cell Sci. 2008, 121, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Hayakawa, K.; Tatsumi, H.; Ono, S. Actin-interacting Protein 1 Promotes Disassembly of Actin-depolymerizing Factor/Cofilin-bound Actin Filaments in a pH-dependent Manner. J. Biol. Chem. 2016, 291, 5146–5156. [Google Scholar] [CrossRef]
- Ono, K.; Ono, S. Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans. Cytoskeleton 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rodal, A.A.; Tetreault, J.W.; Lappalainen, P.; Drubin, D.G.; Amberg, D.C. Aip1p Interacts with Cofilin to Disassemble Actin Filaments. J. Cell Biol. 1999, 145, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Ono, S. Functions of actin-interacting protein 1 (AIP1)/WD repeat protein 1 (WDR1) in actin filament dynamics and cytoskeletal regulation. Biochem. Biophys. Res. Commun. 2018, 506, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Joyce, M.J.; Lynch, A.M.; Witte, K.; Audhya, A.; Hardin, J. The F-BAR domain of SRGP-1 facilitates cell-cell adhesion during C. elegans morphogenesis. J. Cell Biol. 2010, 191, 761–769. [Google Scholar] [CrossRef]
- Hong, F.; Haldeman, B.D.; Jackson, D.; Carter, M.; Baker, J.E.; Cremo, C.R. Biochemistry of Smooth Muscle Myosin Light Chain Kinase. Arch. Biochem. Biophys. 2011, 510, 135–146. [Google Scholar] [CrossRef]
- Kovacevic, I.; Orozco, J.M.; Cram, E.J. Filamin and phospholipase C-ε are required for calcium signaling in the Caenorhabditis elegans spermatheca. PLoS Genet. 2013, 9, e1003510. [Google Scholar] [CrossRef]
- Kelley, C.A.; Wirshing, A.C.E.; Zaidel-Bar, R.; Cram, E.J. The myosin light-chain kinase MLCK-1 relocalizes during Caenorhabditis elegans ovulation to promote actomyosin bundle assembly and drive contraction. Mol. Biol. Cell 2018, 29, 1975–1991. [Google Scholar] [CrossRef]
- Wissmann, A.; Ingles, J.; McGhee, J.D.; Mains, P.E. Caenorhabditis elegans LET-502 is related to Rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev. 1997, 11, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Wissmann, A.; Ingles, J.; Mains, P.E. The Caenorhabditis elegans mel-11 Myosin Phosphatase Regulatory Subunit Affects Tissue Contraction in the Somatic Gonad and the Embryonic Epidermis and Genetically Interacts with the Rac Signaling Pathway. Dev. Biol. 1999, 209, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Zaidel-Bar, R. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C. elegans spermatheca. Curr. Biol. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Wirshing, A.C.E.; Cram, E.J. Myosin activity drives actomyosin bundle formation and organization in contractile cells of the Caenorhabditis elegans spermatheca. Mol. Biol. Cell 2017, 28, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, I.; Cram, E.J. FLN-1/Filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev. Biol. 2010, 347, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, D.; Fang, B.; Zhang, H. The flightless I homolog, fli-1, regulates anterior/posterior polarity, asymmetric cell division and ovulation during Caenorhabditis elegans development. Genetics 2007, 177, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Hegsted, A.; Wright, F.A.; Votra, S.; Pruyne, D. INF2- and FHOD-related formins promote ovulation in the somatic gonad of C. elegans. Cytoskeleton 2016, 73, 712–728. [Google Scholar] [CrossRef]
- Bouffard, J.; Cecchetelli, A.D.; Clifford, C.; Sethi, K.; Zaidel-Bar, R.; Cram, E.J. The RhoGAP SPV-1 regulates calcium signaling to control the contractility of the C. elegans spermatheca during embryo transits. Mol. Biol. Cell 2019. [Google Scholar] [CrossRef]
- Aono, S.; Legouis, R.; Hoose, W.A.; Kemphues, K.J. PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development 2004, 131, 2865–2874. [Google Scholar] [CrossRef]
- Kim, H.; McCulloch, C.A. Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion. FEBS Lett. 2011, 585, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Djinovic-Carugo, K.; Carugo, O. Structural portrait of filamin interaction mechanisms. Curr. Protein Peptide Sci. 2010, 11, 639–650. [Google Scholar] [CrossRef]
- McKeown, C.; Praitis, V.; Austin, J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 1998, 125, 2087–2098. [Google Scholar] [PubMed]
- Buechner, M.; Hall, D.H.; Bhatt, H.; Hedgecock, E.M. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev. Biol. 1999, 214, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.R.; Moerman, D.G. α spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J. Cell Biol. 2002, 157, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, A.; Charron, A.; Sadozai, Y.; Switaj, L.; Szutenbach, A.; Smith, P.A. Multiple phenotypes resulting from a mutagenesis screen for pharynx muscle mutations in Caenorhabditis elegans. PLoS ONE 2011, 6, e26594. [Google Scholar] [CrossRef] [PubMed]
- Wirshing, A.C.E.; Cram, E.J. Spectrin regulates cell contractility through production and maintenance of actin bundles in the Caenorhabditis elegans spermatheca. Mol. Biol. Cell 2018, 29, 2433–2449. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fischman, D.A.; Steck, T.L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J. Supramol. Struct. 1973, 1, 233–248. [Google Scholar] [CrossRef]
- Greenquist, A.C.; Shohet, S.B.; Bernstein, S.E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood 1978, 51, 1149–1155. [Google Scholar]
- Tse, W.T.; Lecomte, M.C.; Costa, F.F.; Garbarz, M.; Feo, C.; Boivin, P.; Dhermy, D.; Forget, B.G. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J. Clin. Investig. 1990, 86, 909–916. [Google Scholar] [CrossRef]
- Hammarlund, M.; Jorgensen, E.M.; Bastiani, M.J. Axons break in animals lacking β-spectrin. J. Cell Biol. 2007, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, R.; Velarde, N.V.; Klancer, R.; Gordon, S.; Kadandale, P.; Parry, J.M.; Hang, J.S.; Rubin, J.; Stewart-Michaelis, A.; Schweinsberg, P.; et al. EGG-3 Regulates Cell-Surface and Cortex Rearrangements during Egg Activation in Caenorhabditis elegans. Curr. Biol. 2007, 17, 1555–1560. [Google Scholar] [CrossRef]
- Rattan, S.; De Godoy, M.A.F.; Patel, C.A. Rho Kinase as a Novel Molecular Therapeutic Target for Hypertensive Internal Anal Sphincter. Gastroenterology 2006, 131, 108–116. [Google Scholar] [CrossRef]
- DeMaso, C.R.; Kovacevic, I.; Uzun, A.; Cram, E.J. Structural and functional evaluation of C. elegans filamins FLN-1 and FLN-2. PLoS ONE 2011, 6, e22428. [Google Scholar] [CrossRef]
- Sawa, M.; Suetsugu, S.; Sugimoto, A.; Miki, H.; Yamamoto, M.; Takenawa, T. Essential role of the C. elegans Arp2/3 complex in cell migration during ventral enclosure. J. Cell Sci. 2003, 116, 1505–1518. [Google Scholar] [CrossRef]
- Vanneste, C.A.; Pruyne, D.; Mains, P.E. The role of the formin gene fhod-1 in C. elegans embryonic morphogenesis. Worm 2013, 2, e25040. [Google Scholar] [CrossRef] [PubMed]
- Gettner, S.N.; Kenyon, C.; Reichardt, L.E. Characterization of/3pat-3 Heterodimers, a Family of Essential Integrin Receptors in C. elegans. J. Cell Biol. 1995, 129, 1127–1141. [Google Scholar] [CrossRef]
- Lynch, A.M.; Hardin, J. The assembly and maintenance of epithelial junctions in C. elegans. Front. Biosci. 2009, 1, 1414–1432. [Google Scholar] [CrossRef]
- Nance, J.; Munro, E.M.; Priess, J.R. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 2003, 130, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Raich, W.; Agbunag, C.; Leung, B.; Hardin, J.; Priess, J.R. A Putative Catenin-Cadherin System Mediates Morphogenesis of the Caenorhabditis elegans Embryo. J. Cell Biol. 1998, 141, 297–308. [Google Scholar] [CrossRef]
- Pilipiuk, J.; Lefebvre, C.; Wiesenfahrt, T.; Legouis, R.; Bossinger, O. Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev. Biol. 2009, 327, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ward, J.D.; Cheng, Z.; Dernburg, A.F. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 2015, 142, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Furuse, M. Molecular organization and function of invertebrate occluding junctions. Semin. Cell Dev. Biol. 2014, 36, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Babatz, F.; Naffin, E.; Klämbt, C. The Drosophila Blood-Brain Barrier Adapts to Cell Growth by Unfolding of Pre-existing Septate Junctions. Dev. Cell 2018, 47, 697–710.e3. [Google Scholar] [CrossRef]
- Hatan, M.; Shinder, V.; Schnorrer, F.; Volk, T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J. Cell Biol. 2011, 192, 307–319. [Google Scholar] [CrossRef]
- Simske, J.S. Claudins reign: The claudin/EMP/PMP22/γ channel protein family in C. elegans. Tissue Barriers 2013, 1, e25502. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Fehon, R.G. Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include basolateral polarity protein Discs large. J. Cell Sci. 2011, 124, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Spiro, D.; Loewenstein, W.R. Studies on an epithelial (gland) cell junction. II. Surfact Structure. J. Cell Biol. 1964, 22, 587–598. [Google Scholar] [CrossRef]
- Noirot-Timothee, C.; Smith, D.S.; Cayer, M.L.; Noirot, C. Septate Junctions in Insects: Comparison between Intercellular and Intramembranous Structures. Tissue Cell 1978, 10, 125–136. [Google Scholar] [CrossRef]
- Genova, J.L.; Fehon, R.G. Neuroglian, Gliotactin, and the Na/K ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 2003, 161, 979–989. [Google Scholar] [CrossRef]
- Haklai-Topper, L.; Soutschek, J.; Sabanay, H.; Scheel, J.; Hobert, O.; Peles, E. The neurexin superfamily of Caenorhabditis elegans. Gene Expr. Patterns 2011, 11, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Draper, B.W.; Priess, J.R. The Role of Actin Filaments in Patterning the Caenorhabditis elegans Cuticle. Dev. Biol. 1997, 184, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Priess, J.R.; Hirsh, D.I. Caenorhabditis elegans morphogenesis: The role of the cytoskeleton in elongation of the embryo. Dev. Biol. 1986, 117, 156–173. [Google Scholar] [CrossRef]
- Tamiello, C.; Buskermolen, A.B.C.; Baaijens, F.P.T.; Broers, J.L.V.; Bouten, C.V.C. Heading in the Right Direction: Understanding Cellular Orientation Responses to Complex Biophysical Environments. Cell. Mol. Bioeng. 2015, 9, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Plastino, J.; Blanchoin, L. Adaptive Actin Networks. Dev. Cell 2017, 42, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Kaunas, R.; Nguyen, P.; Usami, S.; Chien, S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA 2005, 102, 15895–15900. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, A.S.; Rothenberg, K.E.; Berginski, M.E.; Urs, A.N.; Hoffman, B.D. Construction, imaging, and analysis of FRET-based tension sensors in living cells. Methods Cell Biol. 2015, 125, 161–186. [Google Scholar] [PubMed]
- Kobb, A.B.; Zulueta-Coarasa, T.; Fernandez-Gonzalez, R. Tension regulates myosin dynamics during Drosophila embryonic wound repair. J. Cell Sci. 2017, 130, 689–696. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelley, C.A.; Cram, E.J. Regulation of Actin Dynamics in the C. elegans Somatic Gonad. J. Dev. Biol. 2019, 7, 6. https://doi.org/10.3390/jdb7010006
Kelley CA, Cram EJ. Regulation of Actin Dynamics in the C. elegans Somatic Gonad. Journal of Developmental Biology. 2019; 7(1):6. https://doi.org/10.3390/jdb7010006
Chicago/Turabian StyleKelley, Charlotte A., and Erin J Cram. 2019. "Regulation of Actin Dynamics in the C. elegans Somatic Gonad" Journal of Developmental Biology 7, no. 1: 6. https://doi.org/10.3390/jdb7010006
APA StyleKelley, C. A., & Cram, E. J. (2019). Regulation of Actin Dynamics in the C. elegans Somatic Gonad. Journal of Developmental Biology, 7(1), 6. https://doi.org/10.3390/jdb7010006



