1. Introduction
Chromatin remodelers play important roles during metazoan development through their regulatory role in gene expression. They are usually part of large enzymatic complexes that modulate chromatin structure. The highly conserved, ATP- dependent chromatin remodeler, Mi2 is part of an abundant multi-protein complex in mammalian cells called NuRD (nucleosome remodeling and deacetylase) [
1]. In mouse embryonic stem cells NuRD modulates the activity of pluripotency genes in a way that allow cells to respond to developmental cues [
2,
3]. During skin development and in the mouse hematopoietic cell lineages, Miβ is required for stem cell homeostasis and lineage choice [
4,
5]. Recent molecular studies showed that NuRD acts at gene regulatory sites by increasing nucleosome density and consequently decreasing the accessibility of transcription factors to these regions [
6].
In
C. elegans there are two Mi2 homologs, LET-418 and CHD-3. While
chd-3 mutant worms do not show any phenotype, worms lacking
let-418 activity present important developmental defects, such as sterility, larval arrest and vulval malformations [
7,
8,
9]. LET-418 is also required to maintain normal lifespan and together with the histone demethylase SPR-5, the
C. elegans homolog of LSD1, it was shown to maintain pluripotency of germ cells [
10]. LET-418 also interacts with the nuclear RNAi pathway to silence repetitive elements, including transposons and retrotransposons, to ensure genome stability [
11].
Detailed characterization of the
let-418 associated developmental defects revealed that post-embryonic development is not initiated and blast cells and germ cells do not divide [
12]. In
C. elegans post-embryonic development is not initiated in the absence of food and the freshly hatched L1 larvae go into a diapause stage until food is available [
13]. This developmental arrest is regulated by the insulin signaling, which is sensitive to nutrient availability [
14].
let-418 L1 larvae look superficially similar to diapaused larvae and might be deficient in food sensing. The developmental arrest associated with
let-418 mutation is dependent on a network of chromatin regulators. Most of these chromatin associated proteins are part of large complexes that are involved in activation of transcription [
12]. These findings highlight the importance of chromatin state in cells of the freshly hatched larvae to ensure a proper response to the environment.
In this study, we developed molecular tools to modulate the activity of let-418 in different tissues for the purpose of identifying LET-418 focus of action. We found that LET-418 functions cell non-autonomously in the intestine or in the hypodermis to control the onset of progenitor cell proliferation. However, to support continuous and coordinated development of all tissues, LET-418 activity is further required in the progenitor cells, as well as in adjacent tissues. Furthermore, we show that the cell non-autonomous function of LET-418 in triggering the exit of blast and germ cells from quiescence relies on the insulin signaling pathway.
3. Results
3.1. let-418 Expression Can Be Controlled by Tissue-Specific Promoters
Loss of
let-418 maternal gene activity leads to developmental arrest at the L1 stage [
9,
12]. To determine in which tissues LET-418/Mi2 is required for postembryonic development, we generated transgenes including tissue specific promoters to drive expression of FLAG-tagged LET-418 in the intestine (
pelt-2), the hypodermis (
pdpy-7), the muscles (
pmyo-3), the neurons (
prgef-1), the M cell lineage (
phlh-8) and the germline (
ppie-1). The resulting transgenes were injected into the worm germline and integrated into the genome using the MosSCI technique [
18]. Immunostaining with anti-FLAG antibody revealed that the tissue-specific promoters are driving expression of FLAG-tagged LET-418 in the appropriate tissue (
Figure 1 and
Supplementary Materials). Expression of
let-418 under its native promoter is observed in most if not all cells (
Figure 1). Expression pattern analysis throughout development shows that
let-418 expression driven by its own promoter and by the
pie-1 promoter is seen already in one-cell stage embryos, indicating that the gene product is maternally delivered. Under the control of the
pie-1 promoter
let-418 expression persists in all cells of the embryos with declining levels after the 100 cell-stage. It is only at the larval stage that the expression gets restricted to the germ cells (data not shown). The other transgenes start to be expressed at different stages of embryogenesis (
Figure 1A).
pelt-2::let-418 begins to be expressed at the 2E cell stage (endodermal cell stage) in two cells and is expressed until adulthood in intestinal cells.
pdyp-7::let-418 and
myo-3::let-418 appear at the coma stage in hypodermal cells and muscle cells respectively, and expression persists until adulthood in the corresponding tissues.
rgef-1p::let-418 is observed first at the three-fold stage of the developing embryo and in neurons of the adult worm (
Figure 1A).
hlh-8p::let-418 drives expression in all undifferentiated cells of the M lineage [
17] (
Figure 1B). Altogether, these results show that
let-418 expression is restricted to the tissues targeted by the different promoters.
3.2. LET-418 Triggers Post-Embryonic Development in a Cell Non-Autonomous Manner
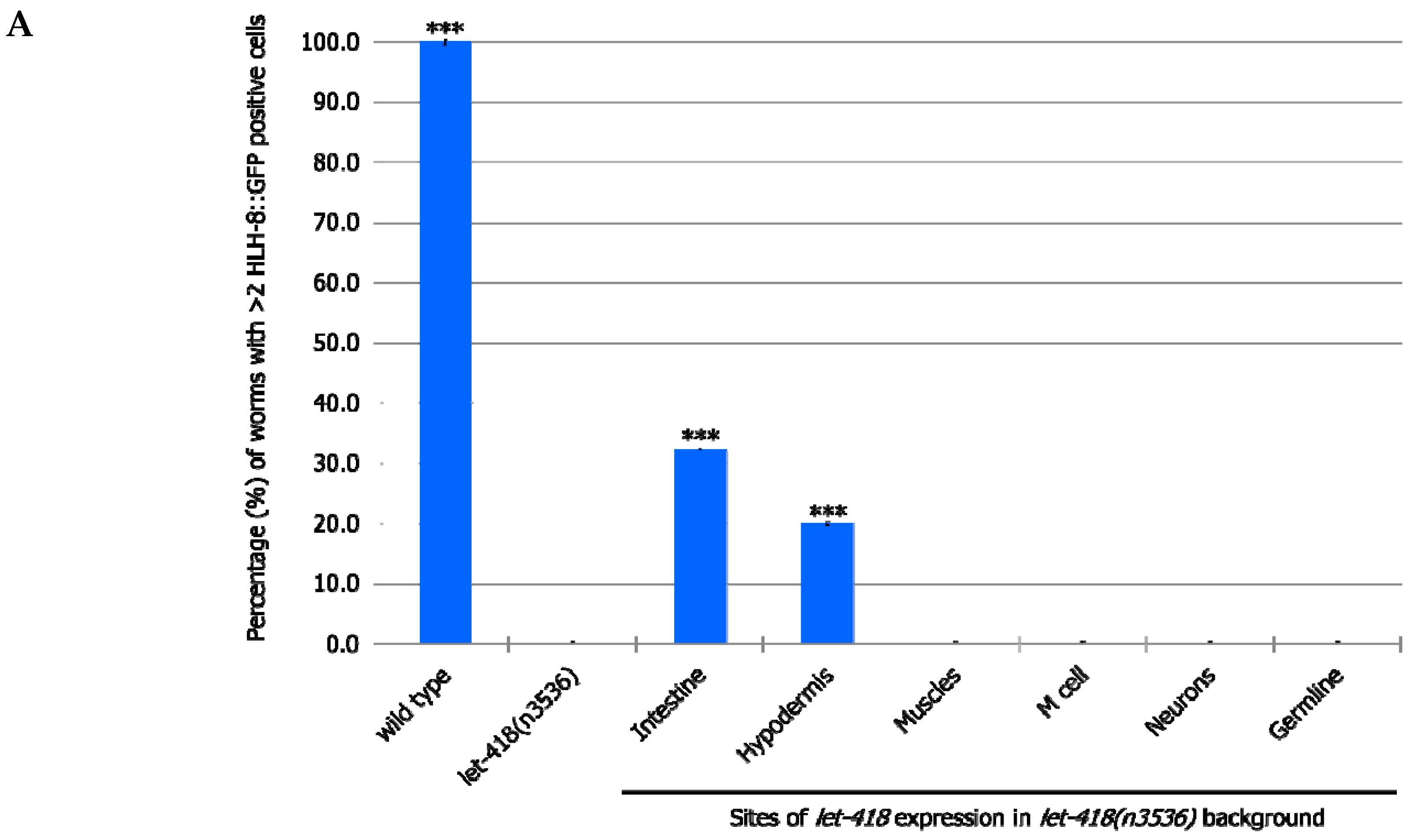
To identify tissues where LET-418/Mi2 is required to promote postembryonic development, we assessed the level of rescue of the different transgenes in the let-418 mutant background. To do so, we examined the overall development of the worms and we monitored the divisions of the blast cells from the V and M lineage and of the germline precursor cells (Z2/Z3) in the different genetic backgrounds.
let-418 expression driven by its own promoter leads to a complete rescue of the developmental defects (
Figure 1A,
Table 1 and [
19]). This indicates an unaltered function of the LET-418 protein by the FLAG tag.
let-418 expression in the intestine or in the hypodermis is sufficient to trigger initiation of postembryonic development (
Table 1).
Based on morphological analysis, we observed that
let-418 expression in the intestine drives development until the L3/L4 stage. Animals expressing
let-418 in the hypodermis are able to bypass L1 arrest and reach the early L2 stage.
let-418 expressed under the control of the
pie-1 promoter allow the worm to develop until adulthood when the transgene is maternally provided. However, when the
pie-1p::let-418 transgene is paternally inherited, the worms arrest their development at L1. None of the other transgenes promote exit from the L1 developmental arrest (
Table 1). These results show that LET-418 functions cell non-autonomously and indicate that LET-418 is required for chromatin function in the intestine and to a lesser extent in the hypodermis to initiate postembryonic development.
To examine the level of development in different tissues, we monitored cells division from three different lineages.
3.2.1. M Lineage
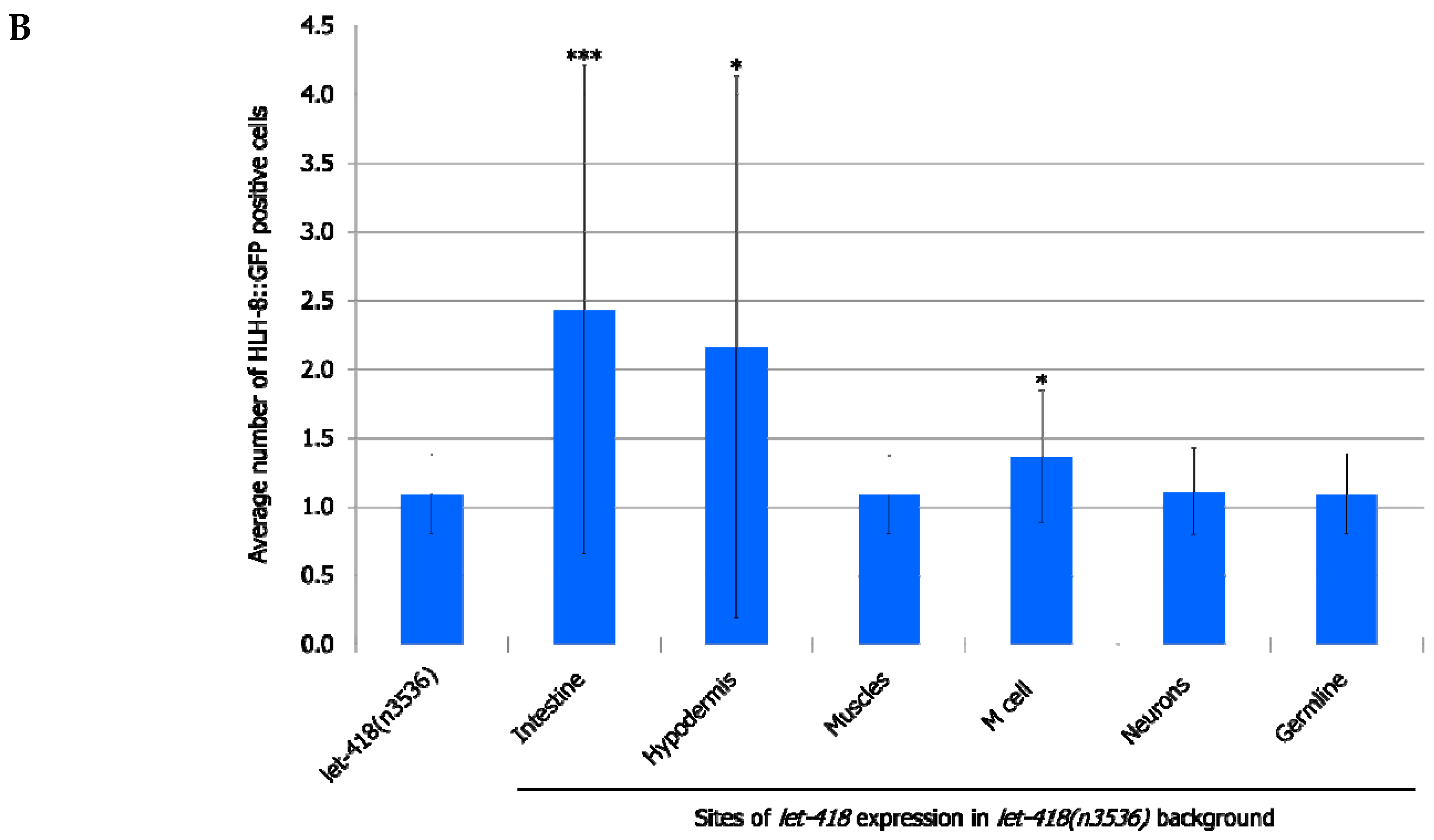
We examined the effect of the different transgenes on the M lineage during postembryonic development. Freshly hatched wild type L1 larvae have a single M cell, which will give birth to 2 coelomocytes, 14 body wall muscle cells and 2 sex myoblast cells [
20]. The M cell lineage was visualized using a
phlh-8::GFP reporter, which is expressed in all undifferentiated cells of the M lineage [
17]. While expression of
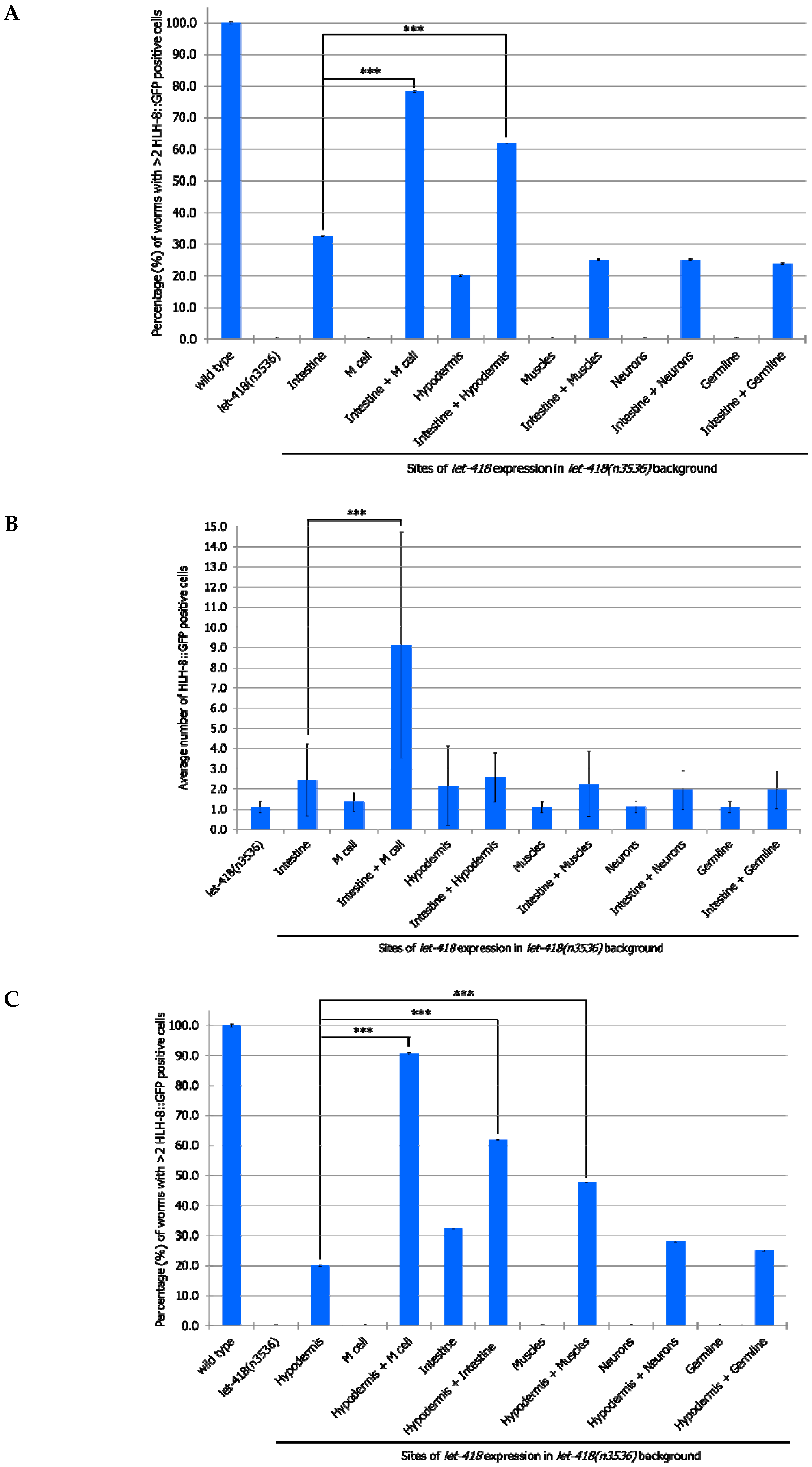
let-418 in the neurons, muscles and germ cells has no effect on M cell division, LET-418 activity in the intestine, the hypodermis and also in the M cell is able to trigger M cell division (
Figure 2,
Supplemental Figure S1, Supplemental Tables S1 and S2). A small proportion of the worms show more than 2 M cell divisions at 30 h after birth when
let-418 was expressed in the intestine or in the hypodermis (
Figure 2A). However, the number of GFP positive cells stays very low in these worms (
Figure 2B). Animals expressing
let-418 in the M cell also show a number of M cell that is significantly higher than in
let-418 mutant (
Figure 2B), however the number of animals showing more than 3 M cell descendants is not significant. This suggests that LET-418 exerts a weak cell autonomous effect on M cell division, however the M cell remains poorly responsive to developmental cues. Altogether these results indicate that LET-418 is predominantly acting cell non-autonomously in the intestine and in the hypodermis to trigger postembryonic development.
3.2.2. V Lineage
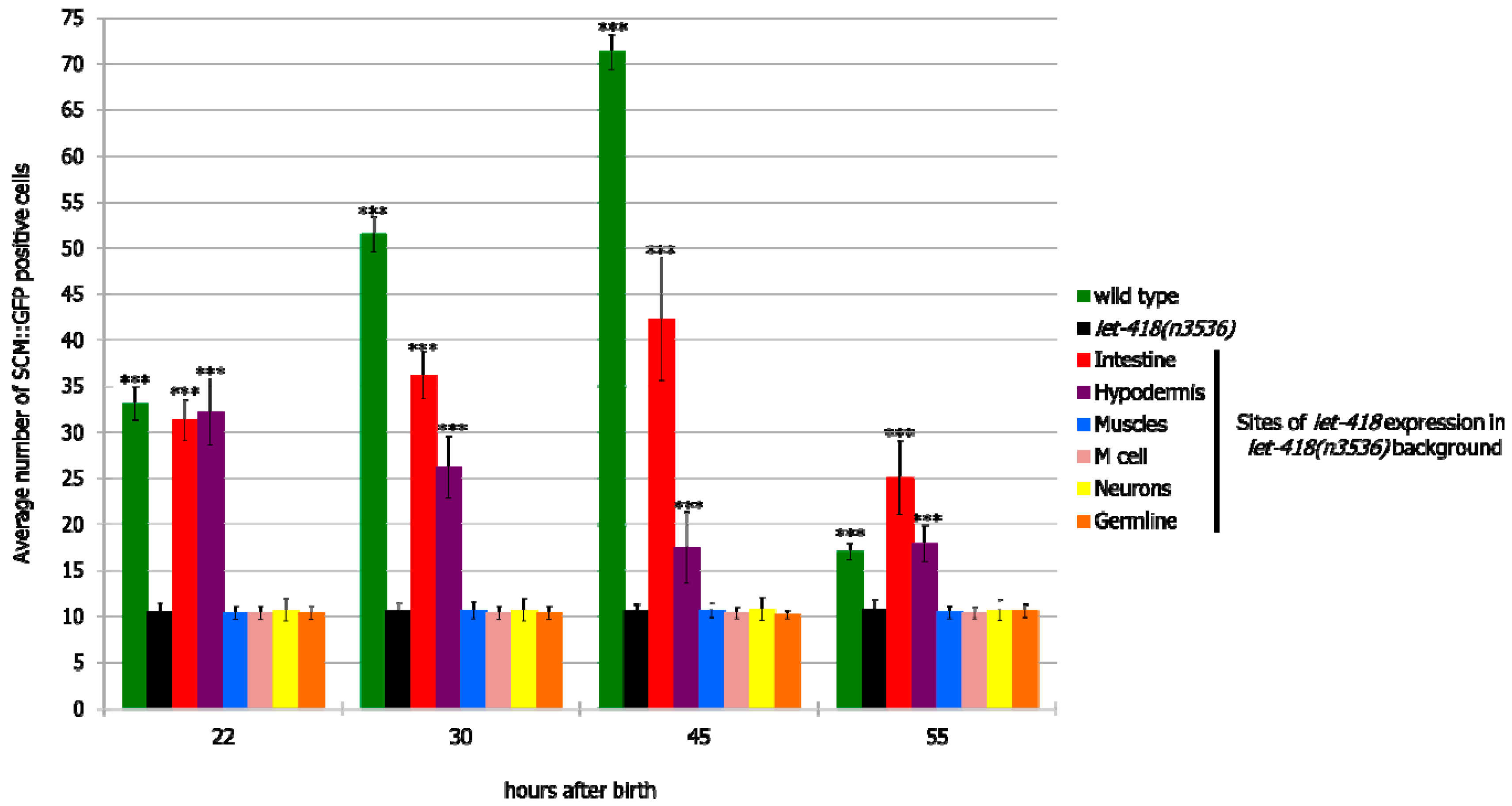
Next, we examined the effect of LET-418 activity on cell division of the V lineage. Freshly hatched wild type larvae possess 10 pairs of seam cells belonging to the V lineage. All of them, except the most anterior ones (H0), are blast cells that will divide during development and give rise to different cell types, such as hypodermal cells, neurons and glial cells [
20]. Seam cells were visualized by the seam cell specific reporter
scm::gfp [
16].
let-418 expression in neurons, germline, M cell and muscle had no effect on V cell divisions (
Figure 3 and
Supplemental Figure S2). However, when
let-418 activity was restored in the hypodermis or the intestine, V cell divisions could be observed in the course of postembryonic development (
Figure 3,
Supplemental Figure S2 and Supplemental Tables S3 and S4). Moreover, the percentage of worms where V cells exit quiescence is higher compared to the M cell in the same genetic background (
Supplemental Tables S1–S4), indicating that these cells are more responsive to the presence of LET-418 in the intestine or the hypodermis compared to cells of the M lineage.
3.2.3. Germ Cells
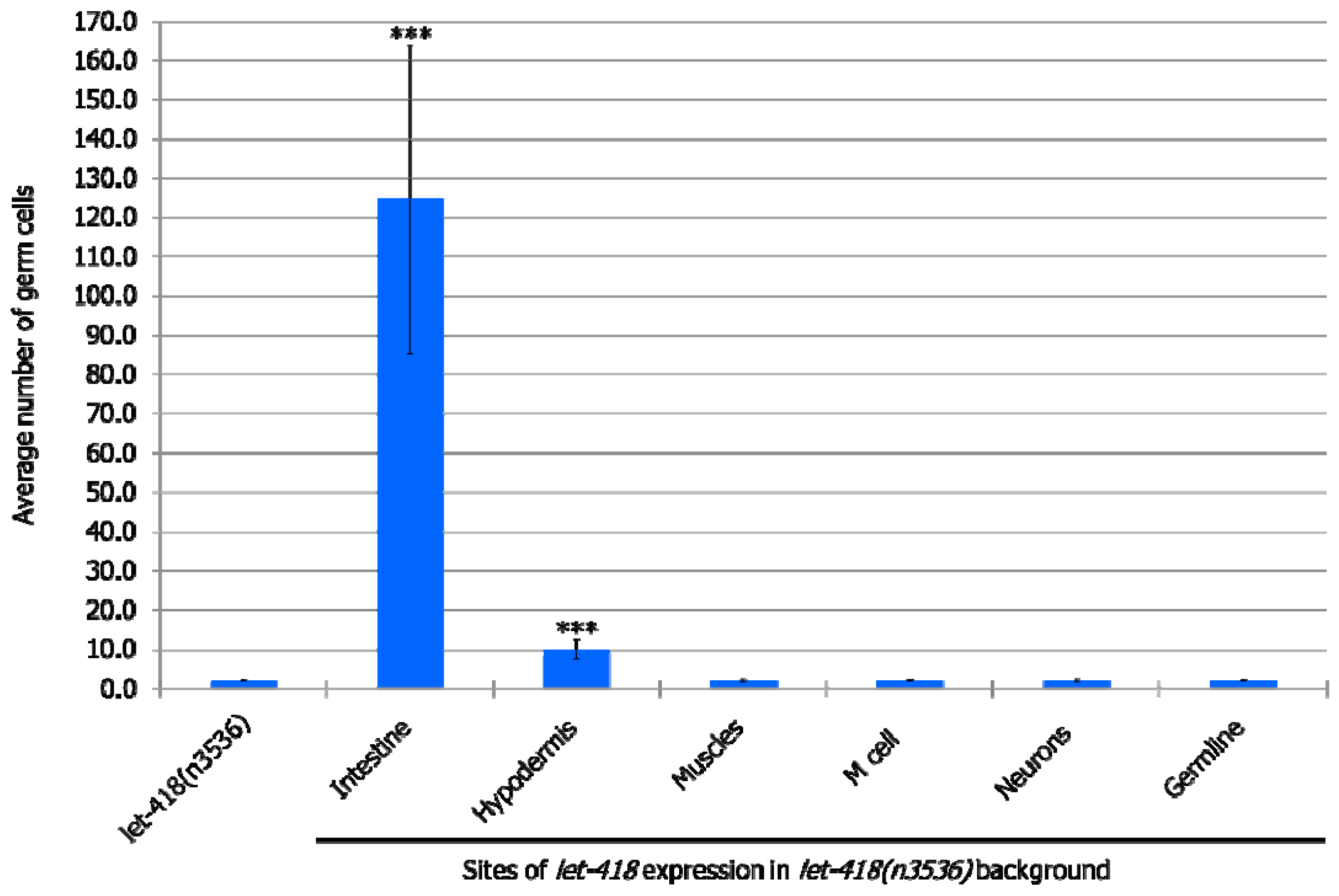
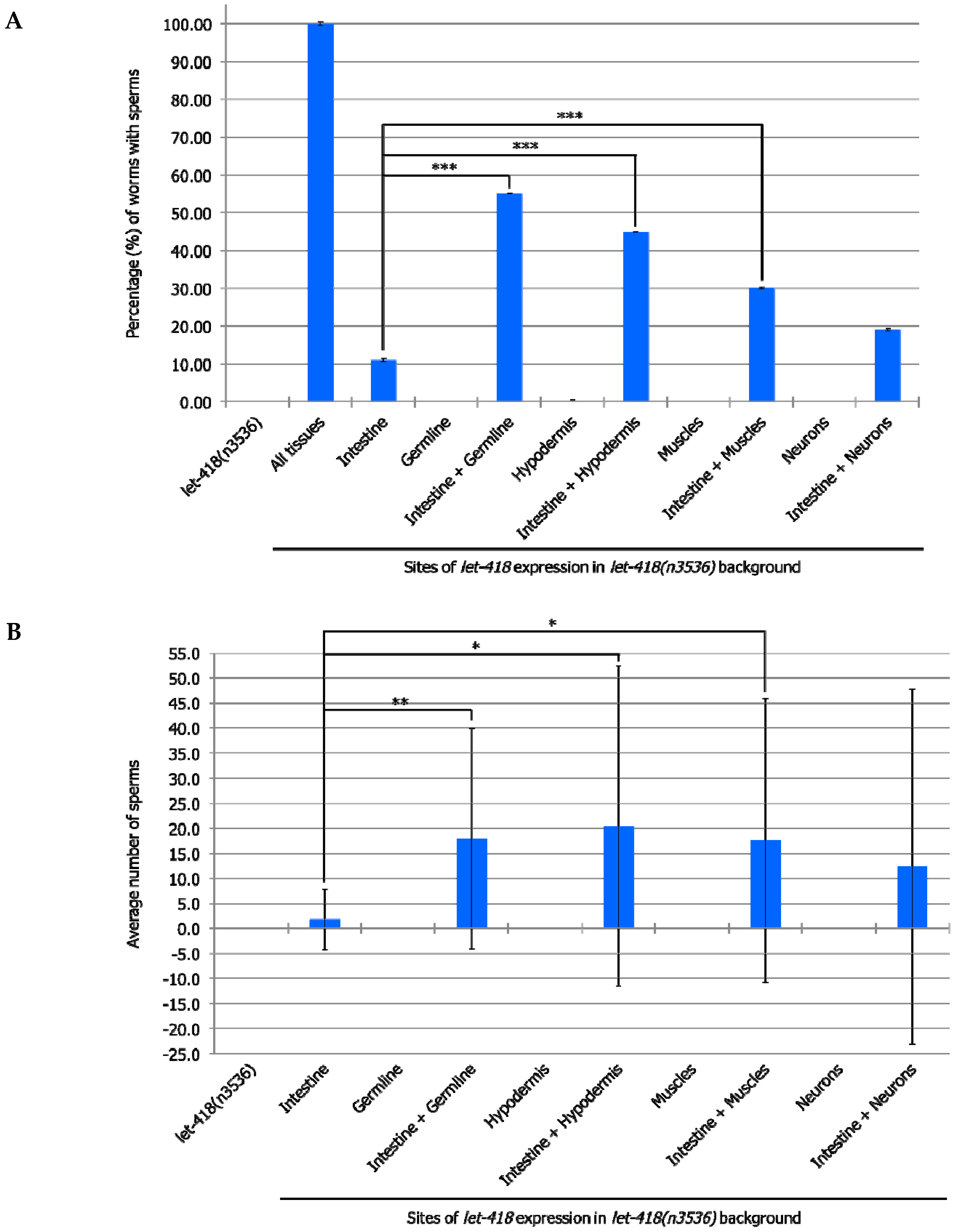
At the non-permissive temperature of 25 °C, in the
let-418 mutant, Z2 and Z3 germline precursor cells are quiescent. When LET-418 activity is present in the intestine, germ cell proliferation is observed to a level that corresponds to the L3 stage in wild type worms (
Figure 4,
Supplemental Figure S3, Supplemental Tables S5 and S6). Furthermore, a small proportion of germ cells could differentiate into sperms (
Supplemental Table S6). We also noted that almost all the worms exhibit Z2/Z3 cells that exit quiescence, indicating that these cells are highly responsive to the presence of LET-418 in the intestine (
Supplemental Table S5). When
let-418 activity is restored in the hypodermis, very low level of Z2/Z3 proliferation is observed (
Figure 4,
Supplemental Figure S3 and Table S6). No proliferation was observed when
let-418 activity was restored in neurons, muscles, M lineage and germline (zygotic expression) (
Figure 4,
Supplemental Figure S3, Tables S5 and S6). To get
let-418 zygotic expression in the germline, the transgene was introduced through the male germline to avoid the maternal contribution, which is sufficient to fully rescue the
let-418 mutant phenotype (data not shown).
Taken together, this suggests that LET-418 dependent chromatin conformation in the intestine is playing a critical role in the developmental control of the entire organism, and in the signaling towards other tissues of the worm. However, lack of correspondence between M, Z and V lineage development in the different strains suggests that LET-418 has also a role in the other tissues to ensure coordinated development.
3.3. Intestine and Hypodermis Are Primary LET-418 Sites of Action to Sustain Coordinated Development
If LET-418 is required for coordinated development, then combined expression of
let-418 from two different tissues should promote further development. In a first set of experiments, we combined all our strains expressing
let-418 from different promoters with the one expressing
let-418 from the intestine and we monitor the effect on M cell division. To do so, we generated heterozygotes worms expressing
let-418 from two different promoters (intestinal promoter and any other promoter). Expressing
let-418 in the intestine and the M cell improve the M cell ability to exit quiescence, as well as the number of divisions (
Figure 5A,B,
Supplemental Tables S7 and S8). This shows that LET-418 has also a cell autonomous effect on M cell development, although a primary trigger should come from the intestine. This result is consistent with the very small cell-autonomous effect which was already observed when
let-418 is expressed solely in the M cell (
Figure 2B).
let-418 expression in both the intestine and the hypodermis leads to a more efficient exit from quiescence. However, there is no significant improvement in the number of M cell descendants, indicating that LET-418 activity is needed in the corresponding tissue to sustain development. No improvement in M cell exit from quiescence and division is observed in the worms expressing
let-418 in the neurons, the muscle and the germline in addition to the intestine. Next, we combined our different strains with the one expressing
let-418 in the hypodermis and assayed the effect on M cell exit of quiescence and division. Expression of
let-418 in the following combinations tissues: hypodermis/M cell, hypodermis/intestine and hypodermis/muscle could trigger M cell exit from quiescence (
Figure 5C,D,
Supplemental Tables S9 and S10). However, a significant level of M cell division was only observed when
let-418 was expressed in the hypodermis and the M cell. This result is consistent with the requirement of a LET-418 cell-autonomous effect in the M cell.
Altogether, we show here that the intestine and the hypodermis are primary sites for LET-418 function to promote M cell exit of quiescence. However, division of the M cell is further enhanced when let-418 is expressed in the M cell, indicating that LET-418 also has a cell-autonomous function.
3.4. Germline Development is Enhanced When let-418 is Expressed in More Than One Tissue
To test if combined expression of LET-418 in two different tissues could also improve germline development, we generated strains where
let-418 was expressed in the intestine and in additional tissues. Enhanced level of germ cell proliferation and differentiation into sperms was observed when
let-418 is expressed in the intestine as well as in the germline (
Figure 6A,B and
Supplemental Tables S11 and S12). Interestingly, combined
let-418 expression in intestine and any of the other somatic tissues, except neurons, also improves germline development (
Figure 6A,B and
Supplemental Tables S11 and S12). Moreover, the development of the somatic gonad is also enhanced in these worms, which show further extension of gonadal arms (
Supplemental Figure S4). Combined
let-418 expression in the hypodermis and any of the other tissues shows comparable results to those obtained with combinations including
let-418 intestinal expression (
Supplemental Table S13). These results indicate that germline development strongly depends on LET-418 activity in all the somatic tissues, except the neurons, with a predominant effect of the intestine and the hypodermis.
3.5. The Cell Non-Autonomous Effect of LET-418 on M Cell Division Depends on the Insulin Signaling Pathway
We show that LET-418 activity in the intestine has the strongest effect on germ cell and blast cell proliferation, suggesting that generation of a pro-developmental signal depends on the presence of LET-418 in the intestine. Insulin signaling is known to be implicated in post-embryonic development in
C. elegans [
21,
22,
23].
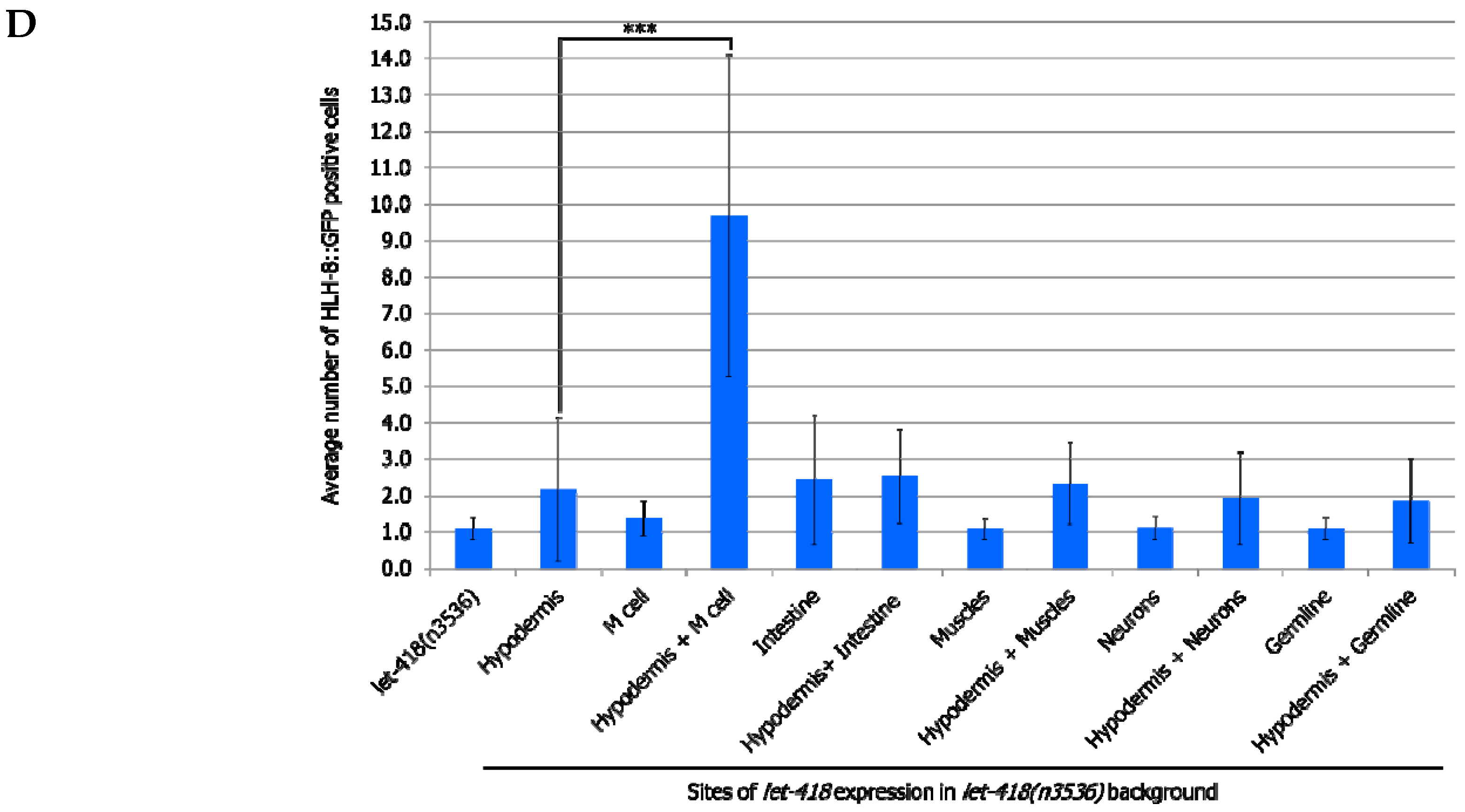
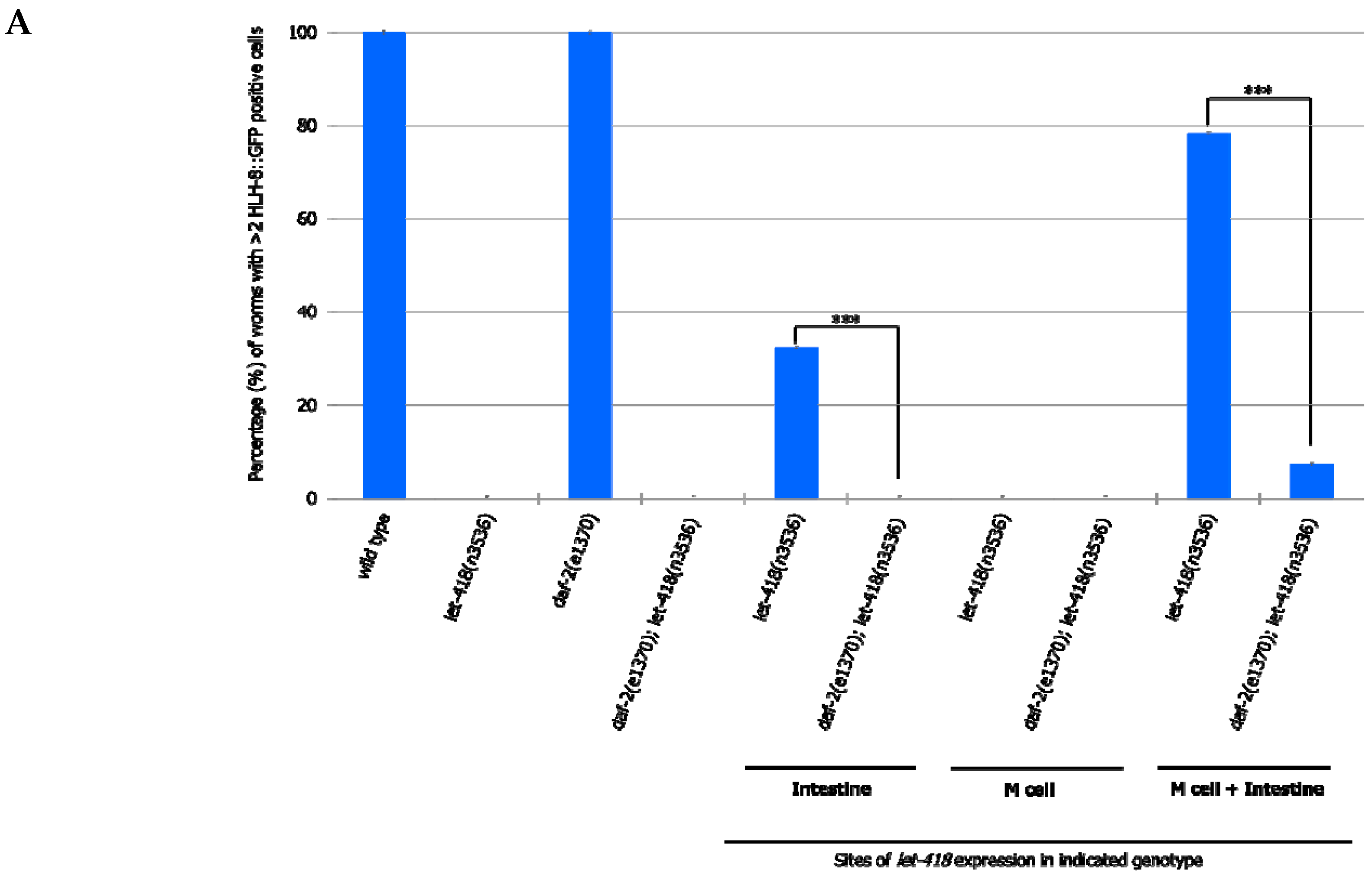
To analyze whether this signaling pathway could be mediating intestinal LET-418 function on M cell division, we used the temperature-sensitive, dauer-constitutive mutant, daf-2(e1370), where the function of the DAF-2/insulin receptor is strongly reduced at restrictive temperature. To test the interaction between LET-418 function and the insulin signaling pathway, we generated strains where let-418 was expressed in the different tissues in a daf-2(e1370) mutant background and monitored M cell division.
In
daf-2 mutants grown at restrictive temperature, the M cell produces 14 BWMs, 2 CCs and 2 SMs, which migrate to the presumptive vulva region but fail to divide further (
Supplemental Figure S5, Tables S14 and S15). This result indicates that
daf-2 activity is not required for the M cell to exit quiescence. In
daf-2;let-418 double mutant post-embryonic development does not initiate. When LET-418 activity is restored in the intestine, in reduced insulin signaling conditions, M cell division is abolished (
Figure 7A,B). These worms exhibit only one
phlh-8::gfp positive cell and are noticeably smaller in body size compared to worms with wild type DAF-2 activity (data not shown). Consistently, M cell division is also strongly reduced, however not totally abolished, in worms with combined LET-418 activity in the intestine and in M cell in a
daf-2 mutant background (
Figure 7A,B). This observation is consistent with a very small cell-autonomous effect of LET-418 in the M cell (
Figure 2B). Altogether, these results indicate that the cell non-autonomous activity of LET-418 relies on insulin signaling to trigger M cell division.
4. Discussion
Our study of LET-418/Mi2 tissue-specific contribution reveals both cell non-autonomous and cell autonomous inputs. LET-418/Mi2 plays a major role in the intestine and the hypodermis for the development of the whole organism and its impact on other tissues is mediated by the insulin signaling pathway.
LET-418 activity in the intestine and to a lesser extend in the hypodermis restores the ability of the worm to initiate post-embryonic development. LET-418 focus of action is in agreement with a role in the food sensing pathway. In the absence of
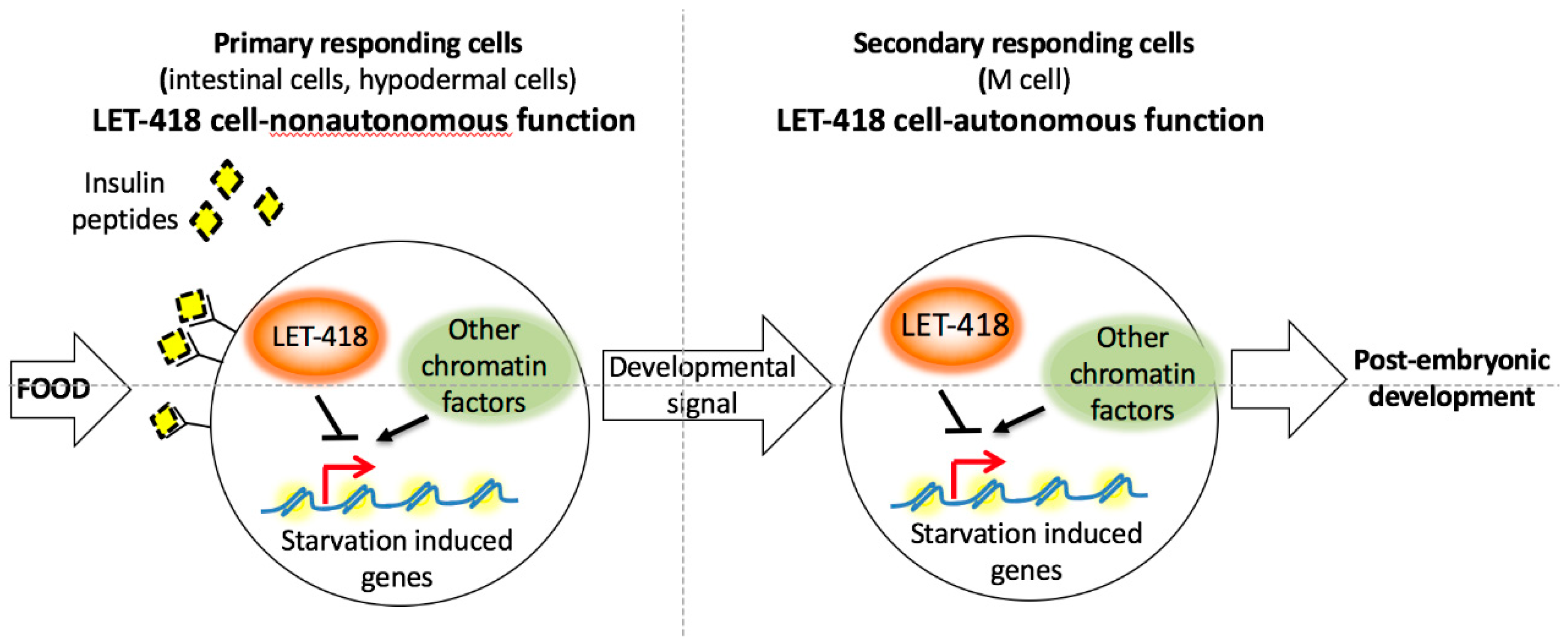
let-418 activity, the L1 arrested larvae look superficially similar to L1 diapaused larvae. The fed larvae might fail to integrate the resulting growth signal at the level of the intestinal chromatin (
Figure 8). The intestinal chromatin might be in a state that constitutively expresses starvation induced genes leading to a developmental arrest and preventing an appropriate response to the nutrient status.
When LET-418 function is restored in other tissues than the intestine and the hypodermis, no rescue of the developmental arrest is observed, suggesting that LET-418 is not required primarily in these tissues for development. However, we cannot rule out that the promoters used in our study do not induce sufficient expression to trigger development.
In mouse embryonic stem cells (ESCs), Mi2, as well as other NuRD components are required for a proper response to developmental cues. In the absence of Mi2, pluripotency genes are misregulated and lineage commitment of the ESCs is compromised [
2,
24]. LET-418 could play a similar role in
C. elegans by shaping the chromatin of intestinal and hypodermal cells to allow them to respond to food. Accordingly, LET-418 is also required in blast cells and germ cells to allow them to respond to the signal coming from the intestine and the hypodermis.
Altogether, these results suggest that the chromatin context in the appropriate tissue is very important to respond to developmental or environmental cues. In line with this proposition, in a genome wide RNAi screen for suppressors of
let-418 developmental defects, we identified mainly chromatin regulators [
12]. Absence of both LET-418 and one of these chromatin factors was able to restore initiation of postembryonic development. It would be interesting to know if these chromatin factors are shaping the chromatin at common loci with LET-418 in the different tissues (
Figure 8).
The insulin signaling pathway is required for the cell non-autonomous function of LET-418. Insulin signaling is known to be activated in response to food and to promote larval development [
14]. It is thought to monitor the internal nutrient status in the intestine and to sense external nutrient status through the neurons [
23,
25]. We propose that in the
let-418 L1 larvae insulin signaling is initiated normally in response to food, but no appropriate transcriptional response is established in the intestine and the hypodermis, because chromatin is not in the proper state. LET-418 function in the intestine and the hypodermis would be important for the response to the nutrient status but also to "organize" the signaling to the other tissues. However, the nature of the developmental signal sent by the intestine has to be different (see below) from the insulin signaling, since we observe no response of the other tissues, when LET-418 activity is restored in these tissues.
The DAF-16 FOXO transcription factor is known to integrate the input of the insulin signaling pathway, which antagonizes its function [
26,
27]. DAF-16 regulates post-embryonic development in the intestine in a cell non-autonomous manner. DAF-16 promotes L1 arrest by inhibiting
daf-12/NHR and
dbl-1/TGFβ Sma/Mab signaling [
22]. In the absence of LET-418, the chromatin context could allow DAF-16 to activate starvation induced genes and trigger developmental arrest. Consistent with this hypothesis, we identified a significant enrichment in DAF-16 target genes that were upregulated in
let-418 L1 arrested larvae [
12]. Moreover, in mouse embryonic stem cells, Mi2 was shown recently to maintain nucleosome density to control the accessibility of specific transcription factors [
6]. However no bypass of L1 arrest is observed in
daf-16;let-418 double mutant suggesting that DAF-16 would contribute only partly to the
let-418 L1 arrest (data not shown).
To summarize, we showed that LET-418 functions cell non-autonomously in the intestine and the hypodermis in the transition to post-embryonic development. Its main role in the energy centers of the worm is consistent with a potential role in food sensing, which is a prerequisite for the initiation of larval development. The cell non-autonomous function of LET-418 is mediated by insulin signaling. Identifying LET-418 tissue-specific target genes will allow to characterize further the link between chromatin and the response to food.