Abstract
Background: The objective of this review is to determine the evidence or, conversely, the absence of evidence regarding the effectiveness of progestogens in treating premenopausal women with uterine fibroids. In particular, the goal is to address recurring questions as to whether they are effective or not for managing symptoms commonly attributed to fibroids. Methods: A review of the most relevant papers (n = 63) on the efficacy of progesterone and progestogens as medical therapy for uterine fibroids. Results: Having reviewed the most significant papers on the relationship between uterine fibroids and progesterone/progestogens, it is clear that there is biochemical, histological and clinical evidence that progesterone and progestogens play a critical role in the pathogenesis of myomas. Conclusion: Since progesterone is already implicated in the pathogenesis of this entity, using progestogens to manage fibroids is like constantly adding fuel to the fire, rendering this treatment ineffective.
1. Introduction
The prevalence of fibroids depends upon ethnic background [,]. It varies widely based on the diagnostic approach, but is estimated to be more than 60% in women over the age of 45 years [,,].
While some fibroids are asymptomatic, others result in symptoms that warrant therapy [,]. The most common symptom is heavy menstrual bleeding (HMB), but pelvic pain, bulk symptoms and infertility are other frequent manifestations that may greatly affect the quality of life of these women [,,,].
1.1. HMB: The Most Common Complaint
A number of theories have been proposed to explain fibroid-related HMB. These include an increase in uterine surface area, endometrial ulceration or an enlarged vascular network on the surface of a submucosal fibroid, greater vascular flow into the myometrium, changes in contractility of the inner junctional zone, and congestion of the endometrium and myometrium by compression of the myometrial venous plexus []. Dysregulation of normal myometrial vascular function in uterine fibroids and surrounding myometrium is due to anomalies in the expression of angiogenic growth factors and their receptors [,]. The presence of uterine fibroids may also impact the composition of the overlying endometrium, particularly the number of uterine natural killer cells and macrophages [,], which are potential producers of angiogenic growth factors.
As stressed by Ikhena and Bulun, uterine fibroids significantly affect gene expression in the endometrium []. The consecutive roles of transforming growth factor beta-3 (TGF-β3) and HOXA-10, leading to impaired endometrial receptivity, were first suggested by Rackow and Taylor []. TGF-β is known to be elevated in leiomyomas and acts as a diffusible signaling molecule to alter bone morphogenetic protein 2 (BMP-2), reducing HOXA-10 expression throughout the endometrium [,]. Moreover, Sinclair et al. and Taylor both identified defective decidualization and hemostasis in the endometrium, which may partially explain the heavier bleeding in women with uterine fibroids [,].
Despite significant advances in understanding the molecular changes in leiomyomas and associated myometrium and endometrium, it remains unclear why clinical symptoms are so diverse. Considering the strong association between uterine fibroids and HMB, ultrasound should be performed as a wholly appropriate diagnostic approach.
1.2. Existing Therapeutic Approach
Numerous treatments are available, including pharmacological, surgical, and radiological interventions, such as uterine embolization [] or MRI-guided focused ultrasound []. However, as reported in a recent editorial, hysterectomy still remains the go-to one-size-fits-all treatment for uterine fibroids. Nonetheless, many women need an effective alternative to hysterectomy for various reasons, including faster recovery and maintenance of fertility []. There is therefore a need for conservative options, and safe and effective medical therapy is one of them.
Among existing medical therapies, tranexamic acid, combined oral contraceptives, oral and injectable progestogens, progestogen-releasing intra uterine systems, antiprogesterone, gonadotropin-releasing hormone (GnRH) agonists and antagonists, selective progesterone receptor modulators (SPRMs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, danazol and gestrinone are frequently cited [,,,].
The majority of these therapies are used for the management of abnormal uterine bleeding but are not specifically indicated for uterine fibroids. Among them, the most commonly used are progestogens.
1.3. The Recurring Question: Are Progestogens Effective?
A recent decision by the EMA’s human medicines committee (CHMP) recommended restricting the use of medicines containing 5 mg ulipristal acetate as they were linked to cases of serious liver injury. If confirmed by the European Commission, this may push gynecologists to go on prescribing progestogens to treat uterine fibroids, so it is high time to evaluate their efficacy.
Progestogen is a natural or synthetic hormone. Progesterone is a natural hormone secreted by the corpus luteum, while progestin is a synthetic progestogen that can be administered orally, vaginally or by intramuscular injection. Progestogens have been used all over the world for many years in the management of uterine fibroids, despite the lack of evidence and absence of adequately designed and powered studies. One of them, depot medroxyprogesterone acetate (DMPA), has been approved for use in more than 100 countries, but other progestogens (lynestrenol, pregnane and nor-pregnane) are still used in uterine fibroids therapy.
The objective of this review is to determine the evidence or otherwise regarding the effectiveness of progestogens in treating premenopausal women with uterine fibroids. In particular, we will try to address recurring questions as to whether they are effective or not for managing symptoms commonly attributed to fibroids.
A literature search was conducted through an electronic database (PubMed, Embase, the Cochrane library) up to September 2020. The following key words were entered: uterine fibroids, progesterone, progestogen, GnRH agonist, GnRH antagonist, heavy menstrual bleeding, add-back therapy (Figure 1).
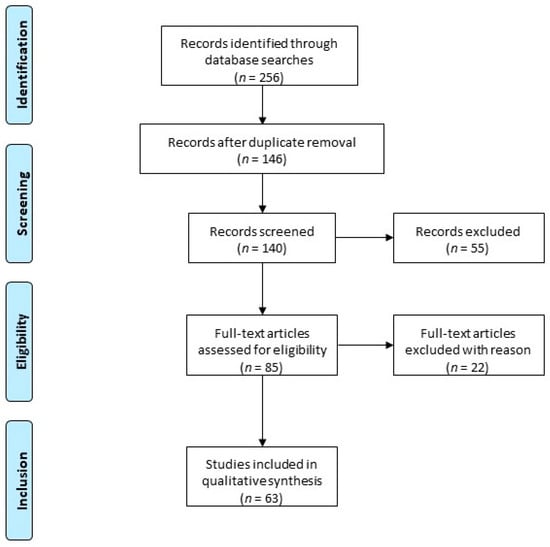
Figure 1.
Article disposition flow diagram: The following keywords were used: uterine fibroids, progesterone, progestogen, GnRH agonist, GnRH antagonist, heavy menstrual bleeding, add-back therapy. Two hundred and fifty-six records were identified. After duplicate removal, 140 records were screened, of which only peer-reviewed articles focusing on the subject were considered for eligibility assessment (n = 63). Among the conducted studies, specific various criteria led to the exclusion of 22 papers due to duplicated results.
2. Biochemical and Histological Evidence Supporting the Critical Role of Progesterone and Progestogens in the Pathogenesis of Myomas
Traditionally, estrogen has been considered the major promoter of myoma growth, but the role of progesterone has become increasingly obvious over the years. Back in 1949, elevated mitotic activity was observed in uterine fibroids removed from women treated with 20 mg of progesterone daily for 1 to 6 months []. In the 1980s, higher mitotic activity was confirmed in myomas treated with medroxyprogesterone acetate (MPA) [] and in those in the secretory phase compared to the proliferative phase [].
During the early 1990s, Lamminen et al. showed that the proliferation index in fibroids from postmenopausal women receiving estrogen and progestin was higher than that in myomas removed from postmenopausal women given estrogen alone []. By the late 1990s, the crucial role of progesterone was abundantly clear. A number of studies reported greater expression of both progesterone receptor A (PR-A) and progesterone receptor B (PR-B) in leiomyoma tissue [,] than in adjacent normal myometrium. Moreover, higher proliferative activity, evidenced by proliferating cell nuclear antigen (PCNA) and the mitotic index, was encountered in leiomyomas during the luteal (secretory) phase [] compared to the proliferative phase.
During the last decade, Kim et al. proved that progesterone promotes growth of uterine fibroids by increasing proliferation, cellular hypertrophy and deposition of the extracellular matrix (ECM) []. In an extensive review, Moravek et al. concluded that progesterone and progestin play key roles in uterine fibroid growth []. Ishikawa et al. determined that estrogen alone is not an in vivo mitogen, but plays a permissive role, acting via the induction of PR expression and thereby allowing leiomyoma responsiveness to progesterone [,]. Concentrations of PR-A and PR-B proteins were also found to be higher in leiomyomas than in matched myometrium [].
Kim and Sefton and Reis et al. described activation of signaling pathways in uterine fibroids by both estrogen and progesterone [,]. Progesterone is able to cause rapid membrane-initiated effects, independent of gene transcription, which alter the production of second messengers involved in cell signaling transduction pathways. The PI3K/AKT pathway is mediated by progesterone, which can quicky activate this pathway through its receptors. PTEN, on the other hand, should be considered a negative regulator of AKT []. Progesterone and growth factor signaling pathways are interconnected and govern numerous physiological processes, such as proliferation, apoptosis and differentiation (Figure 2).
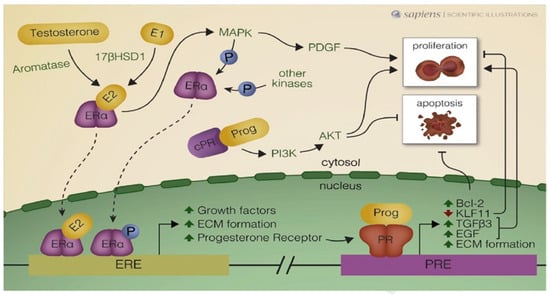
Figure 2.
Schematic illustration of autocrine and paracrine mechanisms activated by estrogen receptor alpha (Era) and progesterone receptors (PRs) in uterine leiomyoma cells. Estradiol (E2) arrives with the blood supply (endocrine), but is also synthesized within cells (autocrine), from precursors such as testosterone and estrone (E1). ERa may be phosphorylated (P) by kinases and interact with estrogen response elements (EREs) in the nucleus. 178HSD1: 178-hydroxysteroid dehydrogenase type 1; MAPK: mitogen-activated protein kinase: PDGF: platelet-derived growth factor; P13K: phosphatidylinositol-3-kinase; AKT: serine/threonine protein kinase: Bcl-2: B-cell leukemia/lymphoma-2 protein; KLF: Kruppel-like transcription factor 11; TGF-83: transforming growth factor beta 3;EGP: epidermal growth factor; ECM: extracellular matrix; Prog: progesterone; R: progesterone receptor in the cytosol and PRE: progesterone response element. From Reis et al. Best Practice & Research, Clinical Obstetrics and Gynaecology (2015), with permission from the editor and author.
As illustrated in Figure 2, numerous autocrine and paracrine mechanisms are activated by ERα and PRs in leiomyoma cells, demonstrating the crucial role of progesterone and progestogens in the pathogenesis of uterine fibroids.
3. Clinical Evidence Supporting the Critical Role of Progesterone and Progestogens in the Pathogenesis of Myomas
3.1. Progestogens as Medical Therapy for Myomas
Back in the early 1960s, it was reported that 15 out of 16 patients with uterine myomas treated with a synthetic progestin (norethynodrel, 20 to 40 mg daily) showed significantly enlarged uterine myomas, which returned to pretreatment size after discontinuation of progestin therapy in 70% of cases []. Only one randomized clinical trial (RCT), comparing lynestrenol and GnRHagonist, was published by Verspyck et al. (2000). This study showed that there was a statistically significant reduction in mean uterine fibroid volume at 16 weeks in the leuprolin group (26.5 +/− 4.5%) compared to lynestrenol (7.3% +/− 5%). However, as pointed out by Sangkomkamhang et al., the quality of the study was very low. Indeed, the risk of bias was judged to be high due to many patients being lost to follow-up (up to 22.7% in the lynestrenol group vs. 9.3% in the leuprolin group) [].
Other studies were performed in France with promegestrone (Surgestone®) [] and nomegestrol acetate []. Neither was able to demonstrate any significant reduction in bleeding or myoma size in most cases. Indeed, no individual study has established that progestogens have a beneficial effect on the different pathogenetic mechanisms involved in fibroid related HMB.
Moreover, Boyd and McCluggage described morphological changes induced by progestogens in myomas. They included small and/or large areas of infarct-type necrosis, with increased surrounding cellularity, mitotic activity, nuclear pyknosis, cytoplasmic eosinophilia, epithelioid morphology, stromal edema, hemorrhage myxoid changes and inflammatory infiltrates, including granulated lymphocytes. As stressed by the authors, pathologists should be aware of these progestogen-associated changes, since erroneous diagnoses of leiomyosarcoma or smooth muscle tumor of uncertain malignant potential cell (STUMP) may otherwise be reached [].
3.2. Association of GnRH Agonist and Add-Back Therapy
Several clinical trials evaluating the association of GnRH agonist plus add-back therapy strongly suggested an important role for progesterone and progestogens in myoma growth. Friedman et al. demonstrated that there were no significant changes in myoma volume during cotreatment with GnRH agonist plus MPA, although a significant reduction was observed in patients treated with GnRH agonist alone (leuprolide) []. These authors concluded that MPA appears to inhibit the ability of GnRH agonist to shrink uterine myomas. In an RCT, Friedman et al. showed that high doses of norethindrone can reverse the effectiveness of GnRH agonist induced myoma shrinkage in a dose-dependent manner [].
In another RCT, Carr et al. compared the effectiveness of administering MPA (20 mg/day) along with GnRH agonist (leuprolide acetate 1 mg/day subcutaneously) []. Total uterine volume, as determined by magnetic resonance imaging, decreased to 73% of the baseline at 12 weeks (p < 0.04) in the group treated with GnRH agonist alone, but did not change in the group treated with GnRH agonist plus MPA. Once again, the effectiveness of GnRH agonist was reversed by a high dose of progestin administration (MPA 20 mg/day).
In 1999, the add-back consensus working group recommended use of appropriate add-back therapy with GnRH agonist treatment to improve the hypoestrogenic symptoms and potentially extend the duration of therapy while preserving therapeutic efficacy []. Based on results from RCTs in women with endometriosis, the progestin norethindrone acetate (NETA), known as norethisterone acetate in Europe, was approved by the Food and Drug Administration at a daily dose of 5 mg, combined with synthetic GnRH agonist (leuprolide acetate), as add-back therapy in women with endometriosis []. The ESHRE guidelines stated that progestogen only as an add-back therapy does not preserve bone mineral density (BMD) [].
Chwalisz et al. believed that the inconsistent results obtained in some studies are due to confusion and the multitude of add-back regimens evaluated to date []. It should nevertheless be stressed that in vivo, NETA exhibits strong tissue-specific progestogenic, estrogenic or antiestrogenic and androgenic effects and the mean conversion ratio by aromatization of NETA to ethynyl estradiol is 0.7% to 1% at doses of 5 mg NETA []. According to Chwalisz et al., the estrogenic activity of NETA may explain its favorable impact on BMD [].
However, endometriosis and uterine fibroids are different diseases. Hence, the optimal dose for each indication should be determined. It is recommended to use the minimal dose of progestogens (combined with 1 mg E2) to reach the primary endpoint (decrease in fibroids, HMB or both), while preventing BMD loss.
3.3. Clinical Evidence in Postmenopausal Women
Having clearly demonstrated the clinical evidence in women of reproductive age, it is also logical to pursue additional investigations into the action of progestogens in postmenopausal women treated with estrogens and progestogens. Indeed, in postmenopausal women with uterine leiomyomas given 2 mg/day of micronized E2, significant changes in mean uterine leiomyoma size were detected in the group treated with 5 MPA mg daily vs. 2.5 mg, revealing the dose-dependent impact of progestogens on fibroid growth. Based on their studies, Palomba et al. and Sener et al. strongly advocated evaluation of different doses of MPA in order to administer the smallest effective dose of progestin during hormone replacement therapy to minimize the risk of fibroid growth [,].
Moro et al. reviewed 17 papers (1122 participants) to assess and ascertain the effects of hormone replacement therapy on leiomyoma development and growth in post-menopausal women []. They reported that some combinations of estrogen and progestins resulted in a significant increase in fibroid size in relation to the dose of progestin compounds [,,]. These studies also confirm the pivotal role of progesterone and progestogens in leiomyoma growth.
3.4. Indirect Proof: Efficacy of Antiprogesterone (Mifepristone) and Selective Progesterone Receptor Modulators
Mifepristone is an antiprogesterone that acts through the inhibition of PRs. Daily administration of 5 and 10 mg of mifepristone yielded uterine volume reduction of 48% after 6 months and 52% after one year []. By modulating the progesterone pathway, SPRMs may exert either an agonistic or antagonistic effect on PRs [,,,,,,,,]. Their binding allows these receptors to interact with coactivators and/or corepressors. This is further impacted by the presence of coregulators in a particular cell type, which will dictate whether an SPRM acts more as an agonist or antagonist [,,].
Ulipristal acetate (UPA) was shown to effectively and significantly reduce menstrual bleeding (as assessed by PBAC scores), induce amenorrhea, and decrease the size of leiomyomas by up to 50% after 6 months via its antagonist action on myomas level [,,]. Courtoy et al. described the specific impact of SPRMs (UPA) on myomas. Increased apoptosis, reduced survival and lower proliferation rates were also evidenced by gene expression changes, as was an increase in ECM resorption due to the high activity of matrix metalloproteinases [,,].
UPA was actually a very effective drug [,,] but, unfortunately, due to very rare (1/150,000) but non-predictable cases of drug-induced liver injury (DILI), the CHMP very recently decided to significantly limit its use to premenopausal women who cannot undergo surgery or in the case of uterine fibroid embolization, or if the surgical procedure fails (still pending EMA confirmation).
4. Evidence from Available and Recent Systematic Reviews
In 2013, Sangkomkamhong conducted a systematic review (Cochrane library) on progestogen use in fibroid therapy []. Progestins have been utilized for many years in the treatment of uterine fibroids and are still used in some countries (Table 1). However, as emphasized in this Cochrane review, the lack of high-quality studies has proved to be a common problem when systematic evaluation of their benefits and potential harms is required. The authors concluded that evidence is insufficient to support the use of progestogens in treating premenopausal women with uterine fibroids. The same conclusion was reached by Lethaby et al. in their systematic review published in 2017 in the Cochrane library [].

Table 1.
Describe the most relevant studies on the topic.
An extensive review by Bitzer et al. on the medical management of HMB found the LNG-IUS (levonorgestrel-releasing intrauterine system) to be the first-line medical therapy for HMB due to dysfunctional uterine bleeding (characterized by the absence of fibroids). In the presence of HMB due to fibroids, however, it shows much more limited efficacy. These authors reported that MPA and NETA are approved in many countries for the treatment of various forms of “abnormal” uterine bleeding, but their long-term use in fibroid-related HMB is not currently supported by solid evidence, because of the absence of benefits reported in the literature [].
In a systematic review and network meta-analysis of RCTs investigating medical therapy for uterine fibroids, Gurusamy et al. identified 75 RCTs among 4237 references []. Only one reported the results of a progestogen, namely the study by Verspijck et al. previously discussed in this manuscript. Since 2000, there have been no reports of RCTs on progestogens in medical therapy for uterine fibroids [].
After appraising all available options, Sohn et al. concluded from a literature review and consensus of expert opinion that GnRH agonist and SPRMs are currently the best effective medical therapies, with the best evidence to support their ability to reduce fibroid volume and HMB. Nevertheless, there is a lack of data on the true efficacy of progestogens, which may even promote uterine fibroid growth [].
5. Conclusions
In this review of the most significant papers on the relationship between uterine fibroids and progesterone/progestogens (Table 1), we have clearly shown biochemical, histological, and clinical evidence that progesterone and progestogens play a critical role in the pathogenesis of myomas. In their manuscript entitled “Practice guidelines on the management of uterine fibroids”, Vilos et al. did not ever include progestogens in their algorithm, as they felt that evidence of their efficacy was still lacking []. Therefore, summarizing studies on progestogens and uterine fibroids overall, we can conclude that the evidence actually points to a lack of evidence of their efficacy.
On the other hand, effective medications such as GnRH agonist and antagonist induce hypoestrogenic symptoms (including progressive BMD loss and vasomotor symptoms), but hormone add-back therapy may well enhance compliance and extend the duration of therapy. The choice of hormone add-back treatment for myomas should aim to exploit the minimal effective dose of progestogens to preserve the therapeutic effects of GnRH agonist and antagonist. Low doses of E2 (1 mg) combined with NETA (0.5 mg) have proved capable of preventing bone loss in early post-menopausal women [] and those with endometriosis undergoing GnRH agonist therapy []. This combination may therefore be considered an option for women with uterine fibroids subjected to long-term GnRH agonist or antagonist therapy.
Funding
This research received no external funding.
Acknowledgments
The author thanks Hasan DONAT for his help in the redaction of this article.
Conflicts of Interest
Jacques Donnez is member of the Scientific Advisory Board of Obseva and Preglem.
References
- Andersen, J. Factors in fibroid growth. Baillieres Clin. Obste. Gynaecol. 1998, 12, 225–243. [Google Scholar]
- Donnez, J.; Dolmans, M.-M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, M.A.; Hamoodi, I.; Gupta, J.; Hickey, M. Fibroids: Diagnosis and management. BMJ 2015, 351, h4887. [Google Scholar] [CrossRef] [PubMed]
- Okolo, S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 571–588. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Hormone therapy for intramural myoma-related infertility from ulipristal acetate to GnRH antagonist: A review. Reprod. Biomed. Online 2020, 41, 431–442. [Google Scholar] [CrossRef]
- Donnez, J.; Courtoy, G.E.; Donnez, O.; Dolmans, M.-M. Ulipristal acetate for the management of large uterine fibroids associated with heavy bleeding: A review. Reprod. Biomed. Online 2018, 37, 216–223. [Google Scholar] [CrossRef]
- Stewart, E.A. Leiomyoma-related bleeding: A classic hypothesis updated for the molecular era. Hum. Reprod. Update 1996, 2, 295–306. [Google Scholar] [CrossRef]
- Weston, G.; Trajstman, A.C.; Gargett, C.E.; Manuelpillai, U.; Vollenhoven, B.J.; Rogers, P.A.W. Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol. Hum. Reprod. 2003, 9, 541–549. [Google Scholar] [CrossRef]
- Kitaya, K.; Matsubayashi, H.; Yamaguchi, K.; Nishiyama, R.; Takaya, Y.; Ishikawa, T.; Yasuo, T.; Yamada, H. Chronic Endometritis: Potential Cause of Infertility and Obstetric and Neonatal Complications. Am. J. Reprod. Immunol. 2015, 75, 13–22. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Bulmer, J.N. Pathophysiology of Heavy Menstrual Bleeding. Women’s Health (Lond.) 2016, 12, 3–13. [Google Scholar] [CrossRef]
- Ikhena, D.E.; Bulun, S.E. Literature Review on the Role of Uterine Fibroids in Endometrial Function. Reprod. Sci. 2017, 25, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Rackow, B.W.; Taylor, H.S. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil. Steril. 2010, 93, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.F.; Taylor, H.S. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil. Steril. 2015, 103, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Fibroids: When should they be removed to improve in vitro fertilization success? Fertil. Steril. 2018, 109, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.C.; Mastroyannis, A.; Taylor, H.S. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-beta3. J. Clin. Endocrinol. Metab. 2011, 96, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Manyonda, I.; Belli, A.-M.; Lumsden, M.-A.; Moss, J.; McKinnon, W.; Middleton, L.J.; Cheed, V.; Wu, O.; Sirkeci, F.; Daniels, J.P.; et al. Uterine-Artery Embolization or Myomectomy for Uterine Fibroids. N. Engl. J. Med. 2020, 383, 440–451. [Google Scholar] [CrossRef]
- Stewart, E.A. Comparing Apples to Apples for Fibroids. N. Engl. J. Med. 2020, 383, 489–490. [Google Scholar] [CrossRef]
- Segaloff, A.; Weed, J.C.; Sternberg, W.H.; Parson, W. The progesterone therapy of human uterine leiomyomas. J. Clin. Endocrinol. Metab. 1949, 9, 1273–1291. [Google Scholar] [CrossRef]
- Tiltman, A.J. The Effect of Progestins on the Mitotic Activity of Uterine Fibromyomas. Int. J. Gynecol. Pathol. 1985, 4, 89–96. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Fujii, S.; Konishi, I.; Nanbu, Y.; Nonogaki, H.; Mori, T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am. J. Obstet. Gynecol. 1989, 160, 637–641. [Google Scholar] [CrossRef]
- Englund, K.; Blanck, A.; Gustavsson, I.; Lundkvist, U.; Sjöblom, P.; Norgren, A.; Lindblom, B. Sex steroid receptors in human myometrium and fibroids: Changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J. Clin. Endocrinol. Metab. 1998, 83, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Gillerot, S.; Casanas-Roux, F.; Squifflet, J.; Berlière, M.; Donnez, J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum. Reprod. 1999, 14, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon V, J.S.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.-J.; et al. Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Fenkci, V.; Fencki, V.; Marsh, E.E.; Yin, P.; Chen, N.; Cheng, Y.-H.; Reisterd, S.; Lin, Z.; Bulun, S.E. CCAAT/enhancer binding protein beta regulates aromatase expression via multiple and novel cis-regulatory sequences in uterine leiomyoma. J. Clin. Endocrinol. Metab. 2008, 93, 981–991. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone Is Essential for Maintenance and Growth of Uterine Leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef]
- Tsigkou, A.; Reis, F.M.; Lee, M.H.; Jiang, B.; Tosti, C.; Centini, G.; Shen, F.-R.; Chen, Y.-G.; Petraglia, F. Increased progesterone receptor expression in uterine leiomyoma: Correlation with age, number of leiomyomas, and clinical symptoms. Fertil. Steril. 2015, 104, 170–175.e1. [Google Scholar] [CrossRef]
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef]
- Reis, F.M.; Bloise, E.; Ortiga-Carvalho, T.M.; Information, P.E.K.F.C. Hormones and pathogenesis of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 13–24. [Google Scholar] [CrossRef]
- Mixson, W.T.; Hammond, D.O. Response of fibromyomas to a progestin. Am. J. Obstet. Gynecol. 1961, 82, 754–760. [Google Scholar] [CrossRef]
- Sangkomkamhang, U.S.; Lumbiganon, P.; Laopaiboon, M.; Mol, B.W.J. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, H. Traitement médical des fibromes utérins par les progestatifs de synthèse du groupe norprégnane. Gynécologie 1989, 40, 175–179. [Google Scholar]
- Audebert, A.; Denis, C. Utilisation de la promegestone dans le traitement des fibromyomes compliqués de ménométrorragies. Bilan d’une étude multicentrique. Gynécologie 1989, 40, 23–26. [Google Scholar]
- Boyd, C.; McCluggage, W.G. Unusual morphological features of uterine leiomyomas treated with progestogens. J. Clin. Pathol. 2011, 64, 485–489. [Google Scholar] [CrossRef]
- Friedman, A.J.; Barbieri, R.L.; Doubilet, P.M.; Fine, C.; Schiff, I. A randomized, double-blind trial of a gonadotropin relcasing-hormone agonist (leuprolide) with or without me medroxyprogesterone acetate in the treatment of leiomyomamata uteri. Fertil. Steril. 1988, 49, 404–409. [Google Scholar] [CrossRef]
- Friedman, A.J.; Daly, M.; Juneau-Norcross, M.; Rein, M.S.; Fine, C.; Gleason, R.; Leboff, M. A pro spective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add back” regimens for women with leiomyomata uteri. J. Clin. Endocrinol. Metab. 1993, 76, 1439–1445. [Google Scholar]
- Carr, B.R.; Marshburn, P.B.; Weatherall, P.T.; Bradshaw, K.D.; Breslau, N.A.; Byrd, W.; Roark, M.; Steinkampf, M.P. An evaluation of the effect of gonadotropin-releasing hormone ana logs and medroxyprogesterone acetate on uterine Jeiomyomata volume by magnetic resonance imaging: A prospective, randomized, double blind, placebo-controlled crossover trial. J. Clin. Endocrinol. Metab. 1993, 76, 1217–1223. [Google Scholar]
- Surrey, E.S. Add-back therapy and gonadotropin-releasing hormone agonists in the treatment of patients with endometriosis: Can a consensus be reached? Add-Back Consensus Working Group. Fertil. Steril. 1999, 71, 420–424. [Google Scholar] [CrossRef]
- Chwalisz, K.; Surrey, E.; Stanczyk, F.Z. The Hormonal Profile of Norethindrone Acetate: Rationale for Add-Back Therapy with Gonadotropin-Releasing Hormone Agonists in Women with Endometriosis. Reprod. Sci. 2012, 19, 563–571. [Google Scholar] [CrossRef]
- European Society of Human Reproduction and Embryology. ESHRE Guideline for the Diagnosis and Treatment of Endometriosis. 2010. Available online: http://guidelines.endometriosis.org/index.html (accessed on 1 October 2020).
- Lamminen, S.; Rantald, I.; Helin, H.; Rorarius, M.; Tuimala, R.; Rantala, I.; Tuimala, R. Proliferative Activity of Human Uterine Leiomyoma Cells as Measured by Automatic Image Analysis. Gynecol. Obstet. Investig. 1992, 34, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kuhnz, W.; Heuner, A.; Hümpel, M.; Seifert, W.; Michaelis, K. In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women. Contraception 1997, 56, 379–385. [Google Scholar] [CrossRef]
- Palomba, S.; Sena, T.; Morelli, M.; Noia, R.; Zullo, F.; Mastrantonio, P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 102, 199–201. [Google Scholar] [CrossRef]
- Sener, A.B.; Seçkin, N.C.; Ozmen, S.; Gökmen, O.; Doğu, N.; Ekici, E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil. Steril. 1996, 65, 354–357. [Google Scholar]
- Moro, E.; Esposti, E.D.; Borghese, G.; Manzara, F.; Zanello, M.; Raimondo, D.; Gava, G.; Arena, A.; Casadio, P.; Meriggiola, M.C.; et al. The Impact of Hormonal Replacement Treatment in Postmenopausal Women with Uterine Fibroids: A State-of-the-Art Review of the Literature. Medicina 2019, 55, 549. [Google Scholar] [CrossRef]
- Polatti, F.; Viazzo, F.; Colleoni, R.; Nappi, R.E. Uterine myoma in postmenopause: A comparison between two therapeutic schedules of HRT. Maturitas 2000, 37, 27–32. [Google Scholar] [CrossRef]
- Palomba, S.; Sena, T.; Noia, R.; Carlo, C.D.; Zullo, F.; Mastrantonio, P. Transdermal Hormone Replacement Therapy in Postmenopausal Women with Uterine Leiomyomas. Obstet. Gynecol. 2001, 98, 1053–1058. [Google Scholar]
- Eisinger, S.H.; Bonfiglio, T.; Fiscella, K.; Meldrum, S.; Guzick, D.S. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J. Minim. Invasive Gynecol. 2005, 12, 227–233. [Google Scholar] [CrossRef]
- Chabbert-Buffet, N.; Meduri, G.; Bouchard, P.; Spitz, I.M. Selective progesterone receptor modulators and progesterone antagonists: Mechanisms of action and clinical applications. Hum. Reprod. Update 2005, 11, 293–307. [Google Scholar] [CrossRef]
- Chabbert-Buffet, N.; Chassard, D.; Ochsenbein, E.; Thomas, J.-L.; Christin-Maitre, S. Inhibition of ovulation by NOMAC/E2, a novel monophasic oral contraceptive combining nomegestrol acetate and 17β-oestradiol: A double-blind, randomised, dose-finding pilot study. Eur. J. Contracept. Reprod. Health Care 2011, 16, 76–84. [Google Scholar] [CrossRef]
- Chabbert-Buffet, N.; Esber, N.; Bouchard, P. Fibroid growth and medical options for treatment. Fertil. Steril. 2014, 102, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, P.; Chabbert-Buffet, N.; Fauser, B.C. Selective progesterone receptor modulators in reproductive medicine: Pharmacology, clinical efficacy and safety. Fertil. Steril. 2011, 96, 1175–1189. [Google Scholar] [CrossRef]
- Bouchard, P. Selective progesterone receptor modulators: A class with multiple actions and applications in reproductive endocrinology, and gynecology. Gynecol. Endocrinol. 2014, 30, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Bestel, E.; Donnez, J. The potential of selective progesterone receptor modulators for the treatment of uterine fibroids. Expert Rev. Endocrinol. Metab. 2013, 9, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Tatarchuk, T.F.; Bouchard, P.; Puscasiu, L.; Zakharenko, N.F.; Ivanova, T.; Ugocsai, G.; Mara, M.; Jilla, M.P.; Bestel, E.; et al. Ulipristal Acetate versus Placebo for Fibroid Treatment before Surgery. N. Engl. J. Med. 2012, 366, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Tomaszewski, J.; Vázquez, F.; Bouchard, P.; Lemieszczuk, B.; Baró, F.; Nouri, K.; Selvaggi, L.; Sodowski, K.; Bestel, E.; et al. Ulipristal Acetate versus Leuprolide Acetate for Uterine Fibroids. N. Engl. J. Med. 2012, 366, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Vázquez, F.; Tomaszewski, J.; Nouri, K.; Bouchard, P.; Fauser, B.C.; Barlow, D.H.; Palacios, S.; Donnez, O.; Bestel, E.; et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil. Steril. 2014, 101, 1565–1573. [Google Scholar] [CrossRef]
- Courtoy, G.E.; Donnez, J.; Marbaix, E.; Dolmans, M.-M. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil. Steril. 2015, 104, 426–434.e1. [Google Scholar] [CrossRef]
- Courtoy, G.E.; Donnez, J.; Ambroise, J.; Arriagada, P.; Luyckx, M.; Marbaix, E.; Dolmans, M.-M. Gene expression changes in uterine myomas in response to ulipristal acetate treatment. Reprod. Biomed. Online 2018, 37, 224–233. [Google Scholar] [CrossRef]
- Courtoy, G.E.; Donnez, J.; Marbaix, E.; Barreira, M.; Luyckx, M.; Dolmans, M.-M. Progesterone Receptor Isoforms, Nuclear Corepressor-1 and Steroid Receptor Coactivator-1 and B-Cell Lymphoma 2 and Akt and Akt Phosphorylation Status in Uterine Myomas after Ulipristal Acetate Treatment: A Systematic Immunohistochemical Evaluation. Gynecol. Obstet. Investig. 2017, 83, 443–454. [Google Scholar] [CrossRef]
- Lethaby, A.; Puscasiu, L.; Vollenhoven, B. Preoperative medical therapy before surgery for uterine fbroids. Cochrane Database Syst. Rev. 2017, 11, CD000547. [Google Scholar] [PubMed]
- Bitzer, J.; Heikinheimo, O.; Nelson, A.L.; Calaf-Alsina, J.; Fraser, I.S. Medical management of heavy menstrual bleeding: A comprehensive review of the literature. Obstet. Gynecol. Surv. 2015, 70, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, K.S.; Vaughan, J.; Fraser, I.S.; Best, L.M.J.; Richards, T. Medical Therapies for Uterine Fibroids—A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. PLoS ONE 2016, 11, e0149631. [Google Scholar] [CrossRef] [PubMed]
- Verspyck, E.; Marpeau, L.; Lucas, C. Leuprorelin depot 3.75 mg versus lynestrenol in the preoperative treatment of symptomatic uterine myomas: A multicentre randomised trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 7–13. [Google Scholar] [CrossRef]
- Sohn, G.S.; Cho, S.; Kim, Y.M.; Cho, C.; Kim, M.R.; Lee, S.R. Current medical treatment of uterine fibroids for the Working Group of Society of Uterine Leiomyoma. Obstet. Gynecol. Sci. 2018, 61, 192–201. [Google Scholar] [CrossRef]
- Amadio, E. Traitement médical des fibromyomes utérins par le nomégestrol acétate. Abstract Gynecol 1991, 69, 1–4. [Google Scholar]
- Johnson, N.; Fletcher, H.; Reid, M.E. Depo medroxyprogesterone acetate (DMPA) therapy for uterine myomata prior to surgery. Int. J. Gynecol. Obstet. 2003, 85, 174–176. [Google Scholar] [CrossRef]
- Lumbiganon, P.; Rugpao, S.; Phandhu-Fung, S.; Laopaiboon, M.; Vudhikamraksa, N.; Werawatakul, Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: A multicentre case--control study. Br. J. Obstet. Gynaecol. 1996, 103, 909–914. [Google Scholar] [CrossRef]
- Marsh, E.E.; E Bulun, S. Steroid Hormones and Leiomyomas. Obstet. Gynecol. Clin. North Am. 2006, 33, 59–67. [Google Scholar] [CrossRef]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Updat. 2004, 10, 207–220. [Google Scholar] [CrossRef]
- Maruo, T.; Matsuo, H.; Samoto, T.; Shimomura, Y.; Kurachi, O.; Gao, Z.; Wang, Y.; Spitz, I.M.; Johansson, E. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 2000, 65, 585–592. [Google Scholar] [CrossRef]
- Rein, M.S.; Barbieri, R.L.; Friedman, A.J. Progesterone: A critical role in the pathogenesis of uterine myomas. Am. J. Obstet. Gynecol. 1995, 172, 14–18. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Bagratee, J.; Moodley, J. Medical management of uterine fibroids with medroxyprogesterone acetate (Depo Provera): A pilot study. J. Obstet. Gynaecol. 2004, 24, 798–800. [Google Scholar] [CrossRef]
- West, C.P.; Lumsden, M.A.; Hillier, H.; Sweeting, V.; Baird, D.T. Potential role for medroxyprogesterone acetate as an adjuct to goserelin (Zoladex) in the medical management of uterine fibroids. Hum. Reprod. 1992, 7, 328–332. [Google Scholar] [CrossRef]
- E Bulun, S. Uterine Fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef]
- Donnez, J.; Hudeček, R.; Donnez, O.; Matule, D.; Arhendt, H.-J.; Zatik, J.; Kasilovskiene, Z.; Dumitrascu, M.C.; Fernandez, H.; Barlow, D.H.; et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil. Steril. 2015, 103, 519–527. [Google Scholar] [CrossRef]
- Koskas, M.; Chabbert-Buffet, N.; Douvier, S.; Huchon, C.; Paganelli, E.; Derrien, J. Place des traitements médicaux : Indication, durée, efficacité, chez la femme porteuse de fibromes utérins symptomatiques en période d’activité génitale. J. Gynécol. Obstét. Biol. Reprod. 2011, 40, 858–874. [Google Scholar] [CrossRef]
- Moroni, R.; Ferriani, R.; Dos Reis, F.J.C.; Brito, L.; Vieira, C. Pharmacological treatment of uterine fibroids. Ann. Med. Heal. Sci. Res. 2014, 4, 185. [Google Scholar] [CrossRef]
- Moroni, R.M.; Martins, W.P.; A Ferriani, R.; Vieira, C.S.; Nastri, C.; Dos Reis, F.J.C.; Brito, L.G. Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Vilos, G.A.; Allaire, C.; Laberge, P.-Y.; Leyland, N.; Vilos, A.G.; Murji, A.; Chen, I. The Management of Uterine Leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef]
- Greenwald, M.W.; Gluck, O.S.; Lang, E.; Rakov, V. Oral hormone therapy with 17beta-estradiol and 17beta-estradiol in combination with norethindrone acetate in the prevention of bone loss in early postmenopausal women: Dose-dependent effects. Menopause 2005, 12, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Park, H.G.; Yoon, B.-K.; Choi, D. Effects of different add-back regimens on hypoestrogenic problems by postoperative gonadotropin-releasing hormone agonist treatment in endometriosis. Obstet. Gynecol. Sci. 2016, 59, 32–38. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).