Characteristics and Prognosis of COVID-19 in Patients with COPD
Abstract
1. Introduction
2. Methods
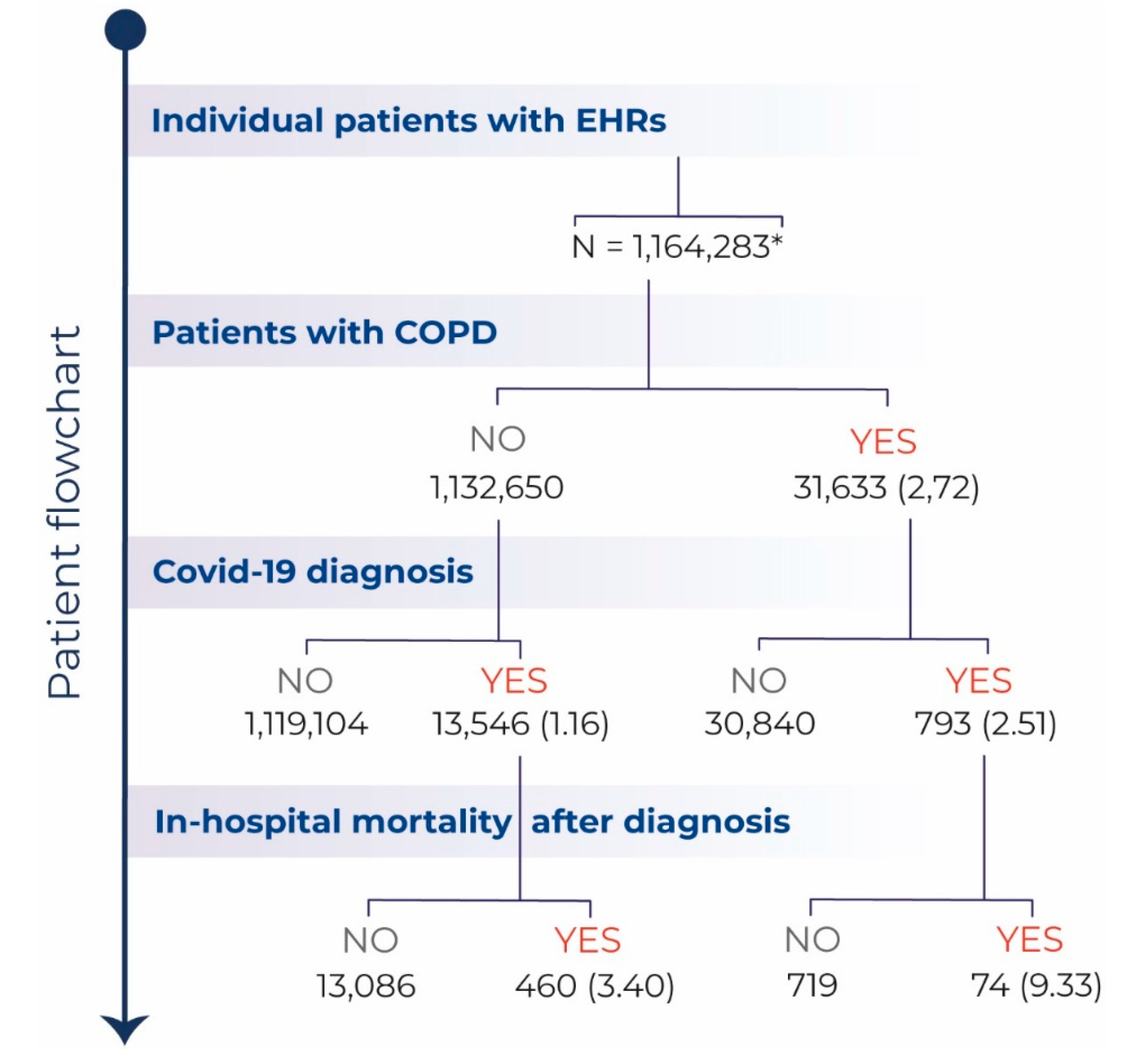
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 Report. Available online: www.goldcopd.org (accessed on 20 May 2020).
- Miravitlles, M. Epidemiology of chronic obstructive pulmonary disease exacerbations. Clin. Pulm. Med. 2002, 9, 191–197. [Google Scholar] [CrossRef]
- Dewan, N.A.; Rafique, S.; Kanwar, B.; Satpathy, H.; Ryschon, K.; Tillotson, G.S.; Niederman, M.S. Acute exacerbation of COPD. Factors associated with poor outcome. Chest 2000, 117, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluna, J.J. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19) Outbreak. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 7 September 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Mes, M.; Leung, S.M.K.; Lau, H.Y.E.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, G.C.; Paul, E.A.; Bestall, J.C.; Jeffries, D.J.; Wedzicha, J.A. Effect of Exacerbation on Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- De Lucas-Ramos, P.; Izquierdo-Alonso, J.L.; Rodriguez-Gonzalez Moro, J.M.; Frances, J.F.; Lozano, P.V.; Bellón-Cano, J.M. CONSISTE study group: Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study). Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 679–686. [Google Scholar]
- Izquierdo, J.L.; Martínez, A.; de Lucas, P.G.; Rodríguez, J.M. Lack of association of ischemic heart disease with COPD when taking into account classical cardiovascular risk factors. Int. J. Chron. Obstruct. Pulmon. Dis. 2010, 5, 387–394. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Benson, T. Principles of Health Interoperability HL7 and SNOMED, 2nd ed.; Springer: London, UK, 2012. [Google Scholar]
- Baeza-Yates, R.A.; Ribeiro-Neto, B. Modern Information Retrieval; Addison-Wesley Longman: Boston, MA, USA, 1999. [Google Scholar]
- Izquierdo, J.L.; Morena, D.; Gonzalez, Y.; Paredero, J.M.; Perez, B.; Graziani, D.; Gutiérrez, M.; Rodríguez, J.M. Clinical Management of COPD in a Real-World Setting. A Big Data Analysis. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Almonacid, C.; González, Y.; Del Rio-Bermúdez, C.; Ancochea, J.; Cárdenas, R.; Soriano, J.B. The impact of covid-19 on patients with asthma. medRxiv 2020. [Google Scholar] [CrossRef]
- Tal-Singer, R.; Crapo, J.D. COPD at the time of COVID-19: A COPD Foundation perspective. Chronic. Obstr. Pulm. Dis. 2020, 7, 73–75. [Google Scholar] [CrossRef]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir. Med. 2020, 167, 105941. [Google Scholar] [CrossRef]
- Faust, J.S.; del Rio, C. Assessment of Deaths From COVID-19 and From Seasonal Influenza. JAMA Int. Med. 2020, 180, 1045–1046. [Google Scholar] [CrossRef]
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N. Engl. J. Med. 2020, 382, e102. [Google Scholar] [CrossRef]
- De Abajo, F.J.; Rodríguez-Martín, S.; Lerma, V.; Mejía-Abril, G.; Aguilar, M.; García-Luque, A.; Laredo, L.; Laosa, O.; Centeno-Soto, G.A.; Gálvez, M.Á.; et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: A case-population study. Lancet 2020, 395, 1705–1714. [Google Scholar] [CrossRef]
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P.G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 Investigators. COVID-19 Related Genes in Sputum Cells in Asthma: Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020, 202, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Anke, L.T.J.; Pardo, A.; Medrano, I.; Ureña, A.; Salcedo, I.; Saggion, H. Savana: A Global Information Extraction and Terminology Expansion Framework in the Medical Domain. Sociedad Española para el Procesamiento del Lenguaje Natural 2016, 57, 23–30. [Google Scholar]
- Ford, E.; Carroll, J.A.; Smith, H.E.; Scott, D.; Cassell, J.A. Extracting information from the text of electronic medical records to improve case detection: A systematic review. J. Am. Med. Inf. Assoc. 2016, 23, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296. [Google Scholar] [CrossRef]
| Characteristics of the Study Population | COVID-19 > 40 Years (1 January–10 May 2020) | COPD-COVID-19 (1 January–10 May 2020) | OR (95% CI) p-Value |
|---|---|---|---|
| N | 13.546 | 793 | |
| Age | |||
| Years (mean ± SD) | 66 ± 15 | 75 ± 12 | <0.001 |
| Sex | |||
| Female (%) | 53 | 17 | 0.18 (0.15–0.22) |
| Comorbidities | |||
| Ischemic heart disease (%) | 8.8 | 22.6 | 3.02 (2.53–3.60) |
| Heart failure (%) | 13.8 | 33.9 | 3.21 (2.75–3.75 |
| Cardiac arrhythmia (%) | 8.0 | 18.5 | 2.61 (2.16–3.15) |
| Diabetes mellitus (%) | 26.1 | 40.6 | 1.93 (1.67–2.24) |
| Arterial Hypertension (%) | 54.2 | 76.4 | 2.74 (2.32–3.24) |
| Dyslipidemia (%) | 15.4 | 20.2 | 1.39 (1.16–1.66) |
| Pulmonary embolism (%) | 2.2 | 5.2 | 2.46 (1.76–3.44) |
| Stroke (%) | 8.3 | 13.1 | 1.46 (1.18–1.81) |
| Smoking (%) | 13 | 33.9 | 3.43 (2.93–4.00) |
| Hospitalization (%) | 31.1 | 39.8 | 1.57 (1.35–1.82) |
| In-hospital mortality (%) | 3.4 | 9.3 | 2.93 (2.27–3.79) |
| Characteristics of the Study Population | COPD-COVID-19 (1 January–10 May 2020) | COPD-Influenza 2019–2020 (1 December 2018–30 April 2019) | OR (95% CI) p-Value * | COPD-Influenza 2018–2019 (1 December 2017–30 April 2018) | OR (95% CI) p-Value ** |
|---|---|---|---|---|---|
| N | 793 | 826 | 1066 | ||
| AgeYears (mean ± SD) | 75 ± 12 | 72 ± 13 | 73 ± 13 | <0.001 | |
| Sex | |||||
| Female (%) | 17 | 19 | 0.87 (0.68–1.13) | 17 | 1.00 (0.79–1.28) |
| Comorbidities | |||||
| Ischemic Heart disease (%) | 22.6 | 20.8 | 1.11 (0.88–1.40) | 23.0 | 0.98 (0.79–1.22) |
| Heart failure (%) | 33.9 | 30.5 | 1.17 (0.95–1.44) | 37.1 | 0.87 (0.72–1.05) |
| Cardiac arrhythmia (%) | 18.5 | 20.5 | 0.89 (0.69–1.13) | 22.1 | 0.74 (0.59–0.93) |
| Diabetes mellitus (%) | 40.6 | 38 | 1.12 (0.91–1.36) | 43.1 | 0.90 (0.75–1.09) |
| Arterial Hypertension (%) | 76.4 | 74.5 | 1.11 (0.89–1.40) | 77.9 | 0.92 (0.74–1.15) |
| Dyslipidemia (%) | 20.2 | 22.9 | 0.85 (0.67–1.08) | 21.4 | 0.93 (0.93–1.17) |
| Pulmonary embolism (%) | 5.2 | 5.0 | 1.04 (0.67–1.63) | 6.4 | 0.80 (0.54–1.19) |
| Stroke (%) | 13.1 | 12.0 | 1.11 (0.83–1.49) | 12.8 | 1.03 (0.79–1.36) |
| Smoking (%) | 33.9 | 43.8 | 0.66 (0.54–0.81) | 39.3 | 0.79 (0.65–0.96) |
| Hospitalization (%) | 39.8 | 5.9 | 10.51 (7.61–14.5) | 6.8 | 9.01 (6.83–11.89) |
| In-hospital mortality (%) | 9.3 | 2.2 | 4.62 (2.73–7.81) | 5.2 | 1.89 (1.32–2.18) |
| Characteristics of the Study Population | COPD-COVID-19 Survivors (1 January–10 May 2020) | COPD-COVID-19 Deceased (1 January–10 May 2020) | OR (95% CI) p-Value |
|---|---|---|---|
| N | 719 | 74 | |
| Age | |||
| Years (mean ± SD) | 74 ± 11 | 77 ± 11 | 0.03 |
| Sex | |||
| Female (%) | 18 | 16 | 1.58 (0.37–3.25) |
| Comorbidities | |||
| Ischemic Heart disease (%) | 24 | 16 | 1.59 (0.84–3.02) |
| Heart failure (%) | 33 | 45 | 0.61 (0.37–0.99) |
| Arrythmia (%) | 18 | 22 | 0.81 (0.45–1.45) |
| Diabetes mellitus (%) | 41 | 41 | 1.00 (0.62–1.63) |
| Arterial Hypertension (%) | 76 | 77 | 0.96 (0.55–1.70) |
| Dyslipidemia (%) | 20 | 23 | 0.83 (0.47–1.47) |
| Pulmonary embolism (%) | 5 | 5 | 0.95 (0.39–1.46) |
| Stroke (%) | 11 | 16 | 0.76 (0.58–1.71) |
| Sleep Apnea (%) | 11 | 11 | 1.06 (0.49–2.29) |
| Smoking (%) | 34 | 28 | 1.33 (0.78–2.25) |
| COPD-COVID19 Survivors (1 January–10 May 2020) | COPD-COVID-19 Deceased (1 January–10 May 2020) | OR (95% CI) | |
|---|---|---|---|
| N | 719 | 74 | |
| Treatment | |||
| Inhaled steroids (%) | 96 | 95 | 0.68 (0.23–2.01) |
| Anticholinergics (%) | 89 | 99 | 8.88 (1.22–64.81) |
| Beta-2 agonists (%) | 85 | 95 | 3.06 (1.09–8.56) |
| Statins (%) | 48 | 42 | 0.77 (0.48–1.26) |
| IECAS (%) | 33 | 23 | 0.60 (0.34–1.05) |
| ARA2 (%) | 37 | 41 | 1.15 (0.71–1.88) |
| Antiaggregant (%) | 79 | 74 | 0.77 (0.44–1.34) |
| Beta-blockers (%) | 27 | 27 | 1.00 (0.59–1.72) |
| Model 1 | Model 2 | Model 2 | |||
|---|---|---|---|---|---|
| COPD | 1.70 (1.29–2.23) | COPD | 1.52 (1.15–2.00) | COPD | 1.42 (1.07–1.88) |
| Sex | 1.86 (1.53–2.26) | Sex | 1.87 (1.54–2.28) | Sex | 1.82 (1.49–2.22) |
| Age | 1.06 (1.05–1.07) | Age | 1.05 (1.04–1.06) | Age | 1.05 (1.04–1.06) |
| HF | 1.65 (1.33–2.03) | Heart failure | 1.46 (1.17–1.81) | ||
| HBP | 1.59 (1.24–2.04) | HBP | 1.43 (1.10–1.84) | ||
| Stroke | 1.02 (0.78–1.32) | ||||
| Arrythmia | 1.30 (1.01–1.71) | ||||
| IHD | 1.05 (0.81–1.36) | ||||
| Diabetes | 1.23 (1.01–1.50) | ||||
| HL | 1.03 (0.81–1.31) | ||||
| Sleep apnoea | 1.27 (0.85–1.90) | ||||
| PTE | 1.72 (1.08–2.75) | ||||
| Smoking | 1.31 (0.99–1.71) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziani, D.; Soriano, J.B.; Del Rio-Bermudez, C.; Morena, D.; Díaz, T.; Castillo, M.; Alonso, M.; Ancochea, J.; Lumbreras, S.; Izquierdo, J.L. Characteristics and Prognosis of COVID-19 in Patients with COPD. J. Clin. Med. 2020, 9, 3259. https://doi.org/10.3390/jcm9103259
Graziani D, Soriano JB, Del Rio-Bermudez C, Morena D, Díaz T, Castillo M, Alonso M, Ancochea J, Lumbreras S, Izquierdo JL. Characteristics and Prognosis of COVID-19 in Patients with COPD. Journal of Clinical Medicine. 2020; 9(10):3259. https://doi.org/10.3390/jcm9103259
Chicago/Turabian StyleGraziani, Desirée, Joan B Soriano, Carlos Del Rio-Bermudez, Diego Morena, Teresa Díaz, María Castillo, Miguel Alonso, Julio Ancochea, Sara Lumbreras, and José Luis Izquierdo. 2020. "Characteristics and Prognosis of COVID-19 in Patients with COPD" Journal of Clinical Medicine 9, no. 10: 3259. https://doi.org/10.3390/jcm9103259
APA StyleGraziani, D., Soriano, J. B., Del Rio-Bermudez, C., Morena, D., Díaz, T., Castillo, M., Alonso, M., Ancochea, J., Lumbreras, S., & Izquierdo, J. L. (2020). Characteristics and Prognosis of COVID-19 in Patients with COPD. Journal of Clinical Medicine, 9(10), 3259. https://doi.org/10.3390/jcm9103259






