The Effect of Heterogeneous Definitions of Massive Transfusion on Using Blood Component Thresholds to Predict Futility in Severely Bleeding Trauma Patients
Abstract
1. Introduction
2. Blood Component Cut-Points of Transfusions to Define Futility
2.1. Historical Evolution of Defining MT Based on Units of Blood Component per Hour
2.2. Critical Administration Threshold (CAT) and Resuscitation Intensity (RI)
2.3. Incorporation of WB as Part of a Cut-Point
2.4. Simplification of Defining Blood Components for FR
2.5. Summary of Literature Defining Transfusion Cut-Points as Predictors of FR
3. Combination of Clinical, Laboratory, and Transfusion Cut-Point Markers to Determine Futility
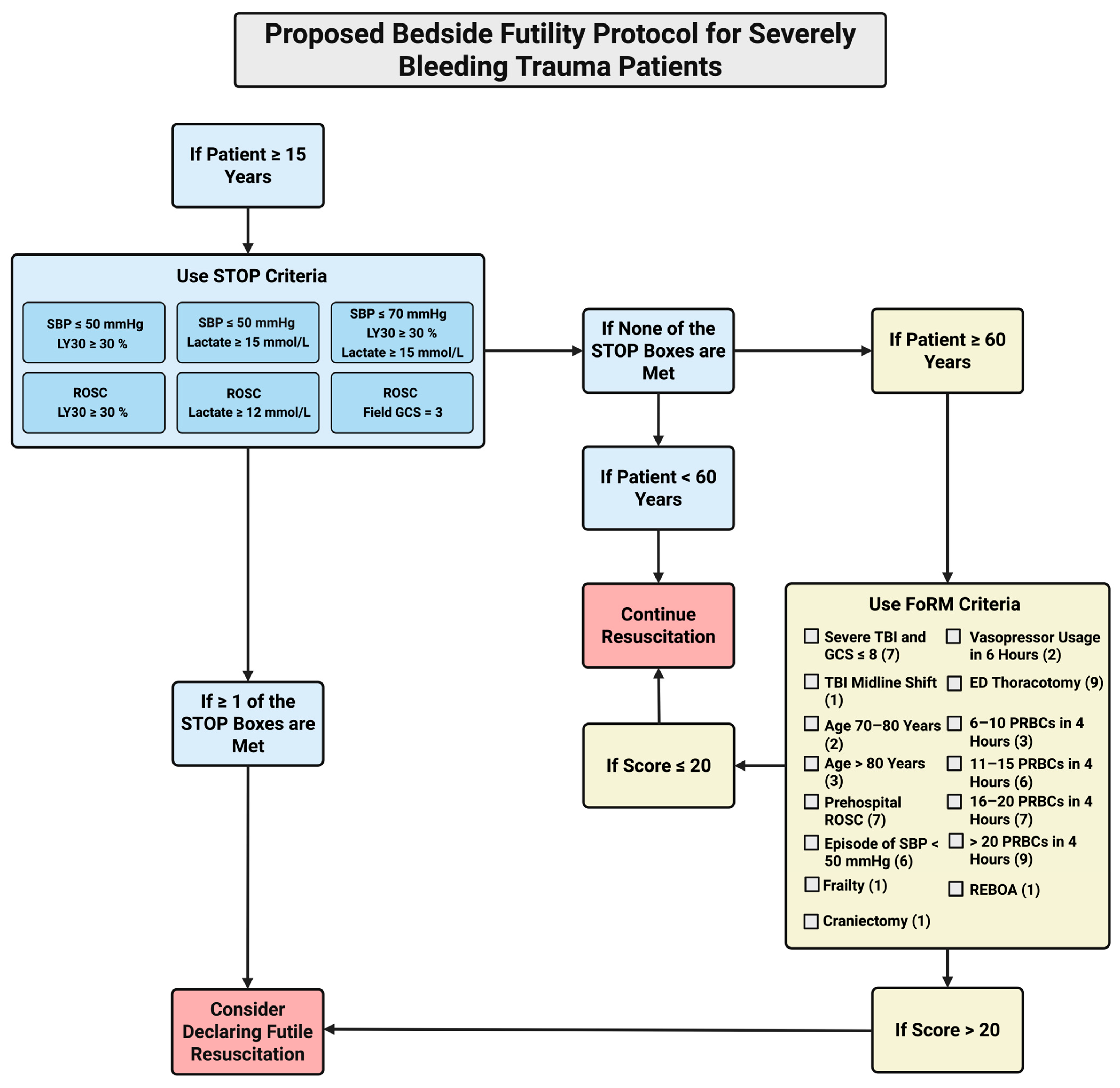
4. Proposal of Protocol for Guiding Declaration of FR for SBTPs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doughty, H.; Green, L.; Callum, J.; Murphy, M.F.; National Blood Transfusion Committee. Triage tool for the rationing of blood for massively bleeding patients during a severe national blood shortage: Guidance from the National Blood Transfusion Committee. Br. J. Haematol. 2020, 191, 340–346. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of COVID-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Casem, C.F.; Baral, E.; Inaba, K.; Kuza, C.M. Narrative Review: Is There a Transfusion Cutoff Value After Which Nonsurvivability Is Inevitable in Trauma Patients Receiving Ultramassive Transfusion? Anesth. Analg. 2023, 137, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Mladinov, D.; Frank, S.M. Massive transfusion and severe blood shortages: Establishing and implementing predictors of futility. Br. J. Anaesth. 2022, 128, e71–e74. [Google Scholar] [CrossRef]
- Nakashima, B.; Schellenberg, M.; Gold, A.I.; Matsushima, K.; Martin, M.J.; Inaba, K. Resuscitative Thoracotomy for Traumatic Cardiac Arrest: Potential Impact of Resource Constraint on Outcomes and Blood Product Utilization. J. Surg. Res. 2024, 295, 683–689. [Google Scholar] [CrossRef]
- Saillant, N.N.; Kornblith, L.Z.; Moore, H.; Barrett, C.; Schreiber, M.A.; Cotton, B.A.; Neal, M.D.; Makar, R.; Cap, A.P. The National Blood Shortage-An Impetus for Change. Ann. Surg. 2022, 275, 641–643. [Google Scholar] [CrossRef]
- Shander, A.; Goobie, S.M.; Warner, M.A.; Aapro, M.; Bisbe, E.; Perez-Calatayud, A.A.; Callum, J.; Cushing, M.M.; Dyer, W.B.; Erhard, J.; et al. Essential Role of Patient Blood Management in a Pandemic: A Call for Action. Anesth. Analg. 2020, 131, 74–85. [Google Scholar] [CrossRef]
- Ngo, A.; Masel, D.; Cahill, C.; Blumberg, N.; Refaai, M.A. Blood Banking and Transfusion Medicine Challenges During the COVID-19 Pandemic. Clin. Lab. Med. 2020, 40, 587–601. [Google Scholar] [CrossRef]
- Riley, W.; Love, K.; McCullough, J. Public Policy Impact of the COVID-19 Pandemic on Blood Supply in the United States. Am. J. Public Health 2021, 111, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Gammon, R.R.; Rosenbaum, L.; Cooke, R.; Friedman, M.; Rockwood, L.; Nichols, T.; Vossoughi, S. Maintaining adequate donations and a sustainable blood supply: Lessons learned. Transfusion 2021, 61, 294–302. [Google Scholar] [CrossRef]
- Clements, T.W.; Van Gent, J.M.; Lubkin, D.E.; Wandling, M.W.; Meyer, D.E.; Moore, L.J.; Cotton, B.A. The Reports of my Death are Greatly Exaggerated: An Evaluation of Futility Cut-Points in Massive Transfusion. J. Trauma Acute Care Surg. 2023, 95, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Loudon, A.M.; Rushing, A.P.; Hue, J.J.; Ziemak, A.; Sarode, A.L.; Moorman, M.L. When is enough enough? Odds of survival by unit transfused. J. Trauma Acute Care Surg. 2023, 94, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, J.M.; Clements, T.W.; Lubkin, D.T.; Wade, C.E.; Cardenas, J.C.; Kao, L.S.; Cotton, B.A. Predicting Futility in Severely Injured Patients: Using Arrival Lab Values and Physiology to Support Evidence-Based Resource Stewardship. J. Am. Coll. Surg. 2023, 236, 874–880. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Thomas, S.G.; Farrell, M.S.; Sixta, S.; Coleman, J.R.; Miller, J.B.; Bunch, C.M.; Waxman, D.; Walsh, M.M. Serial “death diamond” TEGs are a bedside indicator of futile resuscitation during massive transfusion. J. Trauma Acute Care Surg. 2023, 95, e19–e21. [Google Scholar] [CrossRef]
- Bhogadi, S.K.; Ditillo, M.; Khurshid, M.H.; Stewart, C.; Hejazi, O.; Spencer, A.L.; Anand, T.; Nelson, A.; Magnotti, L.J.; Joseph, B. Development and Validation of Futility of Resuscitation Measure in Older Adult Trauma Patients. J. Surg. Res. 2024, 301, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Nelson, A.C.; Obaid, O.; Ditillo, M.F.; El-Qawaqzeh, K.W.M.; Stewart, C.; Reina Limon, R.F.A.; Hosseinpour, H.; Nguyen, L.; Joseph, B. Futility of Resuscitation among Geriatric Trauma Patients: Do We Need to Define When to Withdraw Care? J. Am. Coll. Surg. 2022, 235, S92–S93. [Google Scholar] [CrossRef]
- Dorken-Gallastegi, A.; Spinella, P.C.; Neal, M.D.; Leeper, C.; Sperry, J.; Peitzman, A.B.; Brown, J.B. Whole Blood and Blood Component Resuscitation in Trauma: Interaction and Association with Mortality. Ann. Surg. 2024, 280, 1014–1020. [Google Scholar] [CrossRef]
- Major, F.R.; Pickering, T.A.; Stefanescu, K.; Singh, M.; Clark, D.H.; Inaba, K.; Nahmias, J.T.; Tay-Lasso, E.L.; Alvarez, C.; Chen, J.L.; et al. A Retrospective Study of Ultramassive Transfusion in Trauma Patients: Is There a Value After Which Additional Transfusions Are Futile? Anesth. Analg. 2025. [Google Scholar] [CrossRef]
- Muldowney, M.; Liu, Z.; Stansbury, L.G.; Vavilala, M.S.; Hess, J.R. Ultramassive Transfusion for Trauma in the Age of Hemostatic Resuscitation: A Retrospective Single-Center Cohort From a Large US Level-1 Trauma Center, 2011–2021. Anesth. Analg. 2023, 136, 927–933. [Google Scholar] [CrossRef]
- Quintana, M.T.; Zebley, J.A.; Vincent, A.; Chang, P.; Estroff, J.; Sarani, B.; Forssten, M.P.; Cao, Y.; Chen, M.; Corrado, C.; et al. Cresting mortality: Defining a plateau in ongoing massive transfusion. J. Trauma Acute Care Surg. 2022, 93, 43–51. [Google Scholar] [CrossRef]
- Schneider, A.B.; Adams, U.; Gallaher, J.; Purcell, L.N.; Raff, L.; Eckert, M.; Charles, A. Blood Utilization and Thresholds for Mortality Following Major Trauma. J. Surg. Res. 2023, 281, 82–88. [Google Scholar] [CrossRef]
- Van Gent, J.M.; Clements, T.W.; Rosario-Rivera, B.L.; Wisniewski, S.R.; Cannon, J.W.; Schreiber, M.A.; Moore, E.E.; Namias, N.; Sperry, J.L.; Cotton, B.A. The inability to predict futility in hemorrhaging trauma patients using 4-hour transfusion volumes and rates. J. Trauma Acute Care Surg. 2025, 98, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Kingrey, R.A.; Rizzo, J.A.; April, M.D.; Fisher, A.D.; Braverman, M.A.; Yazer, M.H.; Schauer, S.G. Transfusion quantities associated with 24-h mortality in trauma patients. Transfusion 2025, 65 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Ang, D.; Fakhry, S.M.; Watts, D.D.; Liu, H.; Morse, J.L.; Armstrong, J.; Ziglar, M.; Restivo, J.; Plurad, D.; Kurek, S.; et al. Data-Driven Blood Transfusion Thresholds for Severely Injured Patients During Blood Shortages. J. Surg. Res. 2023, 291, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dorken Gallastegi, A.; Secor, J.D.; Maurer, L.R.; Dzik, W.S.; Saillant, N.N.; Hwabejire, J.O.; Fawley, J.; Parks, J.; Kaafarani, H.M.; Velmahos, G.C. Role of Transfusion Volume and Transfusion Rate as Markers of Futility During Ultramassive Blood Transfusion in Trauma. J. Am. Coll. Surg. 2022, 235, 468–480. [Google Scholar] [CrossRef]
- Grady, Z.J.; Nguyen, J.; Meyer, C.H.; Moran, T.P.; Rowh, M.; Zhang, A.; Harfouche, M.N.; Greiffenstein, P.; Trinh, S.; Inaba, K.; et al. Filling the tank: A multicenter investigation of trauma survival after ultramassive transfusion. J. Trauma Acute Care Surg. 2025, 99, 272–278. [Google Scholar] [CrossRef]
- Lier, H.; Fries, D. Emergency Blood Transfusion for Trauma and Perioperative Resuscitation: Standard of Care. Transfus. Med. Hemother. 2021, 48, 366–376. [Google Scholar] [CrossRef]
- Lier, H.; Hossfeld, B. Massive transfusion in trauma. Curr. Opin. Anaesthesiol. 2024, 37, 117–124. [Google Scholar] [CrossRef]
- Matthay, Z.A.; Hellmann, Z.J.; Callcut, R.A.; Matthay, E.C.; Nunez-Garcia, B.; Duong, W.; Nahmias, J.; LaRiccia, A.K.; Spalding, M.C.; Dalavayi, S.S.; et al. Outcomes after ultramassive transfusion in the modern era: An Eastern Association for the Surgery of Trauma multicenter study. J. Trauma Acute Care Surg. 2021, 91, 24–33. [Google Scholar] [CrossRef]
- Meyer, C.H.; Bailey, N.M.; Leslie, S.L.; Thrasher, K.; Grady, Z.; Sanders, M.; Moore, E.; Nicely, K.W.; Smith, R.N. Defining Ultra-Massive Transfusion through a Systematic Review. Am. J. Surg. 2024, 228, 192–198. [Google Scholar] [CrossRef]
- Rahbar, E.; Fox, E.E.; del Junco, D.J.; Harvin, J.A.; Holcomb, J.B.; Wade, C.E.; Schreiber, M.A.; Rahbar, M.H.; Bulger, E.M.; Phelan, H.A.; et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J. Trauma Acute Care Surg. 2013, 75 (Suppl. S1), S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Wears, R.L. Standardisation and Its Discontents. Cogn. Technol. Work 2015, 17, 89–94. [Google Scholar] [CrossRef]
- Eckstein, M. Termination of resuscitative efforts: Medical futility for the trauma patient. Curr. Opin. Crit. Care 2001, 7, 450–454. [Google Scholar] [CrossRef]
- Pommerening, M.J.; Goodman, M.D.; Holcomb, J.B.; Wade, C.E.; Fox, E.E.; Del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Cohen, M.J.; Alarcon, L.H.; et al. Clinical gestalt and the prediction of massive transfusion after trauma. Injury 2015, 46, 807–813. [Google Scholar] [CrossRef]
- Lin, V.S.; Sun, E.; Yau, S.; Abeyakoon, C.; Seamer, G.; Bhopal, S.; Tucker, H.; Doree, C.; Brunskill, S.J.; McQuilten, Z.K.; et al. Definitions of massive transfusion in adults with critical bleeding: A systematic review. Crit. Care 2023, 27, 265. [Google Scholar] [CrossRef]
- Crosson, J.T. Massive transfusion. Clin. Lab. Med. 1996, 16, 873–882. [Google Scholar] [CrossRef]
- Hu, P.; Uhlich, R.; Black, J.; Jansen, J.O.; Kerby, J.; Holcomb, J.B. A new definition for massive transfusion in the modern era of whole blood resuscitation. Transfusion 2021, 61 (Suppl. S1), S252–S263. [Google Scholar] [CrossRef]
- Meyer, D.E.; Cotton, B.A.; Fox, E.E.; Stein, D.; Holcomb, J.B.; Cohen, M.; Inaba, K.; Rahbar, E. A comparison of resuscitation intensity and critical administration threshold in predicting early mortality among bleeding patients: A multicenter validation in 680 major transfusion patients. J. Trauma Acute Care Surg. 2018, 85, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A.; Zarzaur, B.L.; Croce, M.A.; Fabian, T.C. The new metric to define large-volume hemorrhage: Results of a prospective study of the critical administration threshold. J. Trauma Acute Care Surg. 2015, 78, 224–230; discussion 229–230. [Google Scholar] [CrossRef]
- Savage, S.A.; Sumislawski, J.J.; Zarzaur, B.L.; Dutton, W.P.; Croce, M.A.; Fabian, T.C. Redefining massive transfusion when every second counts. J. Trauma Acute Care Surg. 2013, 74, 396–402; discussion 400–402. [Google Scholar] [CrossRef] [PubMed]
- McQuilten, Z.K.; Flint, A.W.; Green, L.; Sanderson, B.; Winearls, J.; Wood, E.M. Epidemiology of Massive Transfusion—A Common Intervention in Need of a Definition. Transfus. Med. Rev. 2021, 35, 73–79. [Google Scholar] [CrossRef]
- Sim, E.S.; Guyette, F.X.; Brown, J.B.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; Claridge, J.A.; Phelan, H.A.; Neal, M.D.; Forsythe, R.; et al. Massive transfusion and the response to prehospital plasma: It is all in how you define it. J. Trauma Acute Care Surg. 2020, 89, 43–50. [Google Scholar] [CrossRef]
- Dzik, W.S.; Ziman, A.; Cohn, C.; Pai, M.; Lozano, M.; Kaufman, R.M.; Delaney, M.; Selleng, K.; Murphy, M.F.; Hervig, T.; et al. Survival after ultramassive transfusion: A review of 1360 cases. Transfusion 2016, 56, 558–563. [Google Scholar] [CrossRef]
- Huber-Wagner, S.; Qvick, M.; Mussack, T.; Euler, E.; Kay, M.V.; Mutschler, W.; Kanz, K.-G. Massive blood transfusion and outcome in 1062 polytrauma patients: A prospective study based on the Trauma Registry of the German Trauma Society. Vox Sang. 2007, 92, 69–78. [Google Scholar] [CrossRef]
- Liu, S.; Fujii, Q.; Serio, F.; McCague, A. Massive Blood Transfusions and Outcomes in Trauma Patients; An Intention to Treat Analysis. Bull. Emerg. Trauma 2018, 6, 217–220. [Google Scholar] [CrossRef]
- Lo, B.D.; Merkel, K.R.; Dougherty, J.L.; Kajstura, T.J.; Cruz, N.C.; Sikorski, R.A.; Frank, S.M. Assessing predictors of futility in patients receiving massive transfusions. Transfusion 2021, 61, 2082–2089. [Google Scholar] [CrossRef]
- Mitra, B.; Mori, A.; Cameron, P.A.; Fitzgerald, M.; Street, A.; Bailey, M. Massive blood transfusion and trauma resuscitation. Injury 2007, 38, 1023–1029. [Google Scholar] [CrossRef]
- Velmahos, G.C.; Chan, L.; Chan, M.; Tatevossian, R.; Cornwell, E.E., III; Asensio, J.A.; Berne, T.V.; Demetriades, D. Is there a limit to massive blood transfusion after severe trauma? Arch. Surg. 1998, 133, 947–952. [Google Scholar] [CrossRef]
- Malone, D.L.; Hess, J.R.; Fingerhut, A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J. Trauma Inj. Infect. Crit. Care 2006, 60 (Suppl. S6), S91–S96. [Google Scholar] [CrossRef]
- Ruby, K.N.; Dzik, W.H.; Collins, J.J.; Eliason, K.; Makar, R.S. Emergency transfusion with whole blood versus packed red blood cells: A study of 1400 patients. Transfusion 2023, 63, 745–754. [Google Scholar] [CrossRef]
- Braverman, M.A.; Smith, A.; Pokorny, D.; Axtman, B.; Shahan, C.P.; Barry, L.; Corral, H.; Jonas, R.B.; Shiels, M.; Schaefer, R.; et al. Prehospital whole blood reduces early mortality in patients with hemorrhagic shock. Transfusion 2021, 61 (Suppl. S1), S15–S21. [Google Scholar] [CrossRef]
- Braverman, M.A.; Smith, A.A.; Ciaraglia, A.V.; Radowsky, J.S.; Schauer, S.G.; Sams, V.G.; Greebon, L.J.; Shiels, M.D.; Jonas, R.B.; Ngamsuntikul, S.; et al. The regional whole blood program in San Antonio, TX: A 3-year update on prehospital and in-hospital transfusion practices for traumatic and non-traumatic hemorrhage. Transfusion 2022, 62 (Suppl. S1), S80–S89. [Google Scholar] [CrossRef]
- Brill, J.B.; Tang, B.; Hatton, G.; Mueck, K.M.; McCoy, C.C.; Kao, L.S.; Cotton, B.A. Impact of Incorporating Whole Blood into Hemorrhagic Shock Resuscitation: Analysis of 1,377 Consecutive Trauma Patients Receiving Emergency-Release Uncrossmatched Blood Products. J. Am. Coll. Surg. 2022, 234, 408–418. [Google Scholar] [CrossRef]
- Ciaraglia, A.; Brigmon, E.; Braverman, M.; Kidd, E.; Winckler, C.J.; Epley, E.; Flores, J.; Barry, J.; DeLeon, D.; Waltman, E.; et al. Use of whole blood deployment programs for mass casualty incidents: South Texas experience in regional response and preparedness. J. Trauma Acute Care Surg. 2022, 93, e182–e184. [Google Scholar] [CrossRef]
- Ciaraglia, A.; Myers, J.C.; Braverman, M.; Barry, J.; Eastridge, B.; Stewart, R.; Nicholson, S.; Jenkins, D. Transfusion-related cost comparison of trauma patients receiving whole blood versus component therapy. J. Trauma Acute Care Surg. 2023, 95, 62–68. [Google Scholar] [CrossRef]
- Gaines, B.A.; Yazer, M.H.; Triulzi, D.J.; Sperry, J.L.; Neal, M.D.; Billiar, T.R.; Leeper, C.M. Low Titer Group O Whole Blood In Injured Children Requiring Massive Transfusion. Ann. Surg. 2023, 277, e919–e924. [Google Scholar] [CrossRef]
- Guyette, F.X.; Zenati, M.; Triulzi, D.J.; Yazer, M.H.; Skroczky, H.; Early, B.J.; Adams, P.W.; Brown, J.B.; Alarcon, L.; Neal, M.D.; et al. Prehospital low titer group O whole blood is feasible and safe: Results of a prospective randomized pilot trial. J. Trauma Acute Care Surg. 2022, 92, 839–847. [Google Scholar] [CrossRef]
- Hanna, K.; Bible, L.; Chehab, M.; Asmar, S.; Douglas, M.; Ditillo, M.; Castanon, L.; Tang, A.; Joseph, B. Nationwide analysis of whole blood hemostatic resuscitation in civilian trauma. J. Trauma Acute Care Surg. 2020, 89, 329–335. [Google Scholar] [CrossRef]
- Hazelton, J.P.; Ssentongo, A.E.; Oh, J.S.; Ssentongo, P.; Seamon, M.J.; Byrne, J.P.; Armento, I.G.; Jenkins, D.H.; Braverman, M.A.; Mentzer, C.; et al. Use of Cold-Stored Whole Blood is Associated with Improved Mortality in Hemostatic Resuscitation of Major Bleeding: A Multicenter Study. Ann. Surg. 2022, 276, 579–588. [Google Scholar] [CrossRef]
- Sperry, J.L.; Cotton, B.A.; Luther, J.F.; Cannon, J.W.; Schreiber, M.A.; Moore, E.E.; Namias, N.; Minei, J.P.; Wisniewski, S.R.; Guyette, F.X.; et al. Whole Blood Resuscitation and Association with Survival in Injured Patients with an Elevated Probability of Mortality. J. Am. Coll. Surg. 2023, 237, 206–219. [Google Scholar] [CrossRef]
- Walsh, M.; Fries, D.; Moore, E.; Moore, H.; Thomas, S.; Kwaan, H.C.; Marsee, M.K.; Grisoli, A.; McCauley, R.; Vande Lune, S.; et al. Whole blood for civilian urban trauma resuscitation: Historical, present, and future considerations. Semin. Thromb. Hemost. 2020, 46, 221–234. [Google Scholar] [CrossRef]
- Walsh, M.; Moore, E.E.; Moore, H.B.; Thomas, S.; Kwaan, H.C.; Speybroeck, J.; Marsee, M.; Bunch, C.M.; Stillson, J.; Thomas, A.V.; et al. Whole Blood, Fixed Ratio, or Goal-Directed Blood Component Therapy for the Initial Resuscitation of Severely Hemorrhaging Trauma Patients: A Narrative Review. J. Clin. Med. 2021, 10, 320. [Google Scholar] [CrossRef]
- Yazer, M.H.; Cap, A.P.; Glassberg, E.; Green, L.; Holcomb, J.B.; Khan, M.A.; Moore, E.E.; Neal, M.D.; Perkins, G.D.; Sperry, J.L.; et al. Toward a more complete understanding of who will benefit from prehospital transfusion. Transfusion 2022, 62, 1671–1679. [Google Scholar] [CrossRef]
- Yazer, M.H.; Cap, A.P.; Spinella, P.C.; Alarcon, L.; Triulzi, D.J. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018, 58, 622–628. [Google Scholar] [CrossRef]
- Cinat, M.E.; Wallace, W.C.; Nastanski, F.; West, J.; Sloan, S.; Ocariz, J.; Wilson, S.E. Improved survival following massive transfusion in patients who have undergone trauma. Arch. Surg. 1999, 134, 964–968; discussion 968–970. [Google Scholar] [CrossRef]
- Hakala, P.; Hiippala, S.; Syrjala, M.; Randell, T. Massive blood transfusion exceeding 50 units of plasma poor red cells or whole blood: The survival rate and the occurrence of leukopenia and acidosis. Injury 1999, 30, 619–622. [Google Scholar] [CrossRef]
- Criddle, L.M.; Eldredge, D.H.; Walker, J. Variables predicting trauma patient survival following massive transfusion. J. Emerg. Nurs. 2005, 31, 236–242; quiz 320. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.F.; Soulier, G.; Wilson, R.F. Outcome of massive transfusion exceeding two blood volumes in trauma and emergency surgery. J. Trauma Inj. Infect. Crit. Care 1987, 27, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Vaslef, S.N.; Knudsen, N.W.; Neligan, P.J.; Sebastian, M.W. Massive transfusion exceeding 50 units of blood products in trauma patients. J. Trauma Inj. Infect. Crit. Care 2002, 53, 291–296; discussion 295–296. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Curry, N.S.; Davenport, R.A.; Yu, L.M.; Stanworth, S.J. A Delphi study to establish consensus on a definition of major bleeding in adult trauma. Transfusion 2020, 60, 3028–3038. [Google Scholar] [CrossRef]
- Bouzat, P.; Bosson, J.-L.; David, J.-S.; Riou, B.; Duranteau, J.; Payen, J.-F.; PROCOAG Study Group. Four-factor prothrombin complex concentrate to reduce allogenic blood product transfusion in patients with major trauma, the PROCOAG trial: Study protocol for a randomized multicenter double-blind superiority study. Trials 2021, 22, 634. [Google Scholar] [CrossRef]
- Bouzat, P.; Charbit, J.; Abback, P.-S.; Huet-Garrigue, D.; Delhaye, N.; Leone, M.; Marcotte, G.; David, J.-S.; Levrat, A.; Asehnoune, K.; et al. Efficacy and Safety of Early Administration of 4-Factor Prothrombin Complex Concentrate in Patients with Trauma at Risk of Massive Transfusion: The PROCOAG Randomized Clinical Trial. JAMA 2023, 329, 1367–1375. [Google Scholar] [CrossRef]
- Nunns, G.R.; Moore, E.E.; Stettler, G.R.; Moore, H.B.; Ghasabyan, A.; Cohen, M.; Huebner, B.R.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Empiric transfusion strategies during life-threatening hemorrhage. Surgery 2018, 164, 306–311. [Google Scholar] [CrossRef]
- Giancarelli, A.; Birrer, K.L.; Alban, R.F.; Hobbs, B.P.; Liu-DeRyke, X. Hypocalcemia in trauma patients receiving massive transfusion. J. Surg. Res. 2016, 202, 182–187. [Google Scholar] [CrossRef]
- Jeong, T.S.; Lee, G.J.; Kim, W.S.; Kim, J.; Jang, M.J. Epidemiology and Outcomes of Moderate-to-Severe Trauma Patients in a Regional Trauma Center: Challenges and Future Directions. Korean J. Neurotrauma 2025, 21, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Martinaud, C.; Tiberghien, P.; Bégué, S.; Sailliol, A.; Gross, S.; Pouget, T.; Ausset, S. Rational and design of the T-STORHM Study: A prospective randomized trial comparing fresh whole blood to blood components for acutely bleeding trauma patients. Transfus. Clin. Biol. 2019, 26, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Nathens, A.B.; Tiberghien, P.; Bégué, S.; Sailliol, A.; Gross, S.; Pouget, T.; Ausset, S. The effects of leukoreduced blood transfusion on infection risk following injury: A randomized controlled trial. Shock 2006, 26, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nunez, T.C.; Dutton, W.D.; May, A.K.; Holcomb, J.B.; Young, P.P.; Cotton, B.A. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion 2010, 50, 1914–1920. [Google Scholar] [CrossRef]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med. 2021, 47, 49–59. [Google Scholar] [CrossRef]
- Bulger, E.M.; Jurkovich, G.J.; Nathens, A.B.; Copass, M.K.; Hanson, S.; Cooper, C.; Liu, P.-Y.; Neff, M.; Awan, A.B.; Warner, K.; et al. Hypertonic resuscitation of hypovolemic shock after blunt trauma: A randomized controlled trial. Arch. Surg. 2008, 143, 139–148; discussion 149. [Google Scholar] [CrossRef]
- Hauser, C.J.; Boffard, K.; Dutton, R.; Bernard, G.R.; Croce, M.A.; Holcomb, J.B.; Leppaniemi, A.; Parr, M.; Vincent, J.-L.; Tortella, B.J.; et al. Results of the CONTROL trial: Efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J. Trauma Inj. Infect. Crit. Care 2010, 69, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Innerhofer, P.; Fries, D.; Mittermayr, M.; Innerhofer, N.; von Langen, D.; Hell, T.; Gruber, G.; Schmid, S.; Friesenecker, B.; Lorenz, I.H.; et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): A single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017, 4, e258–e271. [Google Scholar] [CrossRef]
- Jost, D.; Lemoine, S.; Lemoine, F.; Derkenne, C.; Beaume, S.; Lanoë, V.; Maurin, O.; Louis-Delaurière, E.; Delacote, M.; Dang-Minh, P.; et al. Prehospital Lyophilized Plasma Transfusion for Trauma-Induced Coagulopathy in Patients at Risk for Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2223619. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Shin, I.S.; Pyo, J.S.; Ahn, S.; Chung, S.; Ki, Y.J.; Seok, J.; Park, C.Y.; Lee, S. Prognostic Accuracy of Massive Transfusion, Critical Administration Threshold, and Resuscitation Intensity in Assessing Mortality in Traumatic Patients with Severe Hemorrhage: A Meta-Analysis. J. Korean Med. Sci. 2019, 34, e318. [Google Scholar] [CrossRef]
- L’Huillier, J.C.; Hua, S.; Logghe, H.J.; Yu, J.; Myneni, A.A.; Noyes, K.; Guo, W.A. Transfusion futility thresholds and mortality in geriatric trauma: Does frailty matter? Am. J. Surg. 2024, 228, 113–121. [Google Scholar] [CrossRef]
- Nascimento, B.; Callum, J.; Tien, H.; Rubenfeld, G.; Pinto, R.; Lin, Y.; Rizoli, S. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: A randomized feasibility trial. CMAJ 2013, 185, E583–E589. [Google Scholar] [CrossRef]
- Sperry, J.L.; Guyette, F.X.; Brown, J.B.; Yazer, M.H.; Triulzi, D.J.; Early-Young, B.J.; Adams, P.W.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N. Engl. J. Med. 2018, 379, 315–326. [Google Scholar] [CrossRef]
- Asim, M.; El-Menyar, A.; Peralta, R.; Arumugam, S.; Wahlen, B.; Ahmed, K.; Khan, N.A.; Alansari, A.N.; Mollazehi, M.; Ibnas, M.; et al. Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study. Diagnostics 2025, 15, 1148. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Chapman, M.P.; McVaney, K.; Bryskiewicz, G.; Blechar, R.; Chin, T.; Burlew, C.C.; Pieracci, F.; West, F.B.; et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: A randomised trial. Lancet 2018, 392, 283–291. [Google Scholar] [CrossRef]
- Boffard, K.D.; Riou, B.; Warren, B.; Choong, P.I.; Rizoli, S.; Rossaint, R.; Axelsen, M.; Kluger, Y. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: Two parallel randomized, placebo-controlled, double-blind clinical trials. J. Trauma Inj. Infect. Crit. Care 2005, 59, 8–18; discussion 15–18. [Google Scholar] [CrossRef]
- Yu, A.J.; Inaba, K.; Biswas, S.; de Leon, L.A.; Wong, M.; Benjamin, E.; Lam, L.; Demetriades, D. Supermassive Transfusion: A 15-Year Single Center Experience and Outcomes. Am. Surg. 2018, 84, 1617–1621. [Google Scholar] [CrossRef]
- Barbosa Rengifo, M.M.; Garcia, A.F.; Gonzalez-Hada, A.; Mejia, N.J. Evaluating the Shock Index, Revised Assessment of Bleeding and Transfusion (RABT), Assessment of Blood Consumption (ABC) and novel PTTrauma score to predict critical transfusion threshold (CAT) in penetrating thoracic trauma. Sci. Rep. 2024, 14, 13395. [Google Scholar] [CrossRef]
- Tran, A.; Nemnom, M.J.; Lampron, J.; Matar, M.; Vaillancourt, C.; Taljaard, M. Accuracy of massive transfusion as a surrogate for significant traumatic bleeding in health administrative datasets. Injury 2019, 50, 318–323. [Google Scholar] [CrossRef]
- Siegel, J.H.; Rivkind, A.I.; Dalal, S.; Goodarzi, S. Early physiologic predictors of injury severity and death in blunt multiple trauma. Arch. Surg. 1990, 125, 498–508. [Google Scholar] [CrossRef]
- Cosgriff, N.; Moore, E.E.; Sauaia, A.; Kenny-Moynihan, M.; Burch, J.M.; Galloway, B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: Hypothermia and acidoses revisited. J. Trauma Inj. Infect. Crit. Care 1997, 42, 857–862; discussion 861–862. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, K.; Subramanian, A.; Pandey, R.M. Determinants of mortality in trauma patients following massive blood transfusion. J. Emerg. Trauma Shock 2011, 4, 58–63. [Google Scholar] [PubMed]
- Morris, M.C.; Niziolek, G.M.; Baker, J.E.; Huebner, B.R.; Hanseman, D.; Makley, A.T.; Pritts, T.A.; Goodman, M.D. Death by Decade: Establishing a Transfusion Ceiling for Futility in Massive Transfusion. J. Surg. Res. 2020, 252, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, J.; Swartz, H.; Albright, J.; Street, W.; Martin, S.; Hagen, C.; Linnaus, M.; Ciresi, D. Achieving optimal massive transfusion ratios: The trauma white board, whole blood, and liquid plasma. Real world low-tech solutions for a high stakes issue. Injury 2022, 53, 2974–2978. [Google Scholar] [CrossRef]
- Barbosa, R.R.; Rowell, S.E.; Diggs, B.S.; Schreiber, M.A.; Group, T.O. Profoundly abnormal initial physiologic and biochemical data cannot be used to determine futility in massively transfused trauma patients. J. Trauma Acute Care Surg. 2011, 71, S364–S369. [Google Scholar] [CrossRef]
- Tzeng, W.J.; Tseng, H.Y.; Hou, T.Y.; Chou, S.E.; Su, W.T.; Hsu, S.Y.; Hsieh, C.H. From Death Triad to Death Tetrad-The Addition of a Hypotension Component to the Death Triad Improves Mortality Risk Stratification in Trauma Patients: A Retrospective Cohort Study. Diagnostics 2022, 12, 2885. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.S.; Karam, B.S.; Murphy, P.B.; Jenkins, P.; Milia, D.J.; Hemmila, M.R.; Haines, K.L.; Puzio, T.J.; de Moya, M.A.; Tignanelli, C.J. Field-Triage, Hospital-Triage and Triage-Assessment: A Literature Review of the Current Phases of Adult Trauma Triage. J. Trauma Acute Care Surg. 2021, 90, e138–e145. [Google Scholar] [CrossRef] [PubMed]
- Al-Fadhl, M.D.; Karam, M.N.; Chen, J.; Zackariya, S.K.; Lain, M.C.; Bales, J.R.; Higgins, A.B.; Laing, J.T.; Wang, H.S.; Andrews, M.G.; et al. Traumatic Brain Injury as an Independent Predictor of Futility in the Early Resuscitation of Patients in Hemorrhagic Shock. J. Clin. Med. 2024, 13, 3915. [Google Scholar] [CrossRef] [PubMed]

| Name | Quantity of Blood Given |
|---|---|
| Massive Transfusion (MT) | ≥3 U packed red blood cells (PRBCs) in 1 h [71,72] |
| >4 U PRBCs in 1 h [73] | |
| ≥4 U PRBCs in 1 h [27,74,75] | |
| ≥4 U blood components in 2 h [70] | |
| ≥4 U PRBCs in 6 h [76] | |
| >5 U blood products in 4 h [24] | |
| >6 U PRBCs in 2 h [73] | |
| ≥4 U of PRBCs, ≥1 U of plasma, and ≥1 U of platelets (PLTs) within 4 h [17] | |
| >6 U PRBCs in 48 h [77] | |
| >8 U PRBCs in 4 h [73] | |
| ≥10 U PRBCs in 4 h [12] | |
| ≥10 U PRBCs in 6 h [27,78] | |
| >10 U PRBCs in 6 h [73] | |
| ≥10 U PRBCs in 24 h [3,21,24,45,49,71,72,74,75,79,80,81,82,83,84,85,86,87,88] | |
| >10 U PRBCs in 24 h [27,89] | |
| >10 U PRBCs [90] | |
| >12 U PRBCs in 12 h [73] | |
| >12 U PRBCs in 24 h [73] | |
| >20 U PRBCs [36,91] | |
| Replacement of half of patient’s entire blood volume within 3 h [21,36] | |
| Replacement of patient’s entire blood volume within 24 h [36] | |
| Transfusion at rate of >150 mL/min [21] | |
| Ultramassive Transfusion (UMT) | ≥20 U PRBCs in 4 h [3,12,18,19] |
| ≥20 U blood components in 24 h [3,22,25,26,29,30] | |
| Dynamic MT | Transfusion of ≥4 U PRBCs in 1 h when ongoing need is foreseeable [36] |
| Supermassive Transfusion (SMT) | ≥25 U PRBCs in 24 h [92] |
| ≥50 U blood components [22] | |
| Critical Administration Threshold (CAT) | CAT+ if ≥3 U PRBCs within a single hour [39,40,93,94] |
| CAT-X where X is either 1 h, 4 h, or 24 h and represents the time to transfuse ≥3 U PRBCs [27] | |
| CAT-X where X is the number of times a patient exceeds CAT (≥3 U PRBCs within a single hour) in 24 h [39,40] | |
| Resuscitation Intensity (RI) | RI+ if ≥4 U PRBCs, fresh frozen plasma (FFP), PLTs, crystalloid (1000 mL equivalent to 1 U), and colloid (500 mL equivalent to 1 U) within 30 min [31] |
| RIX where X is the number of units and 1 U = 1 L crystalloid solution, 0.5 L colloid, 1 U PRBCs, 1 U plasma, or 6 U PLTs transfused in 30 min [31] | |
| Whole Blood Massive Transfusion (WB MT) Score | (3 × U WB) + U RBC within the first hour [37] |
| WB MT (+) = WB MT score ≥ 7 WB MT (−) = WB MT score < 7 [37] |
| Study | Design and Population | Results and Conclusions |
|---|---|---|
| Siegel et al. (1990) [95] | Retrospective study of 185 patients with major hepatic injury caused by blunt trauma | The authors found that base excess (median lethal dose [LD50] = −11.8 mmol/L) and blood volume transfused within the first 24 h (LD50 = 5.4 L) were significant predictors of death. A predictive model they generated using Glasgow Coma Scale and base excess was highly successful in predicting death. |
| Cosgriff et al. (1997) [96] | Retrospective observational study of 58 patients in a two-year period who were older than 15 years (mean = 35.4 years), did not have pre-existing disease or massive head injuries, and received massive transfusion (MT) (>10 U packed red blood cells (PRBCs) in 24 h). | The authors found that U PRBCs/6 h and U PRBCs, U fresh frozen plasma (FFP), and U platelets (PLTs)/24 h were not significant predictors of mortality. |
| Velmahos et al. (1998) [48] | Retrospective observational study of 141 trauma patients (mean injury severity score [ISS] 29, penetrating injury 74%, mortality rate 30.5%) receiving >20 U of PRBCs in the preoperative and intraoperative period. One unit was defined as 1 U of WB or 1 U PRBCs. | The number of units transfused did not differ between survivors and non-survivors. Volume of blood loss and transfusion was less critical than quality and duration of shock. No single clinical, laboratory, or procedural marker was predictive of futility. However, out of a cohort of 13 patients, aortic clamping, use of inotropes, and 90+ minutes of hypotension combined resulted in 100% mortality. The authors concluded that discontinuation of MT cannot be justified for up to 68 U blood. |
| Vaslef et al. (2002) [69] | Retrospective observational study of 44 patients (mean ISS 36.8, blunt injury 61.4%) who received >50 U of all blood components in the first 24 h of admission. | Patients receiving >50 U of blood products had a survival rate of 43%, and thus aggressive transfusion should continue for those trauma patients requiring >50 U at 24 h after admission. The authors concluded that volume and/or total units of blood products in the first 24 h were not independent risk factors for mortality. |
| Rangarajan et al. (2011) [97] | Retrospective observational study analyzing 71 trauma patients (median ISS = 27) who received MT, defined as ≥10 U of PRBCs in 24 h, in a Level I trauma center | The authors concluded that the PRBC units transfused in the first 12 h as well as the total number of blood products transfused were not significant indicators of in-hospital mortality. |
| Liu et al. (2018) [45] | Retrospective observational study analyzing 131 adult trauma patients who received blood transfusion. Mortality was 24% for patients who received 0–9 U PRBCs, 21% for 10–19 U, 38% for 20–29 U, 50% for 30–39 U, and 80% for ≥40 U. | The authors concluded that there was no increased death risk for patients who received 10–39 U PRBCs in the first 24 h compared to patients who received 0–9 U. This suggests that 40 U may be a threshold at which mortality increases significantly. |
| Morris et al. (2020) [98] | Retrospective Trauma Quality Improvement Program (TQIP) database analysis of 16,395 patients receiving MT defined as ≥4 U PRBCs in 4 h. This study looked at transfusion requirements in the first 4 h combined with decade of life to predict futility. | Mortality increased with age and transfusion requirement, which together may be used to guide prognosis. However, many older adults were resuscitated successfully, and thus age alone should not contraindicate large volume transfusion. However, as age increases, the number of units needed to approach futility decreases, and there is futility of transfusion past 51–60 U of PRBC within the first 4 h of admission in the octogenarian population. Also, giving more than 80 U PRBC in 4 h is associated with 100% mortality for all age groups. |
| Quintana et al. (2022) [20] | The TQIP database was used to find adult patients who received one or more U PRBC within the first 4 h of arrival from 2013 to 2017. Patients were analyzed based on the total amount of blood they received at 4 h and 24 h and whether they received blood in a 1:1 to 2:1 ratio of PRBC–plasma. | Mortality rate plateaued in transfusion volumes > 40.5 U at 4 h after admission and plateaued after 52.8 U at 24 h. For patients who received transfusion in a 1:1 to 2:1 ratio of PRBC–plasma, mortality rate plateaued after 39 U at 4 h and 53 U at 24 h. |
| Dorken Gallastegi et al. (2022) [25] | Examined data from the TQIP database between the years of 2013 and 2018. Patients receiving ultramassive (UMT), defined as ≥20 U PRBC in 24 h, were included. Transfusion volume was examined at 4 h and 24 h or time of death. | A transfusion rate of 7 U/hour for the first 24 h after arrival to the hospital is associated with 100% mortality. The authors conclude that defining futility should relate more to transfusion intensity than transfusion volume. |
| Anand et al. (2022) [16] | Retrospective TQIP database analysis of geriatric patients (≥65 years old). Patients were separated into 10-year-wide age groups. Futile resuscitation (FR) was defined as conditions leading to 90% mortality. | The authors conclude that transfusions > 40 U PRBC were futile for patients older than 65 years. The authors have described combined markers to define FR in geriatric patients. PRBC volumes at 4 h associated with FR were >30 U for 65–75-year-olds, >27 U for 75–85-year-olds, and >21 U for those older than 85 years. Also, increasing age was associated with increasing mortality among those who received emergency laparotomy or vasopressors, but did not reach FR. |
| Loudon et al. (2023) [12] | Retrospective observational study of 207 trauma patients who received transfusion in the first 4 h of care. Transfusion groups were defined as 2–9 U PRBC, 10–19 U PRBC (MT), >19 U PRBC (UMT) in 4 h. | Beyond 16 U PRBCs in the first 4 h, odds of mortality exceed survival. Survival approaches near zero with >36 U PRBC in the first 4 h. The authors termed efforts “heroic” at 16 U PRBC/4 h and “futile” at 36 U PRBC/4 h. Also, there was no survival beyond 67 U PRBC/4 h. |
| Ang et al. (2023) [24] | Retrospective cohort study of 1605 patients from 47 Level I or Level II trauma centers within one healthcare system. Data was taken from 2017 to 2019. Patients were stratified by age (16–30, 31–55, and ≥56) in an examination of percent mortality corresponding to varying transfusion volumes (≤ 24 units, 25–36 units, 37–48 units, 49–60 units, 61–72 units, 73–84 units, and >84 units where each unit was composed of an approximate 1:1:1 ratio of PRBCs, FFP, and PLTs). | There was a positive correlation between volume of transfusion and mortality and between age and mortality and a negative association between age and transfusion thresholds. The authors identify transfusion volumes for the age groups that indicate when the odds of mortality significantly increase with greater blood volumes transfused. These thresholds were 60 units for those aged 16–30 (30.3% mortality below the threshold and 67.7% mortality above the threshold), 48 units for those aged 31–55 (32.8% mortality below the threshold and 72.6% mortality above the threshold), and 24 units for those aged 56–100 (36.2% mortality below the threshold and 77.0% mortality above the threshold). |
| Clements et al. (2023) [11] | Retrospective observational study of 2299 trauma patients (median ISS 25, blunt injury 69%, 30-day mortality rate 22%) who received any blood products in the emergency department. First analysis compared those who received >50 U of blood components and those who received ≤ 50 U in the first 4 h. The second analysis compared those who received any WB during their resuscitation to those who received only blood components. One unit WB was defined as equivalent to 2.17 U blood components (1 U PRBC + 1 U FFP + 0.17 U PLT). | Survival rates in patients receiving >50 U of blood products in the first 4 h of care are as high as 50–60%, with survival still at 15–25% after 100 U. The authors concluded that futility should not be declared based on high transfusion volumes alone. Patients who received any WB (n = 1291) trended towards increased survival, but this was not statistically significant compared to the group who only received components. Therefore, futility could not be defined by transfusion volume alone. |
| Muldowney et al. (2023) [19] | Retrospective cohort study of 159 trauma patients (mean ISS 40, blunt trauma 34%, mortality rate 65%) who underwent UMTs defined as receiving ≥20 U PRBC and/or WB in the first 24 h. | 50% of patients who received UMT received ≤ 30 U PRBC and WB. These UMT patients also had 65% mortality, which did not increase as more units of blood were given. The authors conclude that there is no transfusion cut-point for futility because the patients who received the most blood products still survived. |
| Schneider et al. (2023) [21] | Analysis of National Trauma Database to find all patients aged ≥18 who received ≥1 U PRBCs from 2017 to 2019; 61,676 patients were analyzed to arrive at 50% predicted mortality. | A mortality rate of 50% was predicted for all patients who received 31 U PRBCs. However, it was noted that for patients aged 80+, the 50% mortality rate was at 6 U PRBCs. |
| Major et al. (2025) [18] | Retrospective study of 3248 trauma patients aged ≥18 who underwent an operation and received ≥1 U blood products in 24 h. Data was taken from 7 US Level I trauma centers from 2016 to 2022. Patients were grouped into those who received UMT (≥20 U PRBCs in 24 h) and those who did not receive UMT. | The mortality rate for all included patients was 18.9%. Those who received UMT had a higher predicted mortality rate of 39.9% compared to the 6.8% mortality rate of those who did not receive UMT. In further stratification, the authors discovered that groups receiving 20–29 U PRBCs and 30–44 U PRBCs had similar risks of death. However, those who required ≥45 U PRBCs were correlated with a higher risk of death. |
| Van Gent et al. (2025) [22] | Prospective observational study conducted at seven trauma centers. Patients of interest were in need of hemorrhage control and blood transfusion. 1047 patients were included, and transfusion volumes were analyzed at 4 h. | Volumes < 110 U transfusion were not presented as futile and transfusion volumes of >110 U were associated with 100% mortality. Further analysis revealed that only preexisting risk factors could be used to predict futility. |
| Wallace et al. (2025) [23] | Analysis of TQIP database of patients aged ≥15 who received any volume of any blood product within 4 h from 2020 to 2022. 144,379 cases were reviewed to search for predictors of 24 h mortality based on quantities of transfusion. | The authors were unable to discern a difference in mortality predictability from either the quantities of PRBCs and low titer group O WB units transfused or the aggregate volume of transfused products. Thus, transfusion volumes could not effectively predict futility due to significant survival even at large volumes of blood transfused. 90% mortality at 24 h was achieved at >36 L transfused products or >56 U PRBC and WB. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.J.; Patel, V.S.; Schmitt, C.P.; Zielinski, A.T.; Aboukhaled, M.N.; Steinberg, C.A.; Moore, E.E.; Moore, H.B.; Thomas, S.G.; Waxman, D.A.; et al. The Effect of Heterogeneous Definitions of Massive Transfusion on Using Blood Component Thresholds to Predict Futility in Severely Bleeding Trauma Patients. J. Clin. Med. 2025, 14, 5426. https://doi.org/10.3390/jcm14155426
Thomas SJ, Patel VS, Schmitt CP, Zielinski AT, Aboukhaled MN, Steinberg CA, Moore EE, Moore HB, Thomas SG, Waxman DA, et al. The Effect of Heterogeneous Definitions of Massive Transfusion on Using Blood Component Thresholds to Predict Futility in Severely Bleeding Trauma Patients. Journal of Clinical Medicine. 2025; 14(15):5426. https://doi.org/10.3390/jcm14155426
Chicago/Turabian StyleThomas, Samuel J., Vraj S. Patel, Connor P. Schmitt, Aleksey T. Zielinski, Mia N. Aboukhaled, Christopher A. Steinberg, Ernest E. Moore, Hunter B. Moore, Scott G. Thomas, Dan A. Waxman, and et al. 2025. "The Effect of Heterogeneous Definitions of Massive Transfusion on Using Blood Component Thresholds to Predict Futility in Severely Bleeding Trauma Patients" Journal of Clinical Medicine 14, no. 15: 5426. https://doi.org/10.3390/jcm14155426
APA StyleThomas, S. J., Patel, V. S., Schmitt, C. P., Zielinski, A. T., Aboukhaled, M. N., Steinberg, C. A., Moore, E. E., Moore, H. B., Thomas, S. G., Waxman, D. A., Miller, J. B., Bunch, C. M., Aboukhaled, M. W., Thomas, E. J., Zackariya, S. K., Oryakhail, H., Mehreteab, A., Ludwig, R. E., George, S. M., ... Al-Fadhl, M. D. (2025). The Effect of Heterogeneous Definitions of Massive Transfusion on Using Blood Component Thresholds to Predict Futility in Severely Bleeding Trauma Patients. Journal of Clinical Medicine, 14(15), 5426. https://doi.org/10.3390/jcm14155426








