The Evaluation of Blood Prooxidant–Antioxidant Balance Indicators and Cortisol Pre- and Post-Surgery in Patients with Benign Parotid Gland Tumors: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
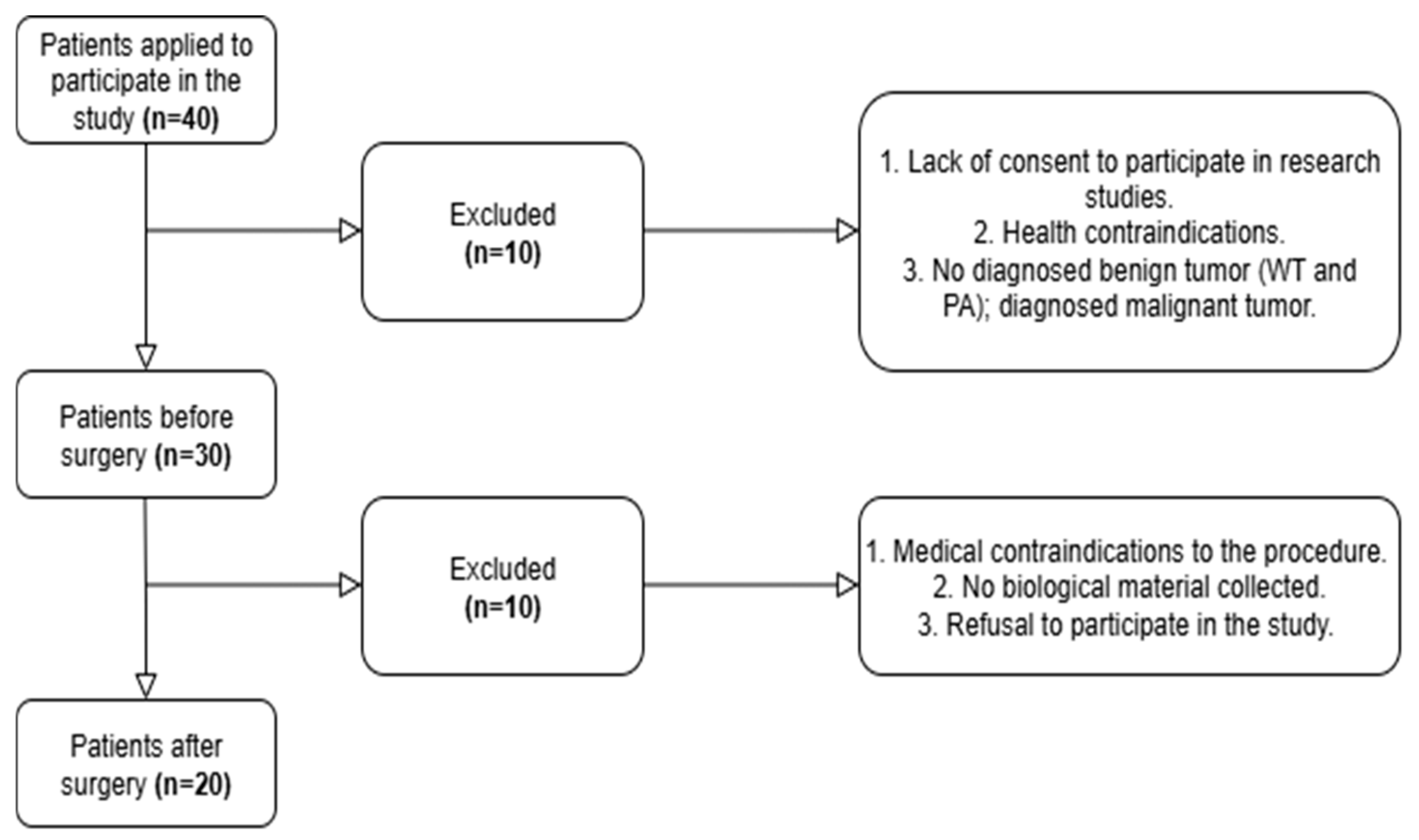
2.1. Participants
2.2. Surgery and Blood Collection
2.2.1. Surgery Methodology
2.2.2. Biochemical Analyses
2.3. Statistical Analysis
3. Results
3.1. Blood Prooxidant–Antioxidant Balance
3.2. Activity of CK and LDH
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANZCTR | Australian New Zealand Clinical Trials Registry |
| AOPPs | Advanced protein oxidation products |
| BMI | Body mass index |
| CAT | Catalase |
| CK | Creatine kinase |
| Cor | Cortisol |
| CT | Computed tomography |
| CV | Coefficients of variation |
| CXPA | Carcinoma ex pleomorphic adenoma |
| GPX | Glutathione peroxidase |
| GSH | Reduced glutathione |
| Hb | Hemoglobin |
| HPV | Human papillomavirus |
| Ht | Hematocrit |
| LDH | Lactate dehydrogenase |
| MDA | Malondialdehyde |
| PA | Pleomorphic adenoma |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TAS | Total antioxidant capacity |
| TBA | The thiobarbituric acid |
| TOS | Total oxidative capacity |
| UA | Uric acid |
| WT | Warthin’s tumor |
References
- Stryjewska-Makuch, G.; Kolebacz, B.; Janik, M.A.; Wolnik, A. Increase in the Incidence of Parotid Gland Tumors in the Years 2005–2014. Otolaryngol. Pol. 2017, 71, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Szydłowski, K.; Puchalski, M.; Ołdziej, S.; Kasprzyk-Tryk, A.; Skorek, A.; Tretiakow, D. The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 12558. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.; Fernández-Coello, A.; Vidal-Sarró, N.; Céspedes, D.; Camins, A.; Taberna, M.; Gabarrós, A. Brain Metastasis of Carcinoma Ex Pleomorphic Adenoma of the Parotid Gland: Case Report and Review of the Literature. Acta Neurochir. 2017, 159, 459–463. [Google Scholar] [CrossRef]
- Sowa, P.; Misiolek, M.; Pasinski, B.; Bartosz, G.; Soszynski, M.; Adamczyk-Sowa, M.; Sadowska-Bartosz, I. Oxidative Stress Markers Patients with Parotid Gland Tumors: A Pilot Study. Biomed. Res. Int. 2018, 2018, 4340871. [Google Scholar] [CrossRef]
- Walsh, H.; Alghamdi, S.; Dave, M.; Alsanie, I.; Khurram, S.A. Diagnostic and Prognostic Biomarkers in Salivary Gland Tumours. Diagn. Histopathol. 2023, 29, 177–187. [Google Scholar] [CrossRef]
- Katabi, N.; Xu, B.; Jungbluth, A.A.; Zhang, L.; Shao, S.Y.; Lane, J.; Ghossein, R.; Antonescu, C.R. PLAG1 Immunohistochemistry Is a Sensitive Marker for Pleomorphic Adenoma: A Comparative Study with PLAG1 Genetic Abnormalities. Histopathology 2017, 72, 285. [Google Scholar] [CrossRef]
- Bratiloveanu, M.; Dumitru, M.; Marinescu, A.N.; Serboiu, C.; Patrascu, O.M.; Costache, A.; Vrinceanu, D. Challenges in the Management of Giant Carcinoma Ex-Pleiomorphic Adenoma of the Parotid Gland in a Single Tertiary Center. Medicina 2025, 61, 37. [Google Scholar] [CrossRef]
- Soares, C.D.; de Lima Morais, T.M.; Carlos, R.; Martins, M.D.; de Almeida, O.P.; Mariano, F.V.; Altemani, A. Immunohistochemical Expression of Mammaglobin in Salivary Duct Carcinomas de Novo and Salivary Duct Carcinoma Ex Pleomorphic Adenoma. Hum. Pathol. 2019, 92, 59–66. [Google Scholar] [CrossRef]
- Khurram, S.A.; Speight, P.M. Characterisation of DOG-1 Expression in Salivary Gland Tumours and Comparison with Myoepithelial Markers. Head Neck Pathol. 2019, 13, 140–148. [Google Scholar] [CrossRef]
- Ohtomo, R.; Mori, T.; Shibata, S.; Tsuta, K.; Maeshima, A.M.; Akazawa, C.; Watabe, Y.; Honda, K.; Yamada, T.; Yoshimoto, S.; et al. SOX10 Is a Novel Marker of Acinus and Intercalated Duct Differentiation in Salivary Gland Tumors: A Clue to the Histogenesis for Tumor Diagnosis. Mod. Pathol. 2013, 26, 1041–1050. [Google Scholar] [CrossRef]
- Adkins, B.D.; Geromes, A.; Zhang, L.Y.; Chernock, R.; Kimmelshue, K.; Lewis, J.; Ely, K. SOX10 and GATA3 in Adenoid Cystic Carcinoma and Polymorphous Adenocarcinoma. Head Neck Pathol. 2020, 14, 406–411. [Google Scholar] [CrossRef]
- Simpson, R.H.W. Salivary Duct Carcinoma: New Developments-Morphological Variants Including Pure In Situ High Grade Lesions; Proposed Molecular Classification. Head Neck Pathol. 2013, 7, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, D.; Epivatianos, A.; Poulopoulos, A.; Nomikos, A.; Papazoglou, G.; Antoniades, D.; Barbatis, C. Detection of C-KIT (CD117) Molecule in Benign and Malignant Salivary Gland Tumours. Oral Oncol. 2006, 42, 56–64. [Google Scholar] [CrossRef]
- Haller, F.; Skálová, A.; Ihrler, S.; Märkl, B.; Bieg, M.; Moskalev, E.A.; Erber, R.; Blank, S.; Winkelmann, C.; Hebele, S.; et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am. J. Surg. Pathol. 2019, 43, 1264–1272. [Google Scholar] [CrossRef]
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. INSM1 Is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am. J. Surg. Pathol. 2018, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Vargas, P.A.; Cheng, Y.; Barrett, A.W.; Craig, G.T.; Speight, P.M. Expression of Mcm-2, Ki-67 and Geminin in Benign and Malignant Salivary Gland Tumours. J. Oral Pathol. Med. 2008, 37, 309–318. [Google Scholar] [CrossRef]
- Bussari, S.; Ganvir, S.M.; Sarode, M.; Jeergal, P.A.; Deshmukh, A.; Srivastava, H. Immunohistochemical Detection of Proliferative Marker Ki-67 in Benign and Malignant Salivary Gland Tumors. J. Contemp. Dent. Pract. 2018, 19, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Vital, D.; Ikenberg, K.; Moch, H.; Rössle, M.; Huber, G.F. The Expression of PD-L1 in Salivary Gland Carcinomas. Sci. Rep. 2019, 9, 12724. [Google Scholar] [CrossRef]
- Szewczyk, M.; Marszałek, A.; Sygut, J.; Golusiński, P.; Golusiński, W. Prognostic Markers in Salivary Gland Cancer and Their Impact on Survival. Head Neck 2019, 41, 3338–3347. [Google Scholar] [CrossRef]
- Alos, L.; Lujan, B.; Castillo, M.; Nadal, A.; Carreras, M.; Caballero, M.; De Bolos, C.; Cardesa, A. Expression of Membrane-Bound Mucins (MUC1 and MUC4) and Secreted Mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in Mucoepidermoid Carcinomas of Salivary Glands. Am. J. Surg. Pathol. 2005, 29, 806–813. [Google Scholar] [CrossRef]
- Plath, M.; Sand, M.; Cavaliere, C.; Plinkert, P.K.; Baumann, I.; Zaoui, K. Long-Term Outcomes and Quality of Life Following Parotidectomy for Benign Disease. Acta Otorhinolaryngol. Ital. 2022, 42, 215–222. [Google Scholar] [CrossRef]
- Committeri, U.; Arena, A.; Iaquino, V.; Salzano, G.; Blasi, F.D.; Esposito, M.; Giovacchini, F.; Calvanese, C.; Abbate, V.; Bonavolontà, P.; et al. Surgical Management and Side Effects of Parotid Gland Surgery for Benign Lesions: A Retrospective Analysis of Our Experience from 2012 to 2021. Br. J. Oral Maxillofac. Surg. 2023, 61, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, W.; Fang, Y.; Cui, X.; Xu, Z. Parotid Tumors and Their Postoperative Complications: A 5-Year Experience. Oral Maxillofac. Surg. Cases 2023, 9, 100319. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Leonart, M.E. Oxidative Stress and Cancer: An Overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Sowa, P.; Misiolek, M.; Zielinski, M.; Mazur, B.; Adamczyk-Sowa, M. Novel Interleukin-33 and Its Soluble ST2 Receptor as Potential Serum Biomarkers in Parotid Gland Tumors. Exp. Biol. Med. 2018, 243, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Misiak, P.; Rzepkowska-Misiak, B.; Wcislo, S.; Dziwińska, K.; Malinowska, K.; Majsterek, I. Evaluation of the Impact of Radical Tumor Resection in Lung Cancer Patients on the Activity of Selected Antioxidant Enzymes. Kardiochirurgia Torakochirurgia Pol. 2014, 11, 414–420. [Google Scholar] [CrossRef]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- Gunning, J.A.; Gilman, K.E.; Zúñiga, T.M.; Simpson, R.J.; Limesand, K.H. Parotid Glands Have a Dysregulated Immune Response Following Radiation Therapy. PLoS ONE 2024, 19, e0297387. [Google Scholar] [CrossRef]
- Liu, G.X.; Lan, J.; Sun, Y.; Hu, Y.J.; Jiang, G.S. Expression of the Chemokine CCL28 in Pleomorphic Adenoma and Adenolymphoma of the Human Salivary Glands. Exp. Ther. Med. 2012, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, K.; Oishi, N.; Kawai, M.; Odate, T.; Tahara, I.; Inoue, T.; Kasai, K.; Kondo, T. Expressions of Cxcl12, Cxcl10 and Ccl18 in Warthin Tumors Characterized Pathologically by Having a Lymphoid Stroma with Germinal Centers. Histol. Histopathol. 2021, 36, 931–938. [Google Scholar] [CrossRef]
- Laohavisudhi, F.; Chunchai, T.; Ketchaikosol, N.; Thosaporn, W.; Chattipakorn, N.; Chattipakorn, S.C. Evaluation of CD44s, CD44v6, CXCR2, CXCL1, and IL-1β in Benign and Malignant Tumors of Salivary Glands. Diagnostics 2022, 12, 1275. [Google Scholar] [CrossRef]
- Khademi, B.; Tajvarpour, M.; Mojtahedi, Z.; Haghshenas, M.R.; Erfani, N. T-helper Type 1 and 2 Cytokine Levels in Patients with Benign and Malignant Salivary Gland Tumors. Iran. J. Immunol. 2016, 13, 9–15. [Google Scholar] [PubMed]
- Haghshenas, M.R.; Khademi, B.; Faghih, Z.; Ghaderi, A.; Erfani, N. Immune Regulatory Cells and IL17-Producing Lymphocytes in Patients with Benign and Malignant Salivary Gland Tumors. Immunol. Lett. 2015, 164, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guardado, J.; Hoffman, R.; Xu, H.; Namas, R.; Vodovotz, Y.; Xu, L.; Ramadan, M.; Brown, J.; Turnquist, H.R.; et al. IL33-Mediated ILC2 Activation and Neutrophil IL5 Production in the Lung Response after Severe Trauma: A Reverse Translation Study from a Human Cohort to a Mouse Trauma Model. PLoS Med. 2017, 14, e1002365. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wu, M.X.; Lin, Y.Q.; Xie, S.L.; Huang, T.C.; Liu, P.M.; Nie, R.Q.; Meng, Q.Q.; Luo, N.S.; Chen, Y.X.; et al. IL-33 Promotes IL-10 Production in Macrophages: A Role for IL-33 in Macrophage Foam Cell Formation. Exp. Mol. Med. 2017, 49, e388. [Google Scholar] [CrossRef]
- Zare, R.; Malekzadeh, M.; Hashemi, M.; Khademi, B.; Andishe-Tadbir, A. Investigation of IL-33 Serum Levels in Patients with Benign and Malignant Salivary Gland Tumors. Cancer Biomark. 2018, 23, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Savic Vujovic, K.; Zivkovic, A.; Dozic, I.; Cirkovic, A.; Medic, B.; Srebro, D.; Vuckovic, S.; Milovanovic, J.; Jotic, A. Oxidative Stress and Inflammation Biomarkers in Postoperative Pain Modulation in Surgically Treated Patients with Laryngeal Cancer—Pilot Study. Cells 2023, 12, 1391. [Google Scholar] [CrossRef]
- Abdalla, M.Y. Glutathione as potential target for cancer; more or less is good? Jordan J. Biol. Sci. 2011, 4, 119–124. [Google Scholar]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Konzack, A.; Jakupovic, M.; Kubaichuk, K.; Görlach, A.; Dombrowski, F.; Miinalainen, I.; Sormunen, R.; Kietzmann, T. Mitochondrial Dysfunction Due to Lack of Manganese Superoxide Dismutase Promotes Hepatocarcinogenesis. Antioxid. Redox Signal. 2015, 23, 1059–1075. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The Thiol Pool in Human Plasma: The Central Contribution of Albumin to Redox Processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, J.; Selander, T.; Purdy, M.; Juvonen, P.; Eskelinen, M. Patients with Increased Levels of the Oxidative Stress Biomarker SOD1 Appear to Have Diminished Postoperative Pain after Midline Laparotomy: A Randomised Trial with Special Reference to Postoperative Pain Score (NRS). Anticancer Res. 2018, 38, 1003–1008. [Google Scholar] [CrossRef]
- Li, D.; Ding, Z.; Du, K.; Ye, X.; Cheng, S. Reactive Oxygen Species as a Link between Antioxidant Pathways and Autophagy. Oxid. Med. Cell. Longev. 2021, 2021, 5583215. [Google Scholar] [CrossRef]
- Orak, Y.; Baylan, F.A.; Kocaslan, A.; Eroglu, E.; Acipayam, M.; Kirisci, M.; Boran, O.F.; Doganer, A. Effect of Mechanical Ventilation during Cardiopulmonary Bypass on Oxidative Stress: A Randomized Clinical Trial. Braz. J. Anesthesiol. 2022, 72, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Didžiapetrienė, J.; Kazbarienė, B.; Tikuišis, R.; Dulskas, A.; Dabkevičienė, D.; Lukosevičienė, V.; Kontrimavičiūtė, E.; Sužiedėlis, K.; Ostapenko, V. Oxidant/Antioxidant Status of Breast Cancer Patients in Pre- and Post-Operative Periods. Medicina 2020, 56, 70. [Google Scholar] [CrossRef]
- Bozan, N.; Demir, H.; Gürsoy, T.; Özkan, H.; Düzenli, U.; Sarıkaya, E.; Turan, M.; Kiroglu, A.F.; Çankaya, H. Alterations in Oxidative Stress Markers in Laryngeal Carcinoma Patients. J. Chin. Med. Assoc. 2018, 81, 811–815. [Google Scholar] [CrossRef]
- Malik, U.U.; Siddiqui, I.A.; Hashim, Z.; Zarina, S. Measurement of Serum Paraoxonase Activity and MDA Concentrations in Patients Suffering with Oral Squamous Cell Carcinoma. Clin. Chim. Acta 2014, 430, 38–42. [Google Scholar] [CrossRef]
- Taysi, S.; Uslu, C.; Akcay, F.; Sutbeyaz, M.Y. Malondialdehyde and Nitric Oxide Levels in the Plasma of Patients with Advanced Laryngeal Cancer. Surg. Today 2003, 33, 651–654. [Google Scholar] [CrossRef]
- Arsalani-Zadeh, R.; Ullah, S.; Khan, S.; MacFie, J. Oxidative Stress in Laparoscopic versus Open Abdominal Surgery: A Systematic Review. J. Surg. Res. 2011, 169, e59–e68. [Google Scholar] [CrossRef]
- Koksal, H.; Kurban, S. Total Oxidant Status, Total Antioxidant Status, and Paraoxonase and Arylesterase Activities during Laparoscopic Cholecystectomy. Clinics 2010, 65, 285–290. [Google Scholar] [CrossRef][Green Version]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Liu, L.; He, Y.; Ge, G.; Li, L.; Zhou, P.; Zhu, Y.; Tang, H.; Huang, Y.; Li, W.; Zhang, L. Lactate Dehydrogenase and Creatine Kinase as Poor Prognostic Factors in Lung Cancer: A Retrospective Observational Study. PLoS ONE 2017, 12, e0182168. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate Dehydrogenase A: A Key Player in Carcinogenesis and Potential Target in Cancer Therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- AL-Daami, H.A. A-H.; Rzaq, S.; Rawaa, G.A. The Significance of Elevated Lactate Dehydrogenase and Creatine Kinase Activities as Prognostic Biomarkers for the Mortality in Patients with Terminal Cancers. Indian J. Forensic Med. 2021, 15, 1536–1542. [Google Scholar]
- Prete, A.; Yan, Q.; Al-Tarrah, K.; Akturk, H.K.; Prokop, L.J.; Alahdab, F.; Foster, M.A.; Lord, J.M.; Karavitaki, N.; Wass, J.A.; et al. The Cortisol Stress Response Induced by Surgery: A Systematic Review and Meta-Analysis. Clin. Endocrinol. 2018, 89, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Jang, J.S.; Hwang, S.M.; Tark, H.; Kim, J.H.; Lee, J.J.; Li, Y. Effects of Surgery Start Time on Postoperative Cortisol, Inflammatory Cytokines, and Postoperative Hospital Day in Hip Surgery: Randomized Controlled Trial. Medicine 2019, 98, e15820. [Google Scholar] [CrossRef] [PubMed]
- Kordzińska-Cisek, I.; Cisek, P.; Grzybowska-Szatkowska, L. The Role of Prognostic Factors in Salivary Gland Tumors Treated by Surgery and Adjuvant Radio-or Chemoradiotherapy—A Single Institution Experience. Cancer Manag. Res. 2020, 12, 1047–1067. [Google Scholar] [CrossRef]
- McCloskey, S.A.; Jaggernauth, W.; Rigual, N.R.; Hicks, W.L.; Popat, S.R.; Sullivan, M.; Mashtare, T.L.; Khan, M.K.; Loree, T.R.; Singh, A.K. Radiation Treatment Interruptions Greater than One Week and Low Hemoglobin Levels (12 g/DL) Are Predictors of Local Regional Failure after Definitive Concurrent Chemotherapy and Intensity-Modulated Radiation Therapy for Squamous Cell Carcinoma of the Head and Neck. Am. J. Clin. Oncol. Cancer Clin. Trials 2009, 32, 587–591. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, H.Z.; Zhu, P.; Feng, X.B. Postoperative Changes in Hemoglobin and Hematocrit in Patients Undergoing Primary Total Hip and Knee Arthroplasty. Chin. Med. J. 2015, 128, 1977–1979. [Google Scholar] [CrossRef] [PubMed]
| Variable | M ± SD |
|---|---|
| Age (years) | 63.9 ± 12.4 |
| Body weight (kg) | 79.9 ± 18.5 |
| Body height (cm) | 168.6 ± 7.67 |
| BMI (kg/m2) | 28.1 ± 5.98 |
| Variables | Time | M ± SD | t | p | dc |
|---|---|---|---|---|---|
| Hb (g/dL) | 1 | 14.2 ± 1.7 | −1.67 | 0.112 | 0.48 |
| 2 | 14.9 ± 1.0 | ||||
| Ht (%) | 1 | 40.5 ± 5.1 | −1.18 | 0.252 | 0.36 |
| 2 | 42.0 ± 2.7 | ||||
| Cor (µg/dL) | 1 | 13.4 ± 4.1 | 2.05 | 0.054 | 0.64 |
| 2 | 10.9 ± 3.5 |
| Variables | Time | M ± SD | Statistics | Significant | Effect Size |
|---|---|---|---|---|---|
| SOD (U/gHb) | 1 | 1414.1 ± 254.4 | t = −0.31 | p = 1.00 | dc = 0.09 |
| 2 | 1436.3 ± 212.7 | ||||
| CAT (U/gHb) | 1 | 167.0 ± 29.4 | t = −0.22 | p = 0.100 | dc = 0.06 |
| 2 | 168.9 ± 30.5 | ||||
| GPx (U/gHb) | 1 | 39.9 ± 8.4 | t = −0.11 | p = 0.916 | dc = 0.04 |
| 2 | 40.2 ± 8.7 | ||||
| GSH (µg/mgHb) | 1 | 3.0 ± 0.4 | t = 3.07 | p = 0.038 | dc = 0.78 |
| 2 | 2.7 ± 0.3 | ||||
| TAS (mmol/L) | 1 | 1.1 ± 0.3 | t = −1.54 | p = 0.563 | dc = 0.48 |
| 2 | 1.2 ± 0.2 | ||||
| UA (mg/dL) | 1 | 5.8 ± 1.5 | t = 2.03 | p = 0.281 | dc = 0.29 |
| 2 | 5.3 ± 1.5 | ||||
| MDA (µmol/L) | 1 | 6.2 ± 1.4 | Z = −3.58 | p = 0.001 | r = 0.80 |
| 2 | 4.3 ± 1.1 | ||||
| TOS (µmol/L) | 1 | 430.0 ± 208.7 | t = −2.15 | p = 0.044 | dc = 0.41 |
| 2 | 520.1 ± 229.6 |
| Variables | Time | M ± SD | Statistics | Significant | Effect Size |
|---|---|---|---|---|---|
| CK (U/L) | 1 | 120.3 ± 99.5 | t = 0.90 | p = 0.760 | dc = 0.23 |
| 2 | 100.0 ± 75.5 | ||||
| LDH (U/L) | 1 | 797.9 ± 335.2 | t = 0.69 | p = 0.500 | dc = 0.22 |
| 2 | 726.4 ± 306.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bańkowski, S.; Pilch, J.; Witek, B.; Markowski, J.; Likus, W.; Rozpara, M.; Sadowska-Krępa, E. The Evaluation of Blood Prooxidant–Antioxidant Balance Indicators and Cortisol Pre- and Post-Surgery in Patients with Benign Parotid Gland Tumors: A Preliminary Study. J. Clin. Med. 2025, 14, 5425. https://doi.org/10.3390/jcm14155425
Bańkowski S, Pilch J, Witek B, Markowski J, Likus W, Rozpara M, Sadowska-Krępa E. The Evaluation of Blood Prooxidant–Antioxidant Balance Indicators and Cortisol Pre- and Post-Surgery in Patients with Benign Parotid Gland Tumors: A Preliminary Study. Journal of Clinical Medicine. 2025; 14(15):5425. https://doi.org/10.3390/jcm14155425
Chicago/Turabian StyleBańkowski, Sebastian, Jan Pilch, Bartosz Witek, Jarosław Markowski, Wirginia Likus, Michał Rozpara, and Ewa Sadowska-Krępa. 2025. "The Evaluation of Blood Prooxidant–Antioxidant Balance Indicators and Cortisol Pre- and Post-Surgery in Patients with Benign Parotid Gland Tumors: A Preliminary Study" Journal of Clinical Medicine 14, no. 15: 5425. https://doi.org/10.3390/jcm14155425
APA StyleBańkowski, S., Pilch, J., Witek, B., Markowski, J., Likus, W., Rozpara, M., & Sadowska-Krępa, E. (2025). The Evaluation of Blood Prooxidant–Antioxidant Balance Indicators and Cortisol Pre- and Post-Surgery in Patients with Benign Parotid Gland Tumors: A Preliminary Study. Journal of Clinical Medicine, 14(15), 5425. https://doi.org/10.3390/jcm14155425






