Association of Epicardial Adipose Tissue with Novel Inflammation and Heart Failure Biomarkers in Type 2 Diabetes Patients: Effect of Metabolic Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. iEAT Volume and Left-Ventricular Function
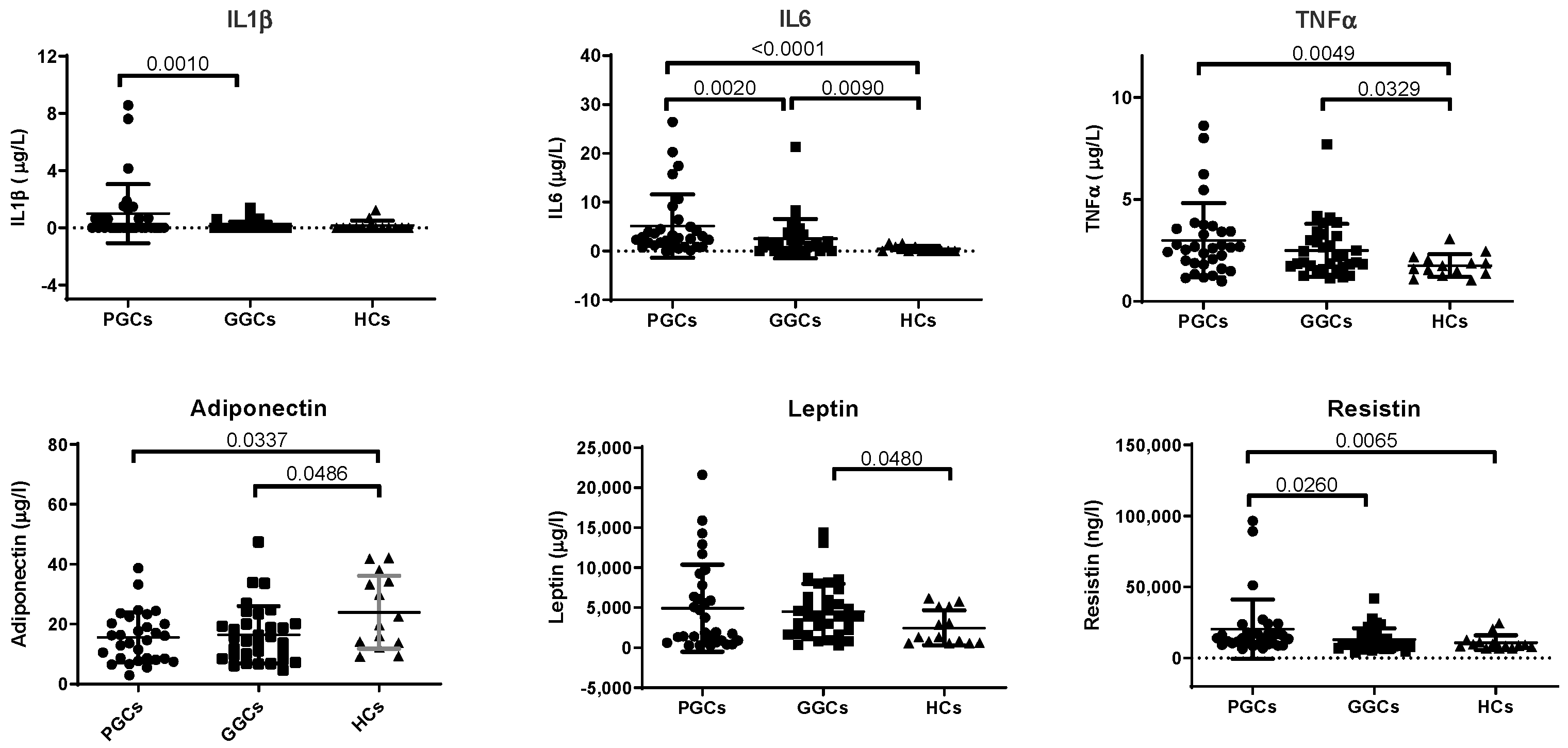
3.3. Biomarkers of Inflammation
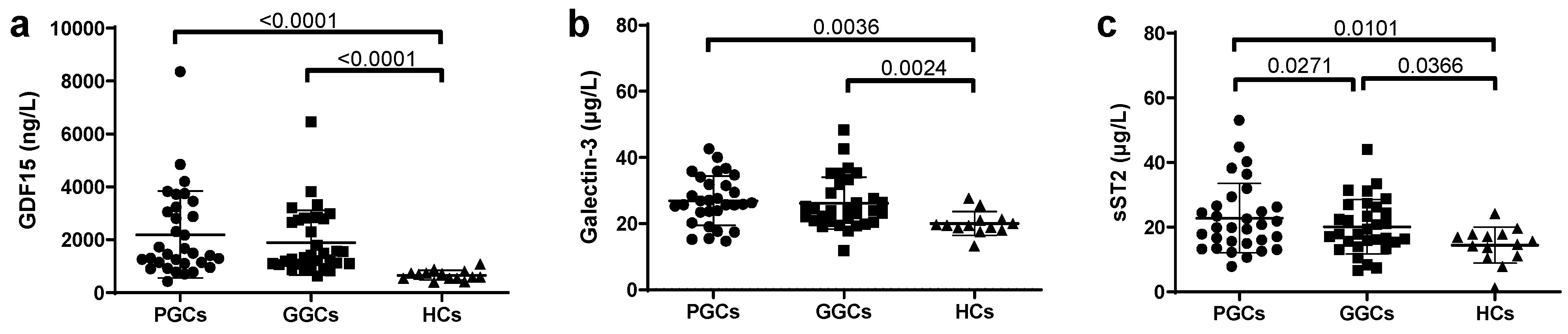
3.4. Novel HF-Related Biomarkers
3.5. EAT and Novel HF-Related Biomarkers
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end-products |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AST | aspartate aminotransferase |
| AW | abdominal waist |
| BMI | body mass index |
| BNP | B-type natriuretic peptide |
| CVD | cardiovascular disease |
| DPP4i | dipeptidyl peptidase-4 inhibitor |
| EAT | epicardial adipose tissue |
| EATv | epicardial adipose tissue volume |
| EDV | end-diastolic volume left ventricular |
| eGFR | estimated glomerular filtration rate |
| ESV | end-systolic volume left ventricular |
| FFAs | free fatty acids |
| GDF15 | growth differentiation factor 15 |
| GGC | good glycemic control |
| GGT | gamma-glutamyl transferase |
| GLP1-ar | glucagon-like peptide 1 agonist receptor |
| HC | healthy controls |
| HDLc | high-density lipoprotein cholesterol |
| HF | heart failure |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| hsCRP | high-sensitivity C-reactive protein |
| hsTnT | high-sensitivity Troponin T |
| iEAT | indexed epicardial adipose tissue |
| IL | interleukin |
| IR | insulin resistance |
| LDLc | low-density lipoprotein cholesterol |
| LVEF | Left-ventricular ejection fraction |
| MDCT | multidetector computed tomography |
| MRI | magnetic resonance imaging |
| NPs | natriuretic peptides |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| PGC | poor glycemic control |
| ROS | reactive oxygen species |
| sHF | subclinical heart failure |
| sST2 | soluble suppression of tumorigenicity 2 |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
| TC | total cholesterol |
| Tg | triglycerides |
| TNFα | tumor necrosis factor alpha |
| VAT | visceral adipose tissue |
| VIF | variance inflation factor |
| VLDLc | very low density lipoprotein cholesterol |
References
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef]
- Nichols, G.A.; Gullion, C.M.; Koro, C.E.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004, 27, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits with Incident HFpEF and HFrEF HHS. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Sciences Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef]
- Okdahl, T.; Wegeberg, A.M.; Pociot, F.; Brock, B.; Størling, J.; Brock, C. Low-grade inflammation in type 2 diabetes: A cross-sectional study from a Danish diabetes outpatient clinic. BMJ Open 2022, 12, e062188. [Google Scholar] [CrossRef]
- Sardu, C.; D’oNofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R.; et al. Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction: Effects of metformin. Cardiovasc. Diabetol. 2019, 18, 126. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Tuñón, J.; Orejas, M.; Cortés, M.; Egido, J.; Lorenzo, Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 28. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218. [Google Scholar] [CrossRef]
- Rossi, V.A.; Gruebler, M.; Monzo, L.; Galluzzo, A.; Beltrami, M. The Different Pathways of Epicardial Adipose Tissue across the Heart Failure Phenotypes: From Pathophysiology to Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 6838. [Google Scholar] [CrossRef]
- Park, J.J. Epidemiology, pathophysiology, diagnosis and treatment of heart failure in diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Colantuoni, S.; Medicamento, G.; et al. Dysregulated Epicardial Adipose Tissue as a Risk Factor and Potential Therapeutic Target of Heart Failure with Preserved Ejection Fraction in Diabetes. Biomolecules 2022, 12, 176. [Google Scholar] [CrossRef]
- Greulich, S.; Maxhera, B.; Vandenplas, G.; de Wiza, D.H.; Smiris, K.; Mueller, H.; Heinrichs, J.; Blumensatt, M.; Cuvelier, C.; Akhyari, P.; et al. Secretory Products from Epicardial Adipose Tissue of Patients with Type 2 Diabetes Mellitus Induce Cardiomyocyte Dysfunction. Circulation 2012, 126, 2324–2334. [Google Scholar] [CrossRef]
- Puig, N.; Rives, J.; Gil-Millan, P.; Miñambres, I.; Ginel, A.; Tauron, M.; Bonaterra-Pastra, A.; Hernández-Guillamon, M.; Pérez, A.; Sánchez-Quesada, J.L.; et al. Apolipoprotein J protects cardiomyocytes from lipid-mediated inflammation and cytotoxicity induced by the epicardial adipose tissue of diabetic patients. Biomed. Pharmacother. 2024, 175, 116779. [Google Scholar] [CrossRef]
- van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Westenbrink, B.D.; de Boer, R.A.; Rienstra, M.; Gorter, T.M. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: Mechanisms, management and modern perspectives. Eur. J. Heart Fail. 2022, 24, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Q.; Li, Z.; Zhou, Y.; Liu, Z.; You, L.; Tao, L.; Dong, Q.; Zuo, Z.; Gao, L.; et al. Epicardial adipose tissue density is a better predictor of cardiometabolic risk in HFpEF patients: A prospective cohort study. Cardiovasc. Diabetol. 2023, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Manzanares, R.; Anguita-Gámez, M.; Muñiz, J.; Barrios, V.; Gimeno-Orna, J.A.; Pérez, A.; Rodríguez-Padial, L.; Anguita, M. Prevalence and incidence of heart failure in type 2 diabetes patients: Results from a nationwide prospective cohort—The DIABET-IC study. Cardiovasc. Diabetol. 2024, 23, 253. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.; Coats, A.J.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Michalska-Kasiczak, M.; Bielecka-Dabrowa, A.; Von Haehling, S.; Anker, S.D.; Rysz, J.; Banach, M. Biomarkers, myocardial fibrosis and co-morbidities in heart failure with preserved ejection fraction: An overview. Arch. Med. Sci. 2018, 14, 890–909. [Google Scholar] [CrossRef]
- Rabkin, S.W. The utility of growth differentiation factor-15, galectin-3, and sST2 as biomarkers for the diagnosis of heart failure with preserved ejection fraction and compared to heart failure with reduced ejection fraction: A systematic review. Heart Fail. Rev. 2021, 26, 799–812. [Google Scholar] [CrossRef]
- Rives, J.; Gil-Millan, P.; Viladés, D.; García-Osuna, Á.; Genua, I.; Miñambres, I.; Grau-Agramunt, M.; Gich, I.; Puig, N.; Benitez, S.; et al. Low-Density Lipoprotein Subfraction Phenotype Is Associated with Epicardial Adipose Tissue Volume in Type 2 Diabetes. J. Clin. Med. 2025, 14, 862. [Google Scholar] [CrossRef]
- Hatle, L. How to diagnose diastolic heart failure—A consensus statement. Eur. Heart J. 2007, 28, 2421–2423. [Google Scholar] [CrossRef]
- Merino-Merino, A.; Gonzlez-Bernal, J.; Fernandez-Zoppino, D.; Saez-Maleta, R.; Perez-Rivera, J.A. The role of galectin-3 and st2 in cardiology: A short review. Biomolecules 2021, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Khadanga, S.; Goel, S.K.; Majumder, S.; Baig, M.S.; Bhatia, V.; Chaudhary, N.; Saluja, R. Evaluation of interleukin-33 & sST2 levels in type-2 diabetic mellitus patients with or without metabolic syndrome. Indian J. Med. Res. 2023, 157, 470–476. [Google Scholar] [CrossRef]
- Castiglione, V.; Chiriaco’, M.; Aimo, A.; Januzzi, J.; Richards, A.; Lam, C.; Latini, R.; Anand, I.; Ueland, T.; Rocca, H.B.-L.; et al. Prognostic Value of SST2 in Heart Failure Patients with Diabetes. Eur. Heart J. 2021, 42, ehab724-0869. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Tang, W.H.W. Reappraisal of Inflammatory Biomarkers in Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The extracellular matrix in ischemic and nonischemic heart failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Yu, L.; Van Veldhuisen, D.J. Galectin-3 in cardiac remodeling and heart failure. Curr. Heart Fail. Rep. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Cai, Y.; Hao, B.; Chen, J.; Li, H.; Liu, H. Prognostic value of soluble suppression of tumorigenesis-2 (sST2) for cardiovascular events in coronary artery disease patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 49. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Gimeno-Orna, J.A.; Rodríguez-Padial, L.; Anguita-Sánchez, M.; Barrios, V.; Muñiz, J.; Pérez, A. Association of the KDIGO risk classification with the prevalence of heart failure in patients with type 2 diabetes. J. Clin. Med. 2021, 10, 4634. [Google Scholar] [CrossRef]
- Gaziano, L.; Sun, L.; Arnold, M.; Bell, S.; Cho, K.; Kaptoge, S.K.; Song, R.J.; Burgess, S.; Posner, D.C.; Mosconi, K.; et al. Mild-to-Moderate Kidney Dysfunction and Cardiovascular Disease: Observational and Mendelian Randomization Analyses. Circulation 2022, 146, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padial, L.; Pérez, A.; Anguita Sánchez, M.; Barrios, V.; Gimeno-Orna, J.A.; Muñiz, J. Characteristics of women with type 2 diabetes and heart failure in Spain. The DIABET-IC study. Cardiol. J. 2023, 31, 103–110. [Google Scholar] [CrossRef] [PubMed]
| Spearman’s Rank Correlation Coefficient Test | |||
|---|---|---|---|
| r | p | ||
| IL1β | HbA1c | 0.295 | 0.006 |
| hsCRP | 0.259 | 0.013 | |
| AST | 0.272 | 0.010 | |
| LVEF | −0.293 | 0.029 | |
| Tg | −0.250 | 0.016 | |
| IL6 | BMI | 0.247 | 0.015 |
| AW | 0.252 | 0.016 | |
| HbA1c | 0.339 | 0.002 | |
| hsCRP | 0.563 | <0.001 | |
| AST | 0.304 | 0.004 | |
| HDL-c | −0.235 | 0.022 | |
| TNFα | AW | 0.208 | 0.038 |
| HbA1c | 0.268 | 0.012 | |
| hsCRP | 0.342 | 0.002 | |
| AST | 0.261 | 0.013 | |
| ALT | 0.232 | 0.023 | |
| ALP | 0.258 | 0.013 | |
| iEATv | 0.242 | 0.021 | |
| LVEF | −0.263 | 0.025 | |
| HDL-c | −0.373 | <0.001 | |
| Adiponectin | Age | 0.192 | 0.046 |
| ALT | −0.439 | <0.001 | |
| GGT | −0.333 | 0.002 | |
| Tg | −0.259 | 0.013 | |
| HDL-c | 0.328 | 0.002 | |
| VLDL-c | −0.241 | 0.020 | |
| Leptin | Weight | 0.468 | <0.001 |
| BMI | 0.653 | <0.001 | |
| AW | 0.553 | <0.001 | |
| ALT | 0.243 | 0.018 | |
| hsCRP | 0.310 | 0.004 | |
| Resistin | BMI | 0.258 | 0.012 |
| AW | 0.251 | 0.016 | |
| HbA1c | 0.268 | 0.012 | |
| hsCRP | 0.298 | 0.005 | |
| Spearman’s Rank Correlation Coefficient Test | |||||||
|---|---|---|---|---|---|---|---|
| GDF15 | Galectin-3 | sST2 | |||||
| r | p | r | p | r | p | ||
| Clinical | Age | 0.56 | <0.0001 | 0.26 | 0.022 | 1 | 1 |
| BMI | 0.23 | 0.04 | 0.27 | 0.02 | 0.28 | 0.015 | |
| Weight | 0.18 | 0.10 | 0.09 | 0.41 | 0.35 | 0.002 | |
| AW | 0.27 | 0.028 | 0.25 | 0.034 | 0.31 | 0.009 | |
| Biochemical | HbA1c | 0.35 | 0.002 | 0.27 | 0.026 | 0.26 | 0.029 |
| Glucose | 0.31 | 0.006 | 0.23 | 0.053 | 0.37 | 0.001 | |
| ALP | 0.29 | 0.010 | 0.25 | 0.033 | 0.14 | 0.22 | |
| GGT | 0.27 | 0.020 | −0.92 | 0.44 | 0.24 | 0.04 | |
| HDL-c | −0.46 | <0.001 | −0.19 | 0.11 | −0.19 | 0.10 | |
| LDL-c | −0.28 | 0.016 | −0.24 | 0.84 | −0.50 | 0.67 | |
| Inflammation | hsCRP | 0.31 | 0.008 | 0.27 | 0.02 | 0.09 | 0.42 |
| TNFα | 0.37 | <0.001 | 0.052 | 0.66 | 0.097 | 0.40 | |
| IL6 | 0.25 | 0.02 | 0.27 | 0.02 | −0.058 | 0.61 | |
| Cardiac Parameters | iEAT | 0.52 | <0.001 | 0.19 | 0.11 | 0.37 | <0.001 |
| LVEF | 0.055 | 0.68 | 0.19 | 0.16 | −0.30 | 0.025 | |
| Multivariable Lineal Regression Analysis (Stepwise) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | p | 95.0% Confidence Interval for B | Collinearity Statistics | ||||
| B | Std. Error | Beta | Lower Bound | Upper Bound | Tolerance | VIF | ||||
| GDF15 | ||||||||||
| 1 | (Constant) | −2623.37 | 1039.69 | −2.52 | 0.014 | −4701.70 | −545.05 | |||
| Age | 80.60 | 18.40 | 0.48 | 4.38 | 0.001 | 43.82 | 117.38 | 1.00 | 1.00 | |
| 2 | (Constant) | −2520.87 | 1007.47 | −2.50 | 0.015 | −4535.44 | −506.31 | 0.79 | 1.25 | |
| Age | 60.03 | 19.98 | 0.36 | 3 | 0.004 | 20.08 | 99.98 | 0.79 | 1.25 | |
| iEAT | 19.41 | 8.54 | 0.27 | 2.27 | 0.027 | 2.32 | 36.51 | |||
| Galectin-3 | ||||||||||
| 1 | (Constant) | 20.57 | 2.36 | 8.70 | 0.001 | 15.85 | 25.30 | |||
| HbA1c | 0.64 | 0.26 | 0.297 | 2.42 | 0.01 | 0.11 | 1.17 | 1.00 | 1.00 | |
| 2 | (Constant) | 20.90 | 2.25 | 9.7 | 0.001 | 16.40 | 25.41 | |||
| HbA1c | 0.32 | 0.28 | 0.14 | 1.5 | 0.25 | −0.23 | 0.88 | 0.81 | 1.22 | |
| hsCRP | 0.44 | 0.16 | 0.34 | 2.69 | 0.009 | 0.11 | 0.77 | 0.81 | 1.22 | |
| sST2 | ||||||||||
| 1 | (Constant) | 12.91 | 3.61 | 3.57 | <0.01 | 5.67 | 20.14 | |||
| HbA1c | 0.90 | 0.40 | 0.28 | 2.21 | 0.031 | 0.08 | 1.72 | 1.00 | 1.00 | |
| 2 | (Constant) | −7.06 | 9.27 | −0.76 | 0.44 | −25.64 | 11.50 | |||
| HbA1c | 0.74 | 0.40 | 0.23 | 1.84 | 0.07 | −0.063 | 1.54 | 0.96 | 1.03 | |
| AW | 0.19 | 0.08 | 0.28 | 2.32 | 0.024 | 0.027 | 0.37 | 0.96 | 1.03 | |
| 3 | (Constant) | −5.27 | 8.99 | −0.58 | 0.56 | −23.29 | 12.74 | |||
| HbA1c | 0.56 | 0.39 | 0.17 | 1.42 | 0.16 | −0.22 | 1.35 | 0.92 | 1.07 | |
| AW | 0.12 | 0.08 | 0.18 | 1.43 | 0.15 | −0.05 | 0.30 | 0.84 | 1.18 | |
| iEAT | 0.14 | 0.06 | 0.29 | 2.24 | 0.02 | 0.01 | 0.26 | 0.81 | 1.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Millan, P.; Rives, J.; Viladés, D.; García-Osuna, Á.; Genua, I.; Miñambres, I.; Grau-Agramunt, M.; Gich, I.; Camacho, M.; Benitez, S.; et al. Association of Epicardial Adipose Tissue with Novel Inflammation and Heart Failure Biomarkers in Type 2 Diabetes Patients: Effect of Metabolic Control. J. Clin. Med. 2025, 14, 4687. https://doi.org/10.3390/jcm14134687
Gil-Millan P, Rives J, Viladés D, García-Osuna Á, Genua I, Miñambres I, Grau-Agramunt M, Gich I, Camacho M, Benitez S, et al. Association of Epicardial Adipose Tissue with Novel Inflammation and Heart Failure Biomarkers in Type 2 Diabetes Patients: Effect of Metabolic Control. Journal of Clinical Medicine. 2025; 14(13):4687. https://doi.org/10.3390/jcm14134687
Chicago/Turabian StyleGil-Millan, Pedro, José Rives, David Viladés, Álvaro García-Osuna, Idoia Genua, Inka Miñambres, Margarita Grau-Agramunt, Ignasi Gich, Mercedes Camacho, Sonia Benitez, and et al. 2025. "Association of Epicardial Adipose Tissue with Novel Inflammation and Heart Failure Biomarkers in Type 2 Diabetes Patients: Effect of Metabolic Control" Journal of Clinical Medicine 14, no. 13: 4687. https://doi.org/10.3390/jcm14134687
APA StyleGil-Millan, P., Rives, J., Viladés, D., García-Osuna, Á., Genua, I., Miñambres, I., Grau-Agramunt, M., Gich, I., Camacho, M., Benitez, S., Julve, J., Sánchez-Quesada, J. L., & Pérez, A. (2025). Association of Epicardial Adipose Tissue with Novel Inflammation and Heart Failure Biomarkers in Type 2 Diabetes Patients: Effect of Metabolic Control. Journal of Clinical Medicine, 14(13), 4687. https://doi.org/10.3390/jcm14134687









