Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Subjects and Ethics Statement
2.2. Statistical Analysis
3. Results
3.1. Ages of In-Hospital Death in Total Individuals, Individuals with Diabetes and Those without Diabetes
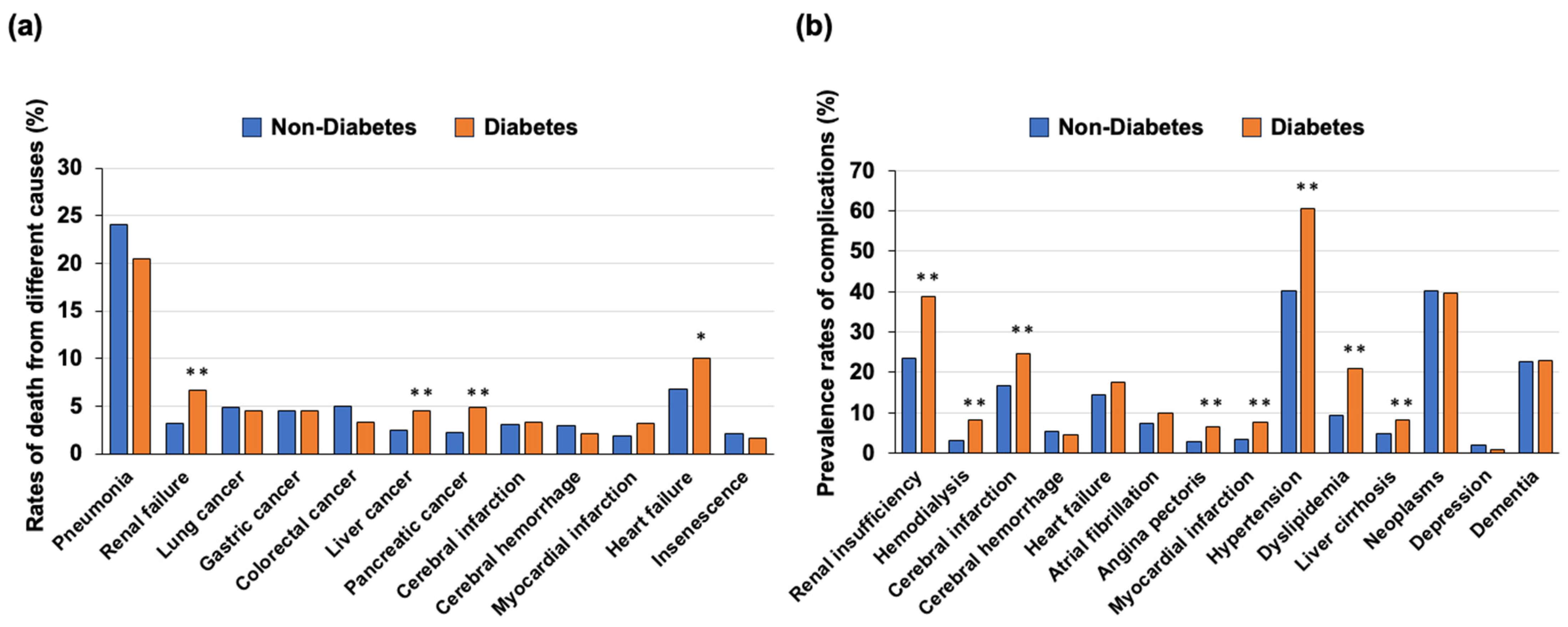
3.2. Causes of Death in Individuals with Diabetes and Those without Diabetes
3.3. Complications in Individuals with Diabetes and Those without Diabetes
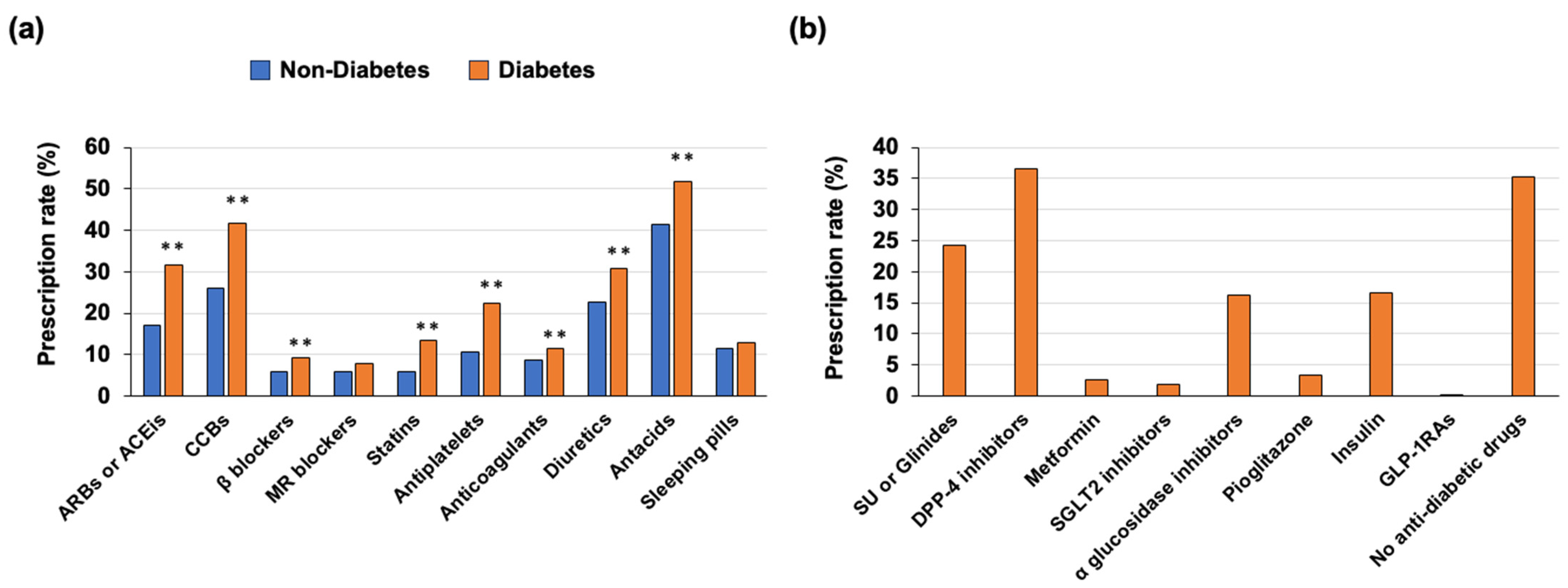
3.4. Medications Used in the Outpatient Clinic before Death
3.5. Identification of the Prescription Drugs Associated with Age of In-Hospital Death in Total Individuals, Individuals with Diabetes and Those without Diabetes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hotta, N.; Nakamura, J.; Iwamoto, Y.; Ohno, Y.; Kasuga, M.; Kikkawa, R.; Toyota, T. Causes of death in Japanese diabetics: A questionnaire survey of 18,385 diabetics over a 10-year period. J. Diabetes Investig. 2010, 1, 66–76. [Google Scholar] [CrossRef]
- Goto, A.; Takao, T.; Yoshida, Y.; Kawazu, S.; Iwamoto, Y.; Terauchi, Y. Causes of death and estimated life expectancy among people with diabetes: A retrospective cohort study in a diabetes clinic. J. Diabetes Investig. 2020, 11, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Khunti, K.; Gillies, C.L.; Dhalwani, N.N.; Davies, M.J.; Yates, T.; Zaccardi, F. Healthy lifestyle and life expectancy in people with multimorbidity in the UK Biobank: A longitudinal cohort study. PLoS Med. 2020, 17, e1003332. [Google Scholar] [CrossRef]
- Ding, M.; Fitzmaurice, G.M.; Arvizu, M.; Willett, W.C.; Manson, J.E.; Rexrode, K.M.; Hu, F.B.; Chavarro, J.E. Associations between patterns of modifiable risk factors in mid-life to late life and longevity: 36 year prospective cohort study. BMJ Med. 2022, 1, e000098. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Catasta, G. Determinants of longevity and age at death in a practically extinct cohort of middle-aged men followed-up for 61 years. Aging Clin. Exp. Res. 2022, 34, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Vedel, P.; Parving, H.H.; Pedersen, O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: The Steno type 2 randomised study. Lancet 1999, 353, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Vedel, P.; Larsen, N.; Jensen, G.V.; Parving, H.H.; Pedersen, O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.J.; Borch-Johnsen, K.; Davies, M.J.; Khunti, K.; Rutten, G.E.; Sandbaek, A.; Sharp, S.J.; Simmons, R.K.; van den Donk, M.; Wareham, N.J.; et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): A cluster-randomised trial. Lancet 2011, 378, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Sasako, T.; Okazaki, Y.; Kato, M.; Okahata, S.; Katsuyama, H.; Haraguchi, M.; Morita, A.; Ohashi, K.; Hara, K.; et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): An open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 951–964. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Shikata, K.; Haneda, M.; Ninomiya, T.; Koya, D.; Suzuki, Y.; Suzuki, D.; Ishida, H.; Akai, H.; Tomino, Y.; Uzu, T.; et al. Randomized trial of an intensified, multifactorial intervention in patients with advanced-stage diabetic kidney disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (DNETT-Japan). J. Diabetes Investig. 2021, 12, 207–216. [Google Scholar] [CrossRef]
- Sasako, T.; Yamauchi, T.; Ueki, K. Intensified Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2023, 47, 185–197. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Bennett, J.; Cheng, Y.J.; Vamos, E.P.; Cross, A.J.; Ezzati, M.; Gregg, E.W. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: An epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021, 9, 165–173. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Bus, S.A.; Monteiro-Soares, M.; Game, F.; van Netten, J.J.; Apelqvist, J.; Fitridge, R.; Senneville, E.; Schaper, N.C.; Board, I.E. Standards for the development and methodology of the 2023 IWGDF guidelines. Diabetes Metab. Res. Rev. 2023, e3656. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Tamaki, N.; Akasaka, T.; Ikeda, T.; Ueshima, K.; Uemura, S.; Otsuji, Y.; Kihara, Y.; Kimura, K.; Kimura, T.; et al. JCS 2018 Guideline on Diagnosis of Chronic Coronary Heart Diseases. Circ. J. 2021, 85, 402–572. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Newton, C.C.; Patel, A.V.; Jacobs, E.J.; Gapstur, S.M. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 2012, 35, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, M.; Aihara, K.; Fujinaka, Y.; Yoshida, S.; Ooguro, Y.; Kurahashi, K.; Kondo, T.; Aki, N.; Kuroda, A.; Endo, I.; et al. Diabetic conditions differentially affect the endothelial function, arterial stiffness and carotid atherosclerosis. J. Atheroscler. Thromb. 2014, 21, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Hara, T.; Yamagami, H.; Mitsui, Y.; Kurahashi, K.; Yoshida, S.; Harada, T.; Otoda, T.; Yuasa, T.; Nakamura, S.; et al. Vascular Endothelial Function Is Associated with eGFR Slope in Female and Non-Smoking Male Individuals with Cardiovascular Risk Factors: A Pilot Study on the Predictive Value of FMD for Renal Prognosis. J. Atheroscler. Thromb. 2023, 30, 1727–1741. [Google Scholar] [CrossRef]
- Parish, A.J.; Swindell, W.R. Metformin has heterogeneous effects on model organism lifespans and is beneficial when started at an early age in Caenorhabditis elegans: A systematic review and meta-analysis. Aging Cell 2022, 21, e13733. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Z.; Zhao, P. The Function of Metformin in Aging-Related Musculoskeletal Disorders. Front. Pharmacol. 2022, 13, 865524. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Samuel, S.M.; Marei, I.; Ding, H.; Triggle, C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014, 171, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.R.; Riester, M.R.; Hayes, K.N.; Munshi, M.N.; Berry, S.D. Comparative safety of sulfonylureas among U.S. nursing home residents. J. Am. Geriatr. Soc. 2023, 71, 1047–1057. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C.; Bigger, J.T., Jr.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Bonds, D.E.; Miller, M.E.; Bergenstal, R.M.; Buse, J.B.; Byington, R.P.; Cutler, J.A.; Dudl, R.J.; Ismail-Beigi, F.; Kimel, A.R.; Hoogwerf, B.; et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: Retrospective epidemiological analysis of the ACCORD study. BMJ 2010, 340, b4909. [Google Scholar] [CrossRef]
- Siraj, E.S.; Rubin, D.J.; Riddle, M.C.; Miller, M.E.; Hsu, F.C.; Ismail-Beigi, F.; Chen, S.H.; Ambrosius, W.T.; Thomas, A.; Bestermann, W.; et al. Insulin Dose and Cardiovascular Mortality in the ACCORD Trial. Diabetes Care 2015, 38, 2000–2008. [Google Scholar] [CrossRef]
- Gamble, J.M.; Chibrikov, E.; Twells, L.K.; Midodzi, W.K.; Young, S.W.; MacDonald, D.; Majumdar, S.R. Association of insulin dosage with mortality or major adverse cardiovascular events: A retrospective cohort study. Lancet Diabetes Endocrinol. 2017, 5, 43–52. [Google Scholar] [CrossRef]
- Boureau, A.S.; Guyomarch, B.; Gourdy, P.; Allix, I.; Annweiler, C.; Cervantes, N.; Chapelet, G.; Delabriere, I.; Guyonnet, S.; Litke, R.; et al. Nocturnal hypoglycemia is underdiagnosed in older people with insulin-treated type 2 diabetes: The HYPOAGE observational study. J. Am. Geriatr. Soc. 2023, 71, 2107–2119. [Google Scholar] [CrossRef]

| Total (2336) | Non-Diabetes (1766) | Diabetes (570) | Non-Diabetes vs. Diabetes | |
|---|---|---|---|---|
| Period | 2011–2020 | |||
| Males (age of death, years (n)) | 79.4 ± 10.4 (1206) | 79.6 ± 10.9 (880) | 78.8 ± 8.9 (326) | ns |
| Females (age of death, years (n)) | 83.4 ± 10.6 (1130) | 83.6 ± 10.9 (886) | 82.5 ± 9.7 (244) | ns |
| Period | 2011–2015 | |||
| Males (age of death, years (n)) | 78.3 ± 10.6 (585) | 78.6 ± 11.1 (429) | 77.6 ± 8.9 (156) | ns |
| Females (age of death, years (n)) | 82.0 ± 11.0 (532) | 82.3 ± 11.2 (418) | 80.8 ± 10.4 (114) | ns |
| Period | 2016–2020 | |||
| Males (age of death, years (n)) | 80.4 ± 10.1 (621) * | 80.6 ± 10.5 (451) * | 80.0 ± 8.8 (170) * | ns |
| Females (age of death, years (n)) | 84.6 ± 10.1 (598) ** | 84.8 ± 10.4 (468) ** | 83.9 ± 8.7 (130) ** | ns |
| Insulin User Group (95) | Anti-Diabetic Drugs Other than Insulin Group (273) | No Anti-Diabetic Drug Group (202) | ||||
|---|---|---|---|---|---|---|
| Males (56) | Females (39) | Males (156) | Females (117) | Males (114) | Females (88) | |
| Age of death (years) | 75.1 ± 10.3 | 76.6 ± 12.4 | 79.2 ± 8.0 * | 83.3 ± 8.7 * | 80.1 ± 9.1 ** | 84.0 ± 8.6 ** |
| HbA1c (%) (sample number) | 7.05 ± 1.73 (47) | 7.63 ± 2.02 (35) | 6.77 ± 1.32 (137) | 6.75 ± 1.44 ** (103) | 6.29 ± 0.96 *# (88) | 6.51 ± 1.12 ** (69) |
| Variables | t Value | VIF | p Value |
|---|---|---|---|
| Male | −8.961 | 1.031 | <0.001 |
| Hypertension | 5.990 | 1.965 | <0.001 |
| Dyslipidemia | −1.967 | 2.132 | 0.049 |
| Diabetes | −0.448 | 1.967 | 0.655 |
| ARBs or ACEis | 0.465 | 1.359 | 0.642 |
| CCBs | 0.505 | 1.691 | 0.614 |
| β blockers | 0.564 | 1.107 | 0.573 |
| MR blockers | −1.158 | 1.166 | 0.247 |
| Statins | −1.570 | 2.036 | 0.117 |
| Ezetimibe | −0.095 | 1.076 | 0.924 |
| Antiplatelets | 3.592 | 1.144 | 0.001 |
| Anticoagulants | 2.720 | 1.097 | 0.007 |
| Diuretics | 2.461 | 1.261 | 0.014 |
| Antacids | −0.396 | 1.089 | 0.693 |
| Sleeping pills | −2.338 | 1.037 | 0.020 |
| SU or Glinides | −2.721 | 1.363 | 0.007 |
| DPP-4 inhibitors | 0.022 | 1.555 | 0.983 |
| Metformin | −0.296 | 1.055 | 0.768 |
| SGLT2 inhibitors | −0.836 | 1.039 | 0.403 |
| α glucosidase inhibitors | 0.029 | 1.259 | 0.977 |
| Pioglitazone | 0.949 | 1.091 | 0.343 |
| Insulin | −4.485 | 1.185 | <0.001 |
| GLP-1RAs | 0.339 | 1.013 | 0.735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoki, M.; Hori, T.; Kaneko, Y.; Mori, K.; Yasui, S.; Tsuji, S.; Yamagami, H.; Kawata, S.; Hara, T.; Masuda, S.; et al. Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital. J. Clin. Med. 2024, 13, 1283. https://doi.org/10.3390/jcm13051283
Hosoki M, Hori T, Kaneko Y, Mori K, Yasui S, Tsuji S, Yamagami H, Kawata S, Hara T, Masuda S, et al. Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital. Journal of Clinical Medicine. 2024; 13(5):1283. https://doi.org/10.3390/jcm13051283
Chicago/Turabian StyleHosoki, Minae, Taiki Hori, Yousuke Kaneko, Kensuke Mori, Saya Yasui, Seijiro Tsuji, Hiroki Yamagami, Saki Kawata, Tomoyo Hara, Shiho Masuda, and et al. 2024. "Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital" Journal of Clinical Medicine 13, no. 5: 1283. https://doi.org/10.3390/jcm13051283
APA StyleHosoki, M., Hori, T., Kaneko, Y., Mori, K., Yasui, S., Tsuji, S., Yamagami, H., Kawata, S., Hara, T., Masuda, S., Mitsui, Y., Kurahashi, K., Harada, T., Nakamura, S., Otoda, T., Yuasa, T., Kuroda, A., Endo, I., Matsuhisa, M., & Aihara, K.-i. (2024). Causes of In-Hospital Death and Pharmaceutical Associations with Age of Death during a 10-Year Period (2011–2020) in Individuals with and without Diabetes at a Japanese Community General Hospital. Journal of Clinical Medicine, 13(5), 1283. https://doi.org/10.3390/jcm13051283






