Impact of COVID-19 Infections among Unvaccinated Patients with Congenital Heart Disease: Results of a Nationwide Analysis in the First Phase of the Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, A.E.; Heinsbroek, E.; Kall, M.M.; Allen, H.; Beebeejaun, K.; Blomquist, P.; Campos-Matos, I.; Campbell, C.N.J.; Mohammed, H.; Sinka, K.; et al. Epidemiology of Confirmed COVID-19 Deaths in Adults, England, March-December 2020. Emerg. Infect. Dis. 2021, 27, 1468–1471. [Google Scholar] [CrossRef]
- Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022, 399, 1513–1536. [CrossRef]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar]
- Del Turco, S.; Vianello, A.; Ragusa, R.; Caselli, C.; Basta, G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb Res. 2020, 196, 143–151. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Naidu, P.; Grigg, L.; Zentner, D. Mortality in adults with congenital heart disease. Int. J. Cardiol. 2017, 245, 125–130. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef]
- Alsaied, T.; Aboulhosn, J.A.; Cotts, T.B.; Daniels, C.J.; Etheridge, S.P.; Feltes, T.F.; Gurvitz, M.Z.; Lewin, M.B.; Oster, M.E.; Saidi, A. Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J. Am. Heart Assoc. 2020, 9, e017224. [Google Scholar] [CrossRef]
- Fong, M.W.; Gao, H.; Wong, J.Y.; Xiao, J.; Shiu, E.Y.C.; Ryu, S.; Cowling, B.J. Nonpharmaceutical Measures for Pandemic Influenza in Nonhealthcare Settings-Social Distancing Measures. Emerg. Infect. Dis. 2020, 26, 976–984. [Google Scholar] [CrossRef]
- Brodeur, A.; Gray, D.; Islam, A.; Bhuiyan, S. A literature review of the economics of COVID-19. J. Econ. Surv. 2021, 35, 1007–1044. [Google Scholar] [CrossRef]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental Health of Children and Adolescents Amidst COVID-19 and Past Pandemics: A Rapid Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 563–645. [Google Scholar] [CrossRef]
- Rommel, A.; von der Lippe, E.; Plaß, D.; Ziese, T.; Diercke, M.; an der Heiden, M.; Haller, S.; Wengler, A. COVID-19-Krankheitslast in Deutschland im Jahr 2020. Dtsch. Ärzteblatt 2021, 118, 145–151. [Google Scholar]
- Diller, G.-P.; Gatzoulis, M.A.; Broberg, C.S.; Aboulhosn, J.; Brida, M.; Schwerzmann, M.; Chessa, M.; Kovacs, A.H.; Roos-Hesselink, J. Coronavirus disease 2019 in adults with congenital heart disease: A position paper from the ESC working group of adult congenital heart disease, and the International Society for Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 1858–1865. [Google Scholar] [CrossRef]
- Sabatino, J.; Ferrero, P.; Chessa, M.; Bianco, F.; Ciliberti, P.; Secinaro, A.; Oreto, L.; Avesani, M.; Bucciarelli, V.; Calcaterra, G.; et al. COVID-19 and Congenital Heart Disease: Results from a Nationwide Survey. J. Clin. Med. 2020, 9, 1774. [Google Scholar] [CrossRef]
- Karsenty, C.; Maury, P.; Blot-Souletie, N.; Ladouceur, M.; Leobon, B.; Senac, V.; Mondoly, P.; Elbaz, M.; Galinier, M.; Dulac, Y.; et al. The medical history of adults with complex congenital heart disease affects their social development and professional activity. Arch Cardiovasc. Dis. 2015, 108, 589–597. [Google Scholar] [CrossRef]
- Clancy, T.; Jordan, B.; de Weerth, C.; Muscara, F. Early Emotional, Behavioural and Social Development of Infants and Young Children with Congenital Heart Disease: A Systematic Review. J. Clin. Psychol. Med. Settings 2020, 27, 686–703. [Google Scholar] [CrossRef]
- Girouard, H.S.; Kovacs, A.H. Congenital heart disease: Education and employment considerations and outcomes. Int. J. Cardiol. Congenit. Heart Dis. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Broberg, C.S.; Kovacs, A.H.; Sadeghi, S.; Rosenbaum, M.S.; Lewis, M.J.; Carazo, M.R.; Rodriguez, F.H., 3rd; Halpern, D.G.; Feinberg, J.; Galilea, F.A.; et al. COVID-19 in Adults with Congenital Heart Disease. J. Am. Coll. Cardiol. 2021, 77, 1644–1655. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Z.; Mo, P.; Li, X.; Ma, Z.; Song, S.; Chen, X.; Luo, M.; Liang, K.; Gao, S.; et al. Clinical Characteristics and Outcomes of Older Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Single-Centered, Retrospective Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1788–1795. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Romero Starke, K.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The isolated effect of age on the risk of COVID-19 severe outcomes: A systematic review with meta-analysis. BMJ Glob. Health 2021, 6, e006434. [Google Scholar] [CrossRef]
- Soleimani, A.; Soleimani, Z. Presentation and Outcome of Congenital Heart Disease During COVID-19 Pandemic: A Review. Curr. Probl. Cardiol. 2022, 47, 100905. [Google Scholar] [CrossRef]
- Sian, C.; Aoife, C.; Rachel, K.; Sonya, V.B.-N.; John, M.S.; Heba, N.; Konstantinos, D.; Michael, A.G.; Dirk, W.; Milos, P.; et al. COVID-19 in congenital heart disease (COaCHeD) study. Open Heart 2023, 10, e002356. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, J.M.; Koolbergen, D.R.; Groenink, M.; Peels, K.C.H.; Reichert, C.L.A.; Post, M.C.; Bosker, H.A.; Wajon, E.M.C.J.; Zwinderman, A.H.; Mulder, B.J.M.; et al. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: Focus on the use of prosthetic material. Eur. Heart J. 2017, 38, 2048–2056. [Google Scholar] [CrossRef]
- Schwerzmann, M.; Ruperti-Repilado, F.J.; Baumgartner, H.; Bouma, B.; Bouchardy, J.; Budts, W.; Campens, L.; Chessa, M.; Del Cerro Marin, M.J.; Gabriel, H.; et al. Clinical outcome of COVID-19 in patients with adult congenital heart disease. Heart 2021, 107, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ammar, L.A.; Nassar, J.E.; Bitar, F.; Arabi, M. COVID-19 in Cyanotic Congenital Heart Disease. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 5561159. [Google Scholar] [CrossRef]
- Fischer, A.J.; Enders, D.; Baumgartner, H.; Diller, G.P. Invasive electrophysiological studies in adult congenital heart disease-evolving use and impact of specialized centre therapy on recurrence of arrhythmia: A nationwird analysis based on 2.433 cases. Eur. Heart J. 2020, 41 (Suppl. 2), ehaa946.2196. [Google Scholar] [CrossRef]
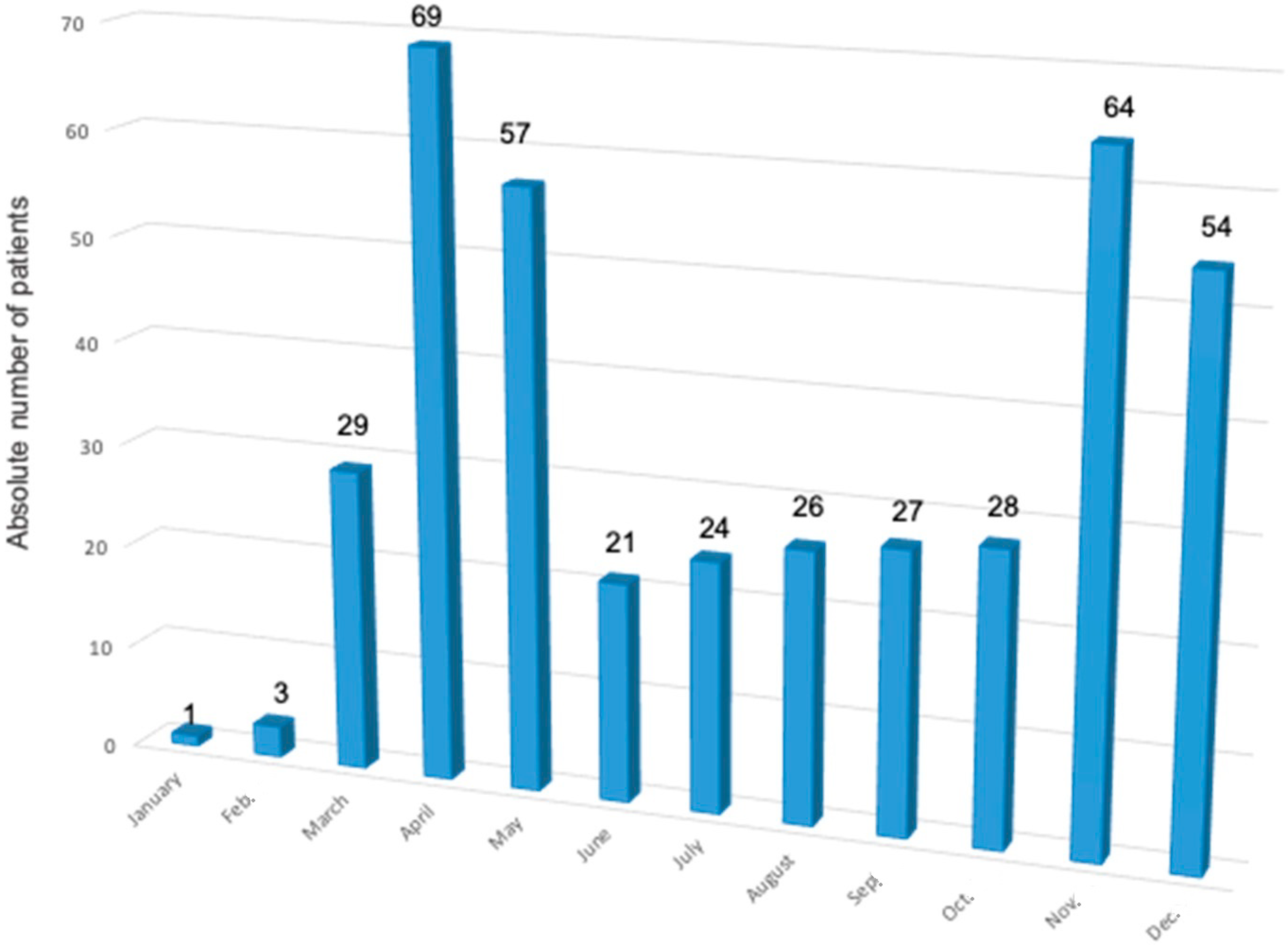
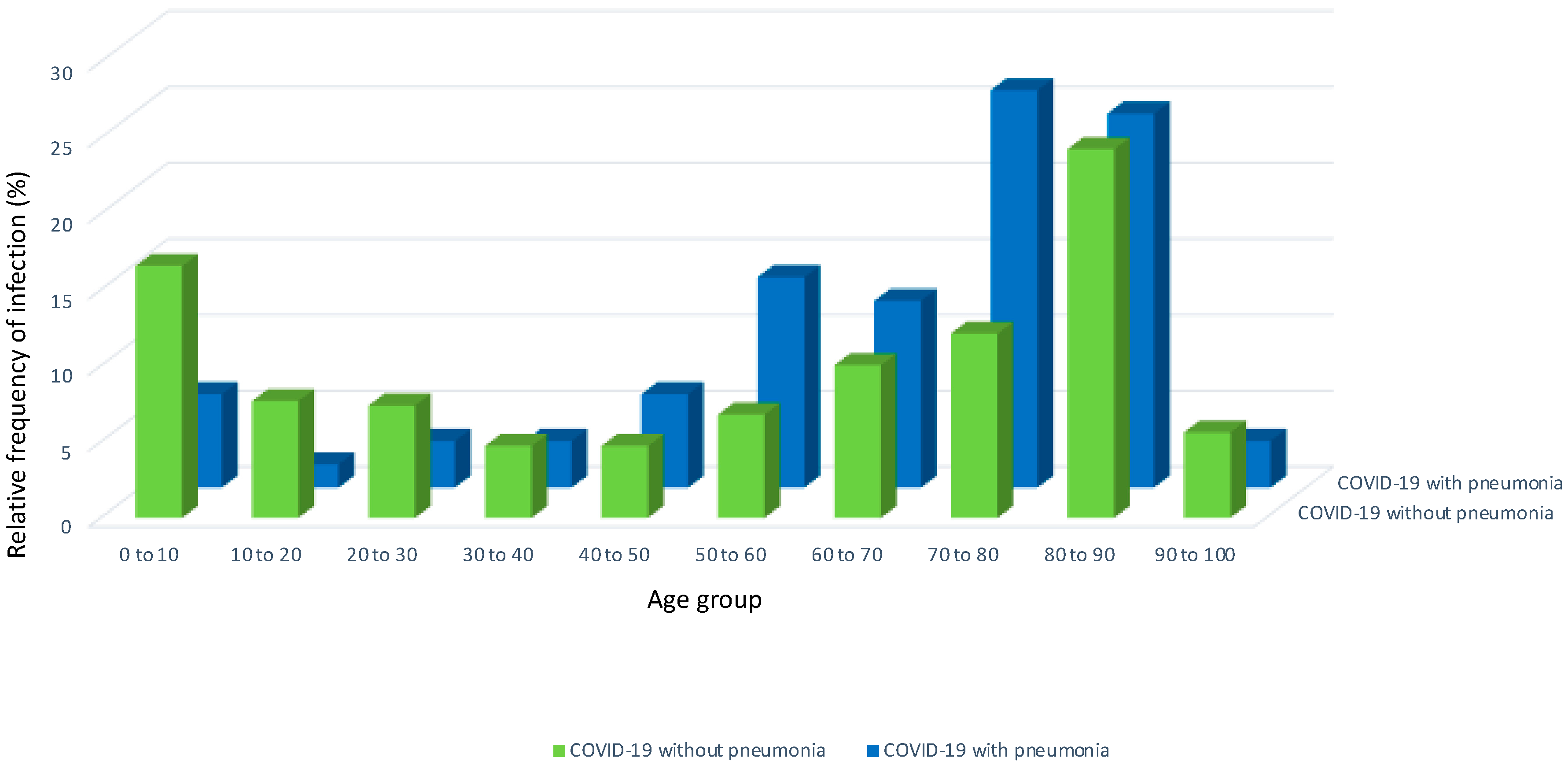
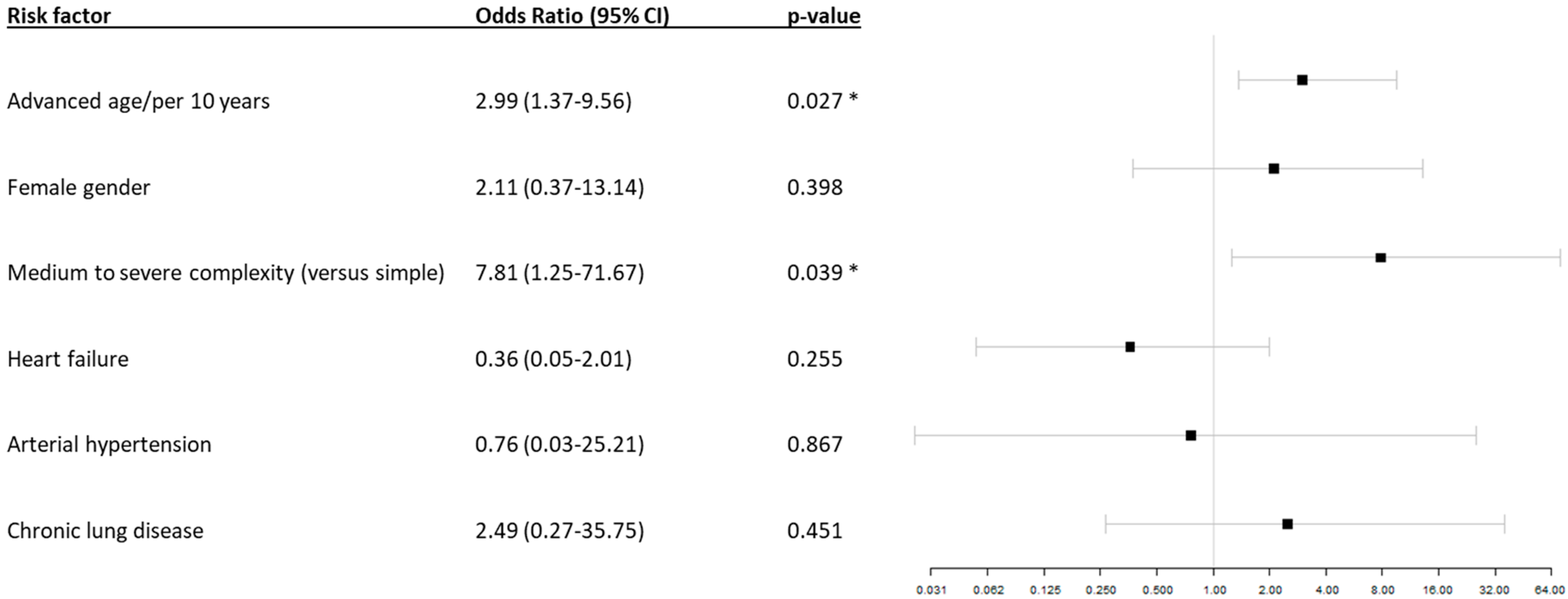
| Patients with COVID-19 without Pneumonia | Patients with COVID-19 and Pneumonia | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median age (Q1, Q3) | 62.1 (21.7, 81.3) | 72.6 (57.3, 80.3) | 0.04 | ||||||
| Age group | All | <18 | 18–65 | >65 | All | <18 | 18–65 | >65 | |
| Number of patients | 338 | 75 | 103 | 160 | 65 | 4 | 23 | 38 | |
| Female sex | 184 (54.4) | 32 | 56 | 96 | 29 (44.6) | 2 | 10 | 17 | 0.18 |
| Congenital heart disease (CHD) | |||||||||
| Simple CHD, n (%) | 243 (71.9) | 49 | 70 | 124 | 48 (73.8) | 3 | 17 | 28 | 0.88 |
| Moderate CHD, n (%) | 52 (15.4) | 11 | 20 | 21 | 12 (18.5) | 1 | 5 | 6 | 0.58 |
| Severe CHD, n (%) | 43 (12.7) | 5 | 13 | 15 | 5 (7.7) | 0 | 1 | 4 | 0.05 |
| Other patient characteristics | |||||||||
| Coronary artery disease, n (%) | 65 (19.2) | 0 | 9 | 56 | 19 (29.2) | 0 | 5 | 14 | 0.09 |
| Congestive heart failure > NYHA II, n (%) | 89 (26.3) | 6 | 18 | 65 | 14 (21.5) | 0 | 4 | 10 | 0.54 |
| Diabetes mellitus, n (%) | 107 (31.7) | 1 | 22 | 84 | 28 (43.1) | 0 | 9 | 19 | 0.09 |
| Chronic kidney disease, n (%) | 123 (36.4) | 1 | 27 | 95 | 28 (43.1) | 0 | 7 | 21 | 0.33 |
| Obesity, n (%) | 109 (32.3) | 4 | 32 | 73 | 29 (44.6) | 0 | 13 | 16 | 0.06 |
| Nicotine abuse, n (%) | 59 (17.5) | 0 | 27 | 32 | 15 (23.1) | 0 | 5 | 10 | 0.30 |
| History of stroke, n (%) | 79 (23.4) | 1 | 18 | 60 | 27 (41.5) | 0 | 6 | 21 | 0.003 |
| Cancer, n (%) | 143 (42.3) | 2 | 30 | 111 | 34 (52.3) | 0 | 8 | 26 | 0.17 |
| Chromosomal anomaly, n (%) | 20 (5.9) | 8 | 11 | 1 | 6 (9.2) | 1 | 5 | 0 | 0.40 |
| Drug therapy of selective cardiac and non-cardiac drugs | |||||||||
| Oral anticoagulants, n (%) | 118 (34.9) | 3 | 28 | 87 | 29 (44.6) | 0 | 6 | 23 | 0.16 |
| Platelet activation inhibitor, n (%) | 117 (34.6) | 7 | 22 | 88 | 28 (43.1) | 0 | 7 | 21 | 0.21 |
| Statins, n (%) | 133 (39.3) | 0 | 24 | 109 | 37 (56.9) | 0 | 7 | 30 | 0.009 |
| Beta blocker, n (%) | 185 (54.7) | 1 | 47 | 137 | 44 (67.7) | 1 | 14 | 29 | 0.06 |
| ACE- inhibitors/Angiotensin II receptor blockers, n (%) | 194 (57.4) | 4 | 47 | 143 | 46 (70.8) | 0 | 12 | 34 | 0.05 |
| Diuretics, n (%) | 180 (53.3) | 10 | 38 | 132 | 42 (64.6) | 0 | 12 | 30 | 0.10 |
| Non-steroidal antirheumatic agents, n (%) | 282 (83.4) | 50 | 89 | 143 | 55 (84.6) | 0 | 20 | 35 | 1 |
| Patients with COVID-19 | Patients with COVID-19 and Pneumonia | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | All | <18 | 18–65 | >65 | All | <18 | 18–65 | >65 | |
| Median length of in-hospital stay, days (Q1, Q3) | 5 (3, 12) | 3 (2, 8.5) | 4 (2.5, 8.5) | 8 (4, 16) | 8 (5, 14) | 3.5 (3, 8) | 7 (5, 11.5) | 10 (4, 15) | 0.01 |
| Median costs, EUR (Q1, Q3) | 3524 (2434, 6843) | 2726 (2078, 6922) | 3253 (2442, 5474) | 4094 (2800, 7266) | 4076 (3151, 7078) | 3041 (2886, 4335) | 3799 (3180, 6734) | 4349 (3102, 9104) | 0.02 |
| Treatment at intensive care unit, n (%) | 15 (4.4) | 0 | 4 | 11 | 8 (12.3) | 0 | 2 | 6 | 0.02 |
| Invasive ventilation, n (%) | 35 (10.4) | 13 | 8 | 14 | 14 (21.5) | 1 | 3 | 10 | 0.02 |
| Circulatory support, n (%) | 8 (2.4) | 6 | 2 | 0 | 1 (1.5) | 0 | 1 | 0 | 1 |
| Resuscitation, n (%) | 12 (3.6) | 1 | 6 | 5 | 3 (4.6) | 0 | 1 | 2 | 0.72 |
| Patients with COVID-19 Only (n = 22) | Patients with COVID-19 and Pneumonia (n = 14) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Median age–years (Q1, Q3) | 82.6 (77.5, 88.7) | 81.9 (77.0, 83.4) | 0.67 | ||||
| Age group | All | 18–65 | >65 | All | 18–65 | >65 | |
| Female sex, n (%) | 11 (50.0) | 1 | 10 | 7 (50.0) | 0 | 7 | 1 |
| Congenital heart disease | |||||||
| Simple, n (%) | 17 (77.3) | 1 | 16 | 7 (50.0) | 0 | 7 | 0.15 |
| Moderate, n (%) | 2 (9.1) | 1 | 1 | 3 (21.4) | 0 | 3 | 0.36 |
| Severe, n (%) | 3 (13.6) | 0 | 3 | 4 (28.6) | 1 | 3 | 0.39 |
| Details on in-hospital treatment | |||||||
| Median length of in hospital stay, days (Q1, Q3) | 9 (6, 10.75) | 9.5 (9.25, 9.75) | 9 (5.3, 11.0) | 4.5 (3.3, 10.8) | 5 (5,5) | 4 (3.0, 11.0) | 0.43 |
| Median costs, EUR (Q1, Q3) | 3946 (3193, 6331) | 4006 (3710, 4301) | 3946 (3097, 6611) | 3896 (3026, 12,753) | 18,144 (18,144, 18,144) | 3631 (2959, 11,466) | 0.71 |
| Treatment at intensive care unit, n (%) | 1 (4.6) | 0 | 1 | 2 (14.3) | 0 | 2 | 0.55 |
| Invasive ventilation, n (%) | 2 (9.1) | 0 | 2 | 5 (35.7) | 1 | 4 | 0.08 |
| Circulatory support, n (%) | 0 (0) | 0 | 0 | 1 (7.1) | 1 | 0 | 0.39 |
| Resuscitation, n (%) | 2 (9.1) | 1 | 1 | 3 (21.4) | 1 | 2 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.J.; Hellmann, A.R.; Diller, G.-P.; Maser, M.; Szardenings, C.; Marschall, U.; Bauer, U.; Baumgartner, H.; Lammers, A.E. Impact of COVID-19 Infections among Unvaccinated Patients with Congenital Heart Disease: Results of a Nationwide Analysis in the First Phase of the Pandemic. J. Clin. Med. 2024, 13, 1282. https://doi.org/10.3390/jcm13051282
Fischer AJ, Hellmann AR, Diller G-P, Maser M, Szardenings C, Marschall U, Bauer U, Baumgartner H, Lammers AE. Impact of COVID-19 Infections among Unvaccinated Patients with Congenital Heart Disease: Results of a Nationwide Analysis in the First Phase of the Pandemic. Journal of Clinical Medicine. 2024; 13(5):1282. https://doi.org/10.3390/jcm13051282
Chicago/Turabian StyleFischer, Alicia Jeanette, Alina Ruth Hellmann, Gerhard-Paul Diller, Maarja Maser, Carsten Szardenings, Ursula Marschall, Ulrike Bauer, Helmut Baumgartner, and Astrid Elisabeth Lammers. 2024. "Impact of COVID-19 Infections among Unvaccinated Patients with Congenital Heart Disease: Results of a Nationwide Analysis in the First Phase of the Pandemic" Journal of Clinical Medicine 13, no. 5: 1282. https://doi.org/10.3390/jcm13051282
APA StyleFischer, A. J., Hellmann, A. R., Diller, G.-P., Maser, M., Szardenings, C., Marschall, U., Bauer, U., Baumgartner, H., & Lammers, A. E. (2024). Impact of COVID-19 Infections among Unvaccinated Patients with Congenital Heart Disease: Results of a Nationwide Analysis in the First Phase of the Pandemic. Journal of Clinical Medicine, 13(5), 1282. https://doi.org/10.3390/jcm13051282






