Whole-Body Cryostimulation as an Adjunctive Treatment for Neurophysiologic Tinnitus and Associated Disorders: Preliminary Evidence from a Case Study
Abstract
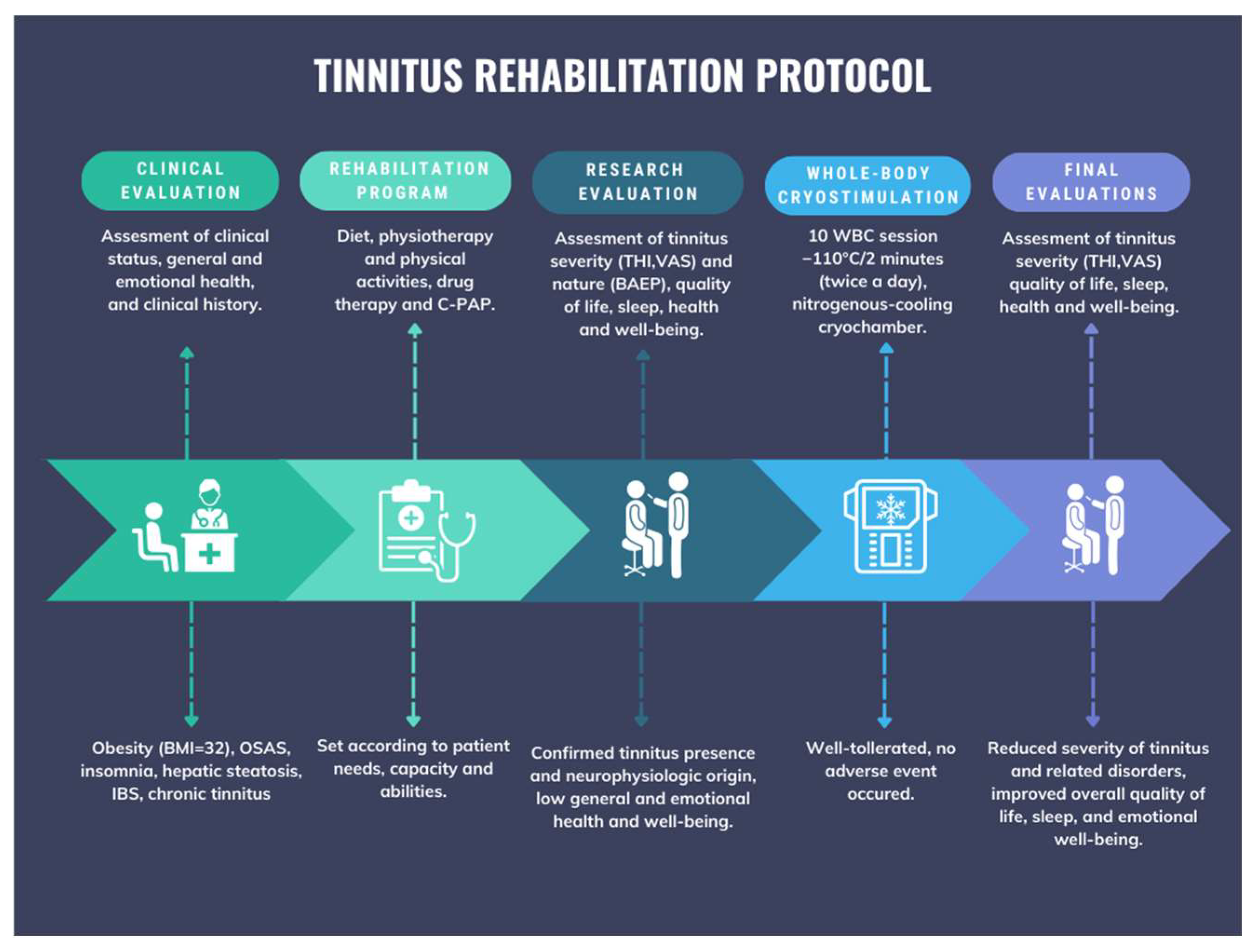
:1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Tinnitus History and Description
2.3. Symptom Assessment
2.4. Intervention
2.4.1. Diet, Physiotherapy, and Physical Activity
- Total caloric intake: 1600 kcal.
- Nutritional composition: proteins 86 g (21%), lipids 50 g (28%), carbohydrates 209 g (51%).
2.4.2. Drug Therapy and CPAP
- Esomeprazole 20 mg;
- Duloxetine 60 mg, twice daily;
- Melatonin Fast, 10 drops at 11:00 PM;
- Clobesol 0.05% cream, applied once in the morning and once in the evening.
2.4.3. Repetitive Transcranial Magnetic Stimulation (rTMS)
2.4.4. Whole-Body Cryostimulation (WBC)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hesse, G. Evidence and evidence gaps in tinnitus therapy. GMS Curr. Top. Otorhinolaryngol. Head. Neck Surg. 2016, 15, Doc04. [Google Scholar]
- Henry, J.A.; Zaugg, T.L.; Myers, P.J.; Kendall, C.J.; Michaelides, E.M. A triage guide for tinnitus. J. Fam. Pract. 2010, 59, 389–393. [Google Scholar] [PubMed]
- Pichora-Fuller, M.K.; Santaguida, P.; Hammill, A.; Oremus, M.; Westerberg, B.; Ali, U.; Patterson, C.; Raina, P. Evaluation and Treatment of Tinnitus: Comparative Effectiveness; AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK158963/ (accessed on 16 November 2023).
- Mennink, L.M.; Aalbers, M.W.; van Dijk, P.; van Dijk, J.M.C. The Role of Inflammation in Tinnitus: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1000. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Geocze, L.; Mucci, S.; Abranches, D.C.; de Marco, M.A.; de Oliveira Penido, N. Systematic review on the evidences of an association between tinnitus and depression. Braz. J. Otorhinolaryngol. 2013, 79, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Milinski, L.; Nodal, F.R.; Vyazovskiy, V.V.; Bajo, V.M. Tinnitus: At a crossroad between phantom perception and sleep. Brain Commun. 2022, 4, fcac089. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, P.J.; Hazell, J.W. A neurophysiological approach to tinnitus: Clinical implications. Br. J. Audiol. 1993, 27, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Knipper, M.; Van Dijk, P.; Nunes, I.; Rüttiger, L.; Zimmermann, U. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Prog. Neurobiol. 2013, 111, 17–33. [Google Scholar] [CrossRef]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation associated with noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 4020. [Google Scholar] [CrossRef]
- Adcock, K.; Vanneste, S. Neuroinflammation in Tinnitus. Curr. Otorhinolaryngol. Rep. 2022, 10, 322–328. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.S.; Zinsmaier, A.K.; Patterson, G.; Leptich, E.J.; Shoemaker, S.L.; Yatskievych, T.A.; Gibboni, R.; Pace, E.; Luo, H.; et al. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLOS Biol. 2019, 17, e3000307. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Lombardi, G.; Colombini, A.; Melegati, G. Whole-body cryotherapy in athletes. Sports Med. 2010, 40, 509. [Google Scholar] [CrossRef]
- Varallo, G.; Piterà, P.; Fontana, J.M.; Gobbi, M.; Arreghini, M.; Giusti, E.M.; Franceschini, C.; Plazzi, G.; Castelnuovo, G.; Capodaglio, P. Is Whole-Body Cryostimulation an Effective Add-On Treatment in Individuals with Fibromyalgia and Obesity? A Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 4324. [Google Scholar]
- Fontana, J.M.; Bozgeyik, S.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Dugué, B.; Lombardi, G.; Capodaglio, P. Whole-body cryostimulation in obesity. A scoping review. J. Therm. Biol. 2022, 106, 103250. [Google Scholar]
- Capodaglio, P.; Cremascoli, R.; Piterà, P.; Fontana, J.M. Whole-Body Cryostimulation: A Rehabilitation Booster. J. Rehabil. Med. Clin. Commun. 2022, 5, 2810. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case-control study. Acta Neurol. Scand. 2016, 134, 420–426. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-Body Cryotherapy in Athletes: From Therapy to Stimulation. An Updated Review of the Literature. Front. Physiol. 2017, 8, 258. [Google Scholar]
- Douzi, W.; Dupuy, O.; Tanneau, M.; Boucard, G.; Bouzigon, R.; Dugué, B. 3-min whole body cryotherapy/cryostimulation after training in the evening improves sleep quality in physically active men. Eur. J. Sport. Sci. 2019, 19, 860–867. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Urbanska, K.; Szcześniak, D.; Pawłowski, T.; Pieniawska-Śmiech, K.; Kokot, I.; Pawlik-Sobecka, L.; Płaczkowska, S.; Zabłocka, A.; Stańczykiewicz, B. Whole-body cryotherapy—Promising add-on treatment of depressive disorders. Psychiatr. Pol. 2019, 53, 1053–1067. [Google Scholar] [CrossRef]
- Szczepańska-Gieracha, J.; Borsuk, P.; Pawik, M.; Rymaszewska, J. Mental state and quality of life after 10 session whole-body cryotherapy. Psychol. Health Med. 2014, 19, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kamińska-Staruch, A.; Olszewski, J. Evaluation of effectiveness of whole-body cryotherapy in patients with tinnitus. Otolaryngol. Pol. 2007, 61, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, P.J. Tinnitus retraining therapy. In Progress in Brain Research; Langguth, B., Hajak, G., Kleinjung, T., Cacace, A., Møller, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 415–423. Available online: https://www.sciencedirect.com/science/article/pii/S0079612307660403 (accessed on 15 November 2023).
- Soleimani, R.; Jalali, M.M.; Hasandokht, T. Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2016, 273, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huo, Y.; Lui, G.; Li, M.; Tyler, R.S.; Ping, H. Reliability and Validity of the Tinnitus Handicap Inventory: A Clinical Study of Questionnaires. J. Int. Adv. Otol. 2022, 18, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Adamchic, I.; Langguth, B.; Hauptmann, C.; Alexander Tass, P. Psychometric Evaluation of Visual Analog Scale for the Assessment of Chronic Tinnitus. Am. J. Audiol. 2012, 21, 215–225. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ciervo, C.A.; Kabat, M. Use of the Beck Anxiety and Depression Inventories for Primary Care with Medical Outpatients. Assessment 1997, 4, 211–219. [Google Scholar] [CrossRef]
- Fydrich, T.; Dowdall, D.; Chambless, D.L. Reliability and validity of the beck anxiety inventory. J. Anxiety Disord. 1992, 6, 55–61. [Google Scholar] [CrossRef]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK564321/ (accessed on 10 December 2023).
- McFerran, D.J.; Stockdale, D.; Holme, R.; Large, C.H.; Baguley, D.M. Why Is There No Cure for Tinnitus? Front. Neurosci. 2019, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Ramsey, D.; Chładzińska-Kiejna, S. Whole-body cryotherapy as adjunct treatment of depressive and anxiety disorders. Arch. Immunol. Ther. Exp. 2008, 56, 63–68. [Google Scholar] [CrossRef] [PubMed]
| Outcome and Range | Pre | Post | Δ% |
|---|---|---|---|
| THI (0–96) | 82 | 44 | −46.3% |
| SF-36 | |||
| Physical functioning | 61.5 | 65 | +4.5% |
| Role limitations due to physical health | 100 | 100 | 0 |
| Role limitations due to emotional problems | 100 | 100 | 0 |
| Energy/fatigue | 30 | 35 | +5% |
| Emotional well-being | 28 | 40 | +12% |
| Social functioning | 0 | 12.5 | +12.5% |
| Pain | 20 | 42.5 | +22.5% |
| General health | 5 | 15 | +10% |
| WHO-5 (0–100) | 24% | 40% | +16% |
| PSQI (0–21) | 12 | 7 | −41.67% |
| BDI (0–63) | 17 | 10 | −41.2% |
| BAI | 3 | 3 | 0 |
| WBC Session | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 |
|---|---|---|---|---|---|---|---|---|---|---|
| VAS tinnitus score pre-WBC | 70 | 60 | 50 | 50 | 35 | 35 | 35 | 35 | 35 | 35 |
| VAS tinnitus score post-WBC | 70 | 60 | 45 | 45 | 35 | 35 | 30 | 35 | 35 | 30 |
| Aud.Stim. dB | I Latency (ms) | II Latency (ms) | III Latency (ms) | IV Latency (ms) | V Latency (ms) | I-V Latency (ms) | |
|---|---|---|---|---|---|---|---|
| ipsilateral | 130 SPL | 0.98 | 1.76 | 2.78 | 3.98 | 5.40 | 4.43 |
| ipsilateral | 125 SPL | 0.94 | 1.66 | 2.80 | 3.98 | 5.12 | 4.18 |
| ipsilateral | 110 SPL | 1.62 | 2.74 | 3.94 | 5 | 5.62 | 4 |
| ipsilateral | 110 SPL | 1.68 | 2.54 | 4.08 | 5.10 | 5.90 | 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piterà, P.; Cremascoli, R.; Alito, A.; Bianchi, L.; Galli, F.; Verme, F.; Fontana, J.M.; Bigoni, M.; Priano, L.; Mauro, A.; et al. Whole-Body Cryostimulation as an Adjunctive Treatment for Neurophysiologic Tinnitus and Associated Disorders: Preliminary Evidence from a Case Study. J. Clin. Med. 2024, 13, 993. https://doi.org/10.3390/jcm13040993
Piterà P, Cremascoli R, Alito A, Bianchi L, Galli F, Verme F, Fontana JM, Bigoni M, Priano L, Mauro A, et al. Whole-Body Cryostimulation as an Adjunctive Treatment for Neurophysiologic Tinnitus and Associated Disorders: Preliminary Evidence from a Case Study. Journal of Clinical Medicine. 2024; 13(4):993. https://doi.org/10.3390/jcm13040993
Chicago/Turabian StylePiterà, Paolo, Riccardo Cremascoli, Angelo Alito, Laura Bianchi, Federica Galli, Federica Verme, Jacopo Maria Fontana, Matteo Bigoni, Lorenzo Priano, Alessandro Mauro, and et al. 2024. "Whole-Body Cryostimulation as an Adjunctive Treatment for Neurophysiologic Tinnitus and Associated Disorders: Preliminary Evidence from a Case Study" Journal of Clinical Medicine 13, no. 4: 993. https://doi.org/10.3390/jcm13040993








